Back to Journals » International Journal of General Medicine » Volume 14
Prevalence of Anemia and Associated Risk Factors Among Non-Pregnant Women in Riyadh, Saudi Arabia: A Cross-Sectional Study
Authors AlFaris N , ALTamimi J, AlKehayez N , AlMushawah F, AlNaeem A, AlAmri N, AlMudawah E, Alsemari M , Alzahrani J, Alqahtani II L, Alenazi W, Almuteb II A, Alotibi H
Received 29 December 2020
Accepted for publication 12 February 2021
Published 5 March 2021 Volume 2021:14 Pages 765—777
DOI https://doi.org/10.2147/IJGM.S299450
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nora AlFaris,1 Jozaa ALTamimi,1 Nora AlKehayez,1 Fatema AlMushawah,2 AbdulRhman AlNaeem,2 Nadia AlAmri,3 Ebtisam AlMudawah,3 Malak Alsemari,4 Jawaher Alzahrani,5 Layla Alqahtani II,6 Wedad Alenazi,2 Ashwaq Almuteb II,3 Hessa Alotibi3
1Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia; 2King Fahd Medical City, Riyadh, Saudi Arabia; 3King Saud Medical City, Riyadh, Saudi Arabia; 4King Abdullah Bin Abdulaziz University Hospital (KAAUH),Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia; 5Ministry of Health (Saudi Arabia), Riyadh, Saudi Arabia; 6King Saud University, Riyadh, Saudi Arabia
Correspondence: Nora AlFaris; Jozaa ALTamimi
Princess Nourah Bint Abdulrahman University, P.O. Box 84428, Riyadh, 11671, Saudi Arabia
Email [email protected]; [email protected]
Background: Iron deficiency is known to be the most common nutritional disorder. About 30% of the world’s population is iron deficient (ID). Women are more likely to be exposed to anemia than men, which is an epidemic public health problem.
Objective: A cross-sectional study was carried out to investigate the prevalence of anemia and associated risk factors among non-pregnant women in Riyadh, Saudi Arabia.
Methods: Non-pregnant women (n = 250) aged 20– 65 years were involved in this study. Sociodemographic, nutritional status, menstrual history, anthropometric and haematological properties were calculated. Anaemia proxies including haemoglobin (HB), serum ferritin (IDA), Haematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were determined as well as BMI.
Results: The respondents were varied according to demographic factors and according to anemia proxies, the majority of them suffered from anemia. The Student’s t-test analysis showed that the average daily food intake was lower than that of the dietary requirement intake (DRI). Correlation and logistic regression analysis between sociodemographic factors and anemia proxies revealed that most of such factors significantly and negatively affected anemia proxies. Moreover, the correlation of daily food intake and anemia proxies showed that the nutrients responsible for the improvement of anemia proxies were not taken in sufficient amount as indicated by a significant and positive correlation.
Conclusion: In conclusion, various factors including demographic factors, daily food intake appeared to be associated with anemia proxies, which are the most important risk factor for anemia among non-pregnant women in Riyadh, Saudi Arabia.
Keywords: anemia, nutrient deficiency, dietary intake, hemoglobin, anthropometric
Introduction
Anemia was reported to affect 2.2 billion people worldwide at a rate of 33%.1 It is defined as the state at which hemoglobin (Hb) and haematocrit (Hct) levels fall under indicated referenced cut-off points depending on age, gender and altitude. Over 30% of women and >50% of pregnant women worldwide are suffering from anemia.2 In developing countries, anemia leads to one of the major public health problems because it can happen at all stages of life. More than one-third of people in the Middle East have anemia due to either iron deficiency (ID) or a combinations of other factors, the majority of which are women.3 In particular, anemia (Hb < 12 g/dl) was observed in 40% of Saudi women aged 15 to 49 years.4
Moreover, IDA is recognized as a condition in which the blood contains inadequate iron to maintain the regular physiological functions of body tissues, such as muscles, bones, and the brain. IDA may occur in the absence of anemia if it has insufficiently persisted or if it is insufficiently severe to cause Hb to drop below specified sex and age thresholds.2 Iron deficiency anemia (IDA) is prevalent among teenagers in the Kingdom of Saudi Arabia (KSA) because of iron deficiency in the diet and scarcity of awareness regarding balanced nutrition and iron-rich food.5 IDA is one of the most proliferating types of under-nutrition. About 50% of anemia cases worldwide are considered caused by iron deficiency, which results in annual mortality of 841,000.6 The prevalence of anemia was associated with haemoglobinopathies (normocytic normochromic anemia), Fe deficiency (microcytic hypochromic anemia) as well as B12/folic acid deficiency (megaloblastic anemia). However, ID is the most significant factor in nutritional anemia.7 Low iron intake and unbalanced diet combined with blood loss due to menstrual cycle put young Mexican women of normal weight and those suffered from obesity at risk of developing IDA.8
Nutritional deficiency can include the deficiency of calories, proteins, vitamins, iron, and other nutrients. The contributing factors of IDA in developing countries include poor intake of dietary iron (low consumption of red meat), and an increased iron requirement for reproduction, low bioavailability of dietary iron (non-heme iron in the cereal-based diet and black tea taken with meals), and iron loss due to parasitic infections.9 Also, a link between ID and obesity was reported. Tijerina-Sáenz et al,8 proposed a multifactorial etiology comprising inadequate iron bioavailability relative to body weight and weakening in iron absorption as reported in subjects with extra adiposity. In addition to nutritional and health status, several scholars reported associations between anemia and biological, socioeconomic, ecological, educational, behavioral, and political factors.10–13 The shortage of data and studies that estimate the prevalence of anemia and micronutrient deficiency and their impacts on healthy women is notable. Therefore, the present study aimed to assess anemia proxies, which lead to the prevalence of anemia and associated risk factors among non-pregnant women in Riyadh, Saudi Arabia.
Materials and Methods
Design and Sampling Method
A cross-sectional study was carried out in King Saud Medical City and King Fahad Medical City, Riyadh, Saudi Arabia, during the spring semester of 2018. A total of 250 women with ages ranging from 20 to 65 years old were involved in this study, after signing a form according to the Helsinki Declaration. The respondents did not include pregnant as indicated by pregnancy test performed at the hospital and did not use any mineral or vitamin supplementation, as well as subjects with a history of any form of cancer, were excluded.
Data Collection
A structural questionnaire and anthropometric measurements were used for data collection. All participants were provided with a written informed consent in their native language before enrollment. The questionnaire was designed to study sociodemographic factors and daily food intake. Before answering the questionnaire, respondents were given an idea about the study and instructed on how to truthfully complete the questionnaire. The nutrients of the respondents’ dietary intake were recorded using the ESHA Program (Elizabeth Stewart Hands and Associates Program) after recording daily food intake of respondents. Fortunately, the respondents are mature enough to communicate well with the data collector. Moreover, the data provided are reliable and the respondents provided good and reliable information. The data collected were reviewed and approved by professionals in this field. According to the ESHA Program menu, a code was given to each type of food. In the case of mixed and cooked food, contents are provided separately. The 24-hour recall food intake data (average of 3 measurements) for each respondent to the ESHA food processor program was entered. Thereafter, the software analyzes the food and gives a percentage of all nutrients including calories that have been taken by the respondents and then compares with the dietary requirement intake (DRI) reported by WHO/FAO dietary guidelines prepared for different populations.
Biochemical Analysis
After 12-hour fasting, blood samples were taken by an expert in the hospital. Thereafter, plasma samples were separated by centrifugation for 20 min at 2000×g. Separated plasma was kept at −70°C for further use. Ferritin was assessed by a two-site immunoradiometric assay14 and by a cobas® 6000 analyzer, Siemens (Erlangen, Germany) using Roche assay. Plasma iron was analyzed by the use of Dimension® RxL Max® Integrated Chemistry System (Siemens, Erlangen, Germany). Hb and hematocrit (Hct) levels were determined by CELL-DYN Ruby Hematology System (Abbott, USA). Normal Hb range is generally defined as 11.6 to 15 grams (g) of Hb per deciliter (dL) of blood. Anemia is classified as; mild = 10.0–10.9 g/dl, moderate = 7.0–9.9 g/dl, and severe = <7.0 g/dl according to WHO cutoff points for anemia based on Hb concentration,15 whereas normal levels of Hct are between 35.5 and 44.9% percent in general. According to the WHO guidelines, female who measured Hb <12.0 g/dl, serum ferritin <15 μg/L, serum iron <10 μmol/L, and TIBC ≥68 μmol/L were defined as IDA. Normal iron level is 60–170 micrograms per deciliter (μcg/dL).
Body Mass Index (BMI) Determination
BMI was calculated as a ratio of weight in kg to height in m2 and classified according to the indices of the WHO.16 According to the WHO classification the BMI categories include: <18.5= underweight; 18.5–24.9= normal; ≥25.0= overweight; 25.0–29.9= pre-obese; 30.0–34.9= obese G1; 35.0–39.9= obese G2; ≥40.0= obese G3.
Data Analysis
The statistical package for social sciences (SPSS Inc., Chicago, IL, USA) version 20 was used for the analysis of the data. The results were expressed as means. The comparison of means between nutrients intake (24-hour recall) and DRI was done using the Student’s t-test. Correlation between anemia proxies and sociodemographic characteristics or food intake was tested using Spearman correlation coefficients and logistic regression analysis.
Ethical Consideration
The study was approved by the ethical review committee of King Saud Medical City (IRB registration number with KACST, KSA: H-01-R-012; IRB Log number: HRC-27-Aug15-01) and King Fahad Medical City (IRB registration number with KACST, KSA: H-01-R-053; Ref. number 15–02E), Riyadh, Saudi Arabia. All participants were provided with written informed consent in their native language before enrollment. Participants were informed of the general-purpose, and benefits of the study. All participants signed a consent form in accordance with the declaration of Helsinki.
Results
Sociodemographic and Lifestyle Characteristics
Table 1 shows the frequency distribution of sociodemographic factors and lifestyle characteristics of study subjects (n = 250). Almost half of the respondents (50.4%) with an age ranged from 40 to 49 years visited the hospital followed by those with age >50 years and those with age ranged from 20 to 39 years. It has been observed that most of the hospital visitors are between the ages of 40 to 49 years old. The level of a monthly income of the family respondents was found to be medium (≤1000 USD) and a low percentage had a high income (>1000 USD).
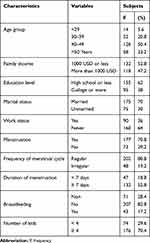 |
Table 1 Frequency Distribution of Sociodemographics and Lifestyle Characteristics of Study Subjects (n = 250) |
The educational level of the respondents was low, and the majority has a high school certificate or less followed by those who have university education which represents a very low percentage. The percentage of married respondents was higher than those unmarried. Most of the respondents dislike to work (64%) compared to those who had a job (36%). About 80.8% of respondents reported regular menstrual cycle, while 19.2% had an irregular cycle and 28.4% had reached the age of menopause. The average duration of menstruation was one week or more in two-thirds of respondents and less than a week for the others. It was found that only 17.2% of the respondents breastfed their children, this is equal to 20.33% of women of childbearing age, while 82.8 did not breastfeed their children. The majority of the respondents (70.4%) had an average number of children ≥4 and 29.6% had less than 4 kids. This reflects the Saudi culture that favored large families.
Average Daily Intake of Respondents
The respondents’ nutritional status was investigated by assessing their daily food intake (nutrients). The daily average amount of food taken by the respondents was determined using the ESHA program to give the average composition (calories, protein, carbohydrates, dietary fiber, total fat, saturated fat, unsaturated fat, cholesterol, vitamins, folate, and minerals). Then the mean of each constituent was compared to the mean of dietary requirement intake (DRI) by using the t-test.
Table 2 shows the average intake of nutrients for respondents compared to that of dietary reference intake (DRI). The results showed that the average intake of calories (902.54 kcal) and carbohydrates (89.28 g) of respondents were significantly (P ≤ 0.01) lower than that of the DRI. The amount of unsaturated fat (23.47 g) was significantly (P ≤ 0.01) lower than that of the DRI, while the amount of saturated fat (19.95 g) was significantly (P ≤ 0.01) higher. Cholesterol, fiber, and vitamins were significantly (P ≤ 0.01) lower than that of the DRI. The average intake of all minerals except Na was significantly (P ≤ 0.01) lower than that of the DRI. The decrease in consumption of iron may be due to low intake of proteins while low consumption of fruits and vegetables by the respondents is the main factor that lowered the intake of some vitamins and minerals.
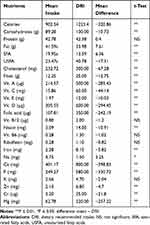 |
Table 2 Average Daily Intake of Nutrients (24-h Recall) in Relation to the Dietary Requirement Intake (DRI) for Respondents Using the t-Test |
Biochemical and Anthropometric Characteristics
Table 3 shows the frequency distribution of anemia proxies and BMI of the respondents under investigation. The data collected showed that 28.40% of the respondents had low hemoglobin (Hb) level while 68.40 had sufficient Hb and 3.20% had a high level. Ferritin is one of the factors that causes anemia. Among the respondents, 36% suffered from low ferritin level (iron depletion), 55.20% had a moderate level and only 8.80% had high level. Hematocrit (Hct) percent varied between respondents with 28% had a lower level and 72% had a higher level than the normal. The analysis of red blood cells showed that 1.20% had a smaller number of blood cells (microcytic) than normal, 88.40% had the normal numbers and 10.40% had a higher level than the normal (macrocytic).
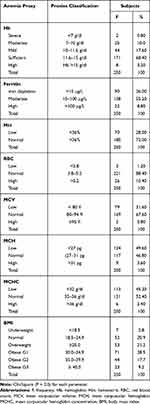 |
Table 3 Frequency Distribution of Anaemia Proxies and BMI of the Respondents |
The average size of the red blood cells measured as mean corpuscular volume (MCV) showed that 31.60% were microcytic, 67.60% were non-microcytic and only 0.80% were observed to be macrocytic. The average amount of the protein (hemoglobin) in red blood cells which carries oxygen around the body as mean corpuscular hemoglobin (MCH) showed that 46.60% had a lower level than the normal, 46.80% had a normal level and 3.60% had a higher level than the normal. The average concentration of hemoglobin inside a single red blood cell measured as mean corpuscular hemoglobin concentration (MCHC) showed that 45.20% of the respondents had a lower level than the normal, 52.40% had a normal level and 0nly 2.40% had a higher level than the normal. Furthermore, BMI was determined and the results are shown in Table 2. According to BMI classification, the study results showed that 2.80% of the respondents are observed to be underweight, 20.90% have a normal BMI, and 21.20% are overweight, while 28.50, 17.7, and 9.2% suffered from obesity G1, G2, and G3, respectively.
Data of Table 4 showed that among each sub-group the anemic respondents were within the age of 30–39 years (34.62%) and those with low income (30.3%) and educational (29.68%) levels. Moreover, married (28.57%) and working (30%) respondents relative to those who were involved in work had been greatly subjected to anemia. Respondents with menstruation (36.16%), especially those with regular menstruation (45.05%), as well as those with prolonged menstruation (35.61%) were observed to be anemic. The respondents who breastfeed their kids (46.51%) or have <4 kids (35.14%) were observed to suffer from anemia. It has been observed that within anemic respondents 57.14% were underweight followed by obese G1 (35.21%) and obese G3 (34.78%). The majority of non-anemic respondents were suffered from overweight and obesity.
 |
Table 4 Frequency Distribution of Anaemic and Non-Anaemic Respondents According to Sociodemographic and BMI Data |
Risk Factors Associated with Anemia
To investigate the risk factors associated with anemia of the respondents, Spearman correlation coefficients and logistic regression analysis between the respondents’ sociodemographic and anemia proxies were calculated. As shown in Table 5, Spearman correlation coefficients and logistic regression analysis between the respondents’ sociodemographic and anemia proxies, in general, showed that the anemia proxies including hemoglobin (Hb) level whether severe, moderate or mild, ferritin whether low, moderate or high, low Hct, low MCV, and low MCHC were significantly (P ≤ 0.05 or P ≤ 0.01) and negatively related to age, work status, menstrual cycle, breastfeeding and the number of kids which indicated that such anemia proxies decrease with these factors as indicated by Spearman correlation and strengthened by logistic regression analysis (Odd ratio and 95% CI). Therefore, lower values of anemia proxies were observed among respondents aged 30–49 years while those of years, ≥50 expected to reach the menopause stage.
 |
Table 5 Spearman Correlation and Multiple Logistic Regression Between Sociodemographic Data and Anaemia Proxies of Respondents (n = 250) |
Family income, educational level and marital status as independent variables were significantly and positively correlated with anemia proxies. According to the data, it was observed that the majority of the demographic factors of the respondents were considered risk factors and significantly associated with anemia proxies. Also, the anemia proxies especially low hemoglobin and MCV was more common among those with menstrual cycles and breastfeeding. However, the prevalence of anemia was high in menstruated respondents and those with regular menstrual cycles compared to those with irregular cycles. Moreover, Spearman correlation coefficients and logistic regression analysis between the respondents’ daily food intake, anemia proxies, and BMI were calculated (Table 6). The results showed that most independent variables were positively or negatively correlated with the dependent variables, but the strength of correlation varied among the variables, nutrient intake was significantly (P ≤ 0.05 or P ≤ 0.01) and positively correlated with all anemia proxies and BMI. In all groups, an increase in the intake of nutrients, significantly (P ≤ 0.01 or P ≤ 0.05) increased the level of anemia proxies as well as the BMI. However, respondents’ intake of such nutrients was found to be very low and therefore, their anemia proxies were low as indicated in Table 2. Other nutrient intake was either positively or negatively correlated with anemia proxies and BMI, but the correlations were not significant.
 |
Table 6 Spearman Correlation and Multiple Logistic Regression Between Food Intake and Anaemia Proxies and Body Mass Index (BMI) of Respondents (n = 250) |
Discussion
The current study aimed to investigate anemia proxies and associated risk factors in non-pregnant women in Riyadh city, Kingdom of Saudi Arabia. The results showed that the majority of the respondents with age 40 to 49 years had medium or low income, low level of education, dislike working, menstruated with regular cycle (<7 days), with ≤4 kids, and did not breastfeed the kids. Most of the respondents’ demographic factors are expected to harm nutritional as well as health status. Educated mothers make better use of health services and provide better childcare including breastfeeding. The level of the monthly income of the families’ respondents was found to be an important factor for the respondents’ nutritional status.
The decrease in the intake of iron and other elements could be due to low intakes of proteins, fruits and vegetables as reported is believed to be the major factor that lowered the level of vitamins and minerals.17 Overall, the results obtained for the respondents indicated that all groups took less food of high carbohydrates, unsaturated fat, and protein and their food intake was insufficient in vitamins and minerals. The results also indicated that most of the respondents had bad food habits. AlQuaiz et al,4 reported that the infrequent intake of meat was associated with the increased risk of anemia according to the results of dietary intake and diversity.
The results revealed that the participants are severely suffering from the deficiency of all nutrients compared to the recommended dietary intake. Anemia proxies expected to be low and the respondents’ will be under risk of anemia due to bad lifestyle that adversely affected their eating habits. Although the respondents enjoy a reasonable level of income and education, the majority of them were found to be anemic based on many parameters including the lower level of Hb, ferritin, Hct, MCV, MHC and MCHC than the recommended level. The anemia proxies were observed to fell below the cutoff points for anemia, which could be attributed to low nutrients intake especially iron, as well as regular menstruation that lasts for a long time. This finding is in contrast with those of Saranaz and Hossein18 who focused on non-pregnant women aged 15 to 45 years. However, these findings were comparable with those of women at the university level in Saudi Arabia and women of reproductive age in Turkey.19,20 Similar results of Hb, MCH, and MCV were observed in women of child-bearing age in Riyadh but lower in terms of MCHC.4
The results of the present study showed that the reduction in anemia proxies that lead to the prevalence of anemia differs from those of previous studies conducted in countries like Egypt,21 Uganda,22 and Ethiopia.23 This variation could be due to variation in the cultural, social, and economic differences as well as differences in nutritional habits, common diseases, health status, and education. According to our data, the reduction in anemia proxy levels and the prevalence of anemia whether low hemoglobin or iron among respondents could be due to insufficient nutrient intake as well as physiological and pathological factors.18 The average daily intake of nutrients was significantly lower than the DRI especially those had great influence on anemia proxies. The physiological factors that affect anemia are related to the intake and need for iron. The need for iron increases in women because of the menstrual cycle as well as how long it takes. The study observed that respondents received less food due to bad habits. Therefore, the intake of iron is less and the prevalence of anemia is more expected.
The study showed that the prevalence of anemia was increased with age, menstruation especially those of regular and prolong cycle, breastfeeding, increased number of kids and BMI. The study observed that the percentage of underweight respondents who suffered from anemia was lower than those of overweight or with obesity G1. The study observed that anemic women were either overweight or obese, whereas those of underweight were normal. The results suggest that anemia is positively related to obesity because anemia was increased with the increase in obesity. Therefore, the authors suggested that obesity could be one of the factors that influence the prevalence and development of anemia. It has been reported that women with anemia reached 40.8% of the participants in normal BMI range, 8.45% were underweight, and 50.7% were overweight.16 Likewise, Kim and Lee24 noted that women with anemia displayed lower anthropometric values compared with non-anemic participants. In contrast to our results, Gautam et al25 found that low risk of developing anemia among those of high BMI compared to those of low BMI.
To determine the risk factors that associated with anemia, we explored the correlation and logistic regression of sociodemographic characteristics and daily food intake with anemia proxies of the respondents. According to iron deficiency anemia proxies such as low hemoglobin, low serum iron, a low serum ferritin, low transferrin saturation, and high total iron-binding capacity, the analyses showed that such proxies decreased significantly with age, working status, menstrual cycle especially regular and prolong cycle, education, and breastfeeding. Also, the analysis showed that the proxies decreased (anemic cases) significantly with the decrease in nutrient intake especially intake of iron by respondents. Moreover, anemia proxies’ levels significantly increased with an increase in monthly income and decreased number of kids. Generally, the results showed that anemia proxies’ levels were significantly affected by demographic factors as well as nutrient intake especially insufficient intake of iron or food rich in iron. Such factors were observed to be responsible for severe anemia of the respondents. Ganapathi and Kumar26 reported that sociodemographic, obstetric, dietary, menstrual, behavioral, contraceptive, and ecological factors were related to anemia for women of reproductive age. Moreover, the prevalence of anemia among women with high levels of economic income was low. This finding is in accordance with the World Bank report on anemia in women, that is, the prevalence of anemia decreases with the increase in income across regions or countries.
Specifically, the prevalence of anemia among people in poor regions is twofold compared with their rich counterparts in numerous countries.27 The result is in line with that of AlQuaiz et al,4 who found that women with five or more children were nearly twice at risk of developing anemia. Women with higher education levels had a significantly high average quantity of hemoglobin in a red blood cell. Similar findings were reported by Aikawa et al,28 Rasheed et al,29 Habib et al.30 Illiteracy in women was six times more likely to promote anemia compared with women who achieved secondary and higher educational levels.31 According to the Pearson correlation and logistic regression analyses, the potential demographic risk factors associated with low anemia proxies in respondents include age, work status, menstrual cycle, breastfeeding and number of kids as well as insufficient intake of nutrients.
Conclusion
The respondents under investigation were varied according to demographic factors. Their intake of nutrients was lower than the recommended dietary intake. The majority of them had low levels of anemia proxies. The educational level, increased blood loss due to menstruation, lack of breastfeeding, work status, and the number of kids were directly and strongly correlated to anemia proxies among Saudi women. The results showed that the demographic factors, obesity, and nutritional inadequacy of micronutrients such as iron, folic acid, and vitamins B12, C, and D are the most important factors that influence anemia proxies and leading to the prevalence of anemia in respondents. Therefore, conducting additional studies and establishing appropriate intervention strategies to improve the nutritional status, reduce obesity, and increase the intake of food that contains adequate quantities of micronutrients, particularly iron intake.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number PNU-DRI-RI-20-024.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kassebaum J, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–624. doi:10.1182/blood-2013-06-508325
2. WHO, UNICEF, UNU. Iron deficiency anaemia: assessment, prevention, and control. A guide for programme managers. Geneva: World Health Organization; 2001. WHO/NHD/01.3.
3. World Health Organization. Regional Committee for the Eastern Mediterranean. Regional strategy on nutrition 2010–2019. EM/RC57/4. Available from: http://applications.emro.who.int/docs/EM_RC57_4_en.pdf.
4. AlQuaiz AM, Mohamed AG, Khoja TAM, et al. Prevalence of anemia and associated factors in child bearing age women in Riyadh, Saudi Arabia. Nutr Metab. 2013;1–7.
5. Mason K, Gibson F, Hambleton I, Serjeant B. Iron deficiency among Jamaican adolescents. W Indian Med J. 2015;63:561–565.
6. Longo D, Fauci KD, Harrison’s AE. Principles of Internal Medicine. New York: McGraw-Hill Medical Publishing Division; 2011.
7. de Sá SA, Willner E, Pereira TA, de Souza VR, Boaventura GT, de Azeredo VB. Anemia in pregnancy: impact on weight and in the development of anemia in newborn. Nutr Hosp. 2015;32:2071–2079. doi:10.3305/nh.2015.32.5.9186
8. Tijerina-Sáenz A, Martínez-Garza NE, Ramírez-López E, Solís-Perez E, Martínez-Báez AZ. Iron status and dietary intakes of iron in normal-weight and obese young Mexican women. Nutr Hosp. 2015;31:2412–2418. doi:10.3305/nh.2015.31.6.8687
9. Kruger M, Dhansay MA, Van SE, et al. Anaemia and iron deficiency in women in the third trimester of pregnancy receiving selective Iron supplementation. SA J Food Sci Nutr. 1994;6:132–137.
10. Kowsalya T, Periyar R, Nadu T, Parimalavalli R; Food Science. Prevalence of overweight/obesity among adolescent girls in Salem district, India. Indian J Health Sci Biomed Res. 2014;7 (2):73–77. doi:10.4103/2349-5006.148799
11. Horton S, Ross J. The economics of iron deficiency. Food Policy. 2003;28:51–75. doi:10.1016/S0306-9192(02)00070-2
12. Balarajan Y, Ramakrishnan UO, Zaltin E, Shankar A, Subramanian S. Anaemia in low-income and middle-income countries. Lancet. 2011;378 (9809):2123–2235. doi:10.1016/S0140-6736(10)62304-5
13. Harding K, Aguayo V, Namirembe G, Webb P. Determinants of anemia among women and children in Nepal and Pakistan: an analysis of recent national survey data. Matern Child Nutr. 2018;14 (S4):e12478–e12484. doi:10.1111/mcn.12478
14. Yoshii M, Kousaka T, Nakajima K, Morita R, Torizuka K. Measurement of serum ferritin values in various diseases by immunoradiometric assay kit. Jpn J Nucl Med. 1979;16 (5):785–794.
15. WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, Switzerland: WHO, World Health Organization; 2011. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
16. WHO, EC. Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. Geneva: WHO; 1995.
17. Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69:727–736. doi:10.1093/ajcn/69.4.727
18. Saranaz J, Hossein I. The prevalence of iron-deficiency anemia in non-pregnant women of reproductive age [14–45] with anemia in Marvdasht’s Shahid Motahari Hospital in 2012–2013. Electron J Biol. 2016;12 (3):294–299.
19. Pala K, Dundar N. Prevalence and risk factors of anaemia among women of reproductive age in Bursa, Turkey. Indian J Med Res. 2008;128:282–286.
20. Al Sayes F, Gari M, Qusti S, et al. Prevalence of iron deficiency and iron deficiency anemia among females at university stage. J Med Lab Diag. 2011;2:5–11.
21. Mbule M, Byaruhanga Y, Kabahenda M, Lubowa A. Determinants of anaemia among pregnant women in rural Uganda. Rural Remote Health. 2013;13:2259–2284.
22. Ibrahim Z, El-Hamid S, Mikhail H, Khattab M. Assessment of adherence to iron and folic acid supplementation and prevalence of anemia in pregnant women. Med J Cair Univ. 2011;79:115–121.
23. FAO & FHI. Minimum Dietary Diversity for Women: A Guide to Measurement. FAO, editor. Rome, Italy: FAO; 2016.
24. Kim H, Lee B. Cross-sectional study on the prevalence of anemia among rural elderly in Asan. Nutr Res Pract. 2008;2:8–12. doi:10.4162/nrp.2008.2.1.8
25. Gautam S, Min H, Kim H, Jeong H-S. Determining factors for the prevalence of anemia in women of reproductive age in Nepal: evidence from recent national survey data. PLoS One. 2019;14 (6):1–17. doi:10.1371/journal.pone.0218288
26. Ganapathi CK, Kumar SK. A cross-sectional study of anemia among women of reproductive age group (15–49 years) in a rural population of Tamil Nadu. Int J Med Sci Publ Health. 2017;6:524–529.
27. Mawani M, Ali S, Bano G, Ali S. Iron deficiency anemia among women of reproductive age, an important public health problem: situation analysis. Reprod Sys Sexual Disord Curr Res. 2016;5:187–193. doi:10.4172/2161-038X.1000187
28. Aikawa R, Ngyen CK, Sasaki S, Binns CW. Risk factors for iron-deficiency anemia among pregnant women living in rural Vietnam. Public Health Nutr. 2006;9:443–448. doi:10.1079/PHN2005851
29. Rasheed P, Koura MR, Al-Dabal BK, Makki SM. Anemia in pregnancy: a study among attendees of primary health care centers. Ann Saudi Med. 2008;28 (6):449–452. doi:10.5144/0256-4947.2008.449
30. Habib F, Alabdin EH, Alenazy M, Nooh R. Compliance to iron supplementation during pregnancy. J Obstet Gynecol. 2009;29:487–492. doi:10.1080/01443610902984961
31. Tesfaye TS, Tessema F, Jarso H. Prevalence of anemia and associated factors among “apparently healthy” urban and rural residents in Ethiopia: a comparative cross-sectional study. J Blood Med. 2020;11:89–96. doi:10.2147/JBM.S239988
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
