Back to Journals » Journal of Pain Research » Volume 11
Prevalence of and risk factors for Modic change in patients with symptomatic cervical spondylosis: an observational study
Authors Bai J, Yu K, Sun Y, Kong L, Shen Y
Received 15 September 2017
Accepted for publication 27 December 2017
Published 14 February 2018 Volume 2018:11 Pages 355—360
DOI https://doi.org/10.2147/JPR.S151795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Jiangbo Bai,* Kunlun Yu,* Yaning Sun, Lingde Kong, Yong Shen
Department of Orthopedics, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
*These authors contributed equally to this work
Background: The aim of this study was to assess the prevalence of cervical Modic change (MC) in patients with cervical spondylosis and to develop a better understanding of the possible risk factors for the prevalence of MC.
Methods: Between January 2014 and April 2017, patients with cervical spondylosis were included in our study. All patients underwent magnetic resonance imaging (MRI) to evaluate the presence of MC. The MC was classified into three types according to the Modic classification. Potential risk factors were collected from demographic data, lifestyle variables, laboratory tests, and radiographic images. Both univariate and multivariate analysis were used to detect factors associated with MC. We further compared several variables related to fat metabolism between patients with Type 1 and Type 2 MC.
Results: The prevalence of MC in patients with cervical spondylosis was 9.24%. The MC was most frequent at C5–6, followed by C6–7, C4–5, and C3–4. The proportion of Type 1 MC in patients with neck pain was significantly higher than that in patients without neck pain (46.2% vs 13.6%, P=0.027). However, none of the variables associated with fat metabolism showed a significant difference between Type 1 and Type 2 MC. Multivariate logistic analysis showed that age ≥55 years (odds ratio [OR], 1.91; 95% confidence interval [CI], 1.22–2.98) and body mass index (BMI) ≥25 kg/m2 (OR, 2.41; 95% CI, 1.62–3.59) were two significant independent factors that are associated with cervical MC in patients with cervical spondylosis (P<0.05).
Conclusion: It appears that advanced age and high BMI were two factors that may be responsible for cervical MC. Type 1 MC is associated with the prevalence of neck pain. However, we cannot confirm that Type 2 MC is correlated with fat metabolism.
Keywords: Modic change, cervical spine, cervical spondylosis, risk factor, multivariable analysis
Introduction
Modic change (MC) refers to abnormal bone signals under the vertebral endplate on spinal magnetic resonance imaging (MRI), suggesting lesions of vertebral endplate as well as adjacent bone marrow in the vertebral body. It was first noted by Roos et al1 in 1987, and a formal classification of it was subsequently provided by Modic et al2 in 1988. According to the study by Modic et al,3 three types of MC have been identified on the basis of MRI: Type 1 MC shows a hypointense signal on T1 sequences and a hyperintense signal on T2 sequences; Type 2 MC shows a hyperintense signal on T1 sequences and a hyper- or isointense signal on T2 sequences; and Type 3 MC shows a hypointense signal on both T1 and T2 sequences. Histological and radiological studies have demonstrated that Type 1 MC represents vascularized bone marrow and/or edema; Type 2 MC represents the conversion of normal red hemopoietic bone marrow into yellow fatty marrow; and Type 3 MC is rare and represents subchondral bone sclerosis.3 Types 1 and 2 MC are interconvertible over time and can eventually convert to Type 3 MC.4,5 All these changes are believed to be associated with rapidly advancing degeneration of the spine.6,7
Elucidation of MC etiology is hindered by the dynamic clinical presentation of patients and multifactorial pathophysiology.8,9 Although appropriate investigations that reveal the relationship between MC and potential risk factors are not too much, several other studies have provided some clues. For example, Frymoyer et al10 hypothesized that smoking can reduce bone mineral content because of the contraction of small arteries in the vertebral disks and then increase the possibility of developing microfractures, which may enhance the likelihood of developing MC. Leboeuf-Yde et al11 proposed that heavy labor can increase the burden on the disks and weaken the bone structure, which would eventually lead to the incidence of MC. Mok et al12 speculated that MC is possibly induced by intervertebral disk herniation. However, nearly all of the literature on MC focus on the lumbar spine, and only a few studies report on the prevalence of and risk factors for MC in the cervical spine. Thus, we performed this study to assess the classification and distribution of cervical MC in patients with cervical spondylosis, and to acquire a better understanding of possible risk factors to the development of MC.
Methods
Patient population
The patients who presented to our hospital from January 2014 to April 2017 because of neurologic symptoms from the cervical spine, such as radiculopathy or myelopathy, were included in our study. The inclusion criteria were adult patients with cervical disk herniation or cervical spondylotic myelopathy. Patients were excluded if they had prior cervical spinal surgery, ossification of the posterior longitudinal ligament, amyotrophic lateral sclerosis, spinal tuberculosis, or spinal infection. The Ethics Committee of the Third Hospital of Hebei Medical University approved this research and waived the informed consent because this was a cross-sectional study and all data were collected and analyzed anonymously.
Assessment of MC
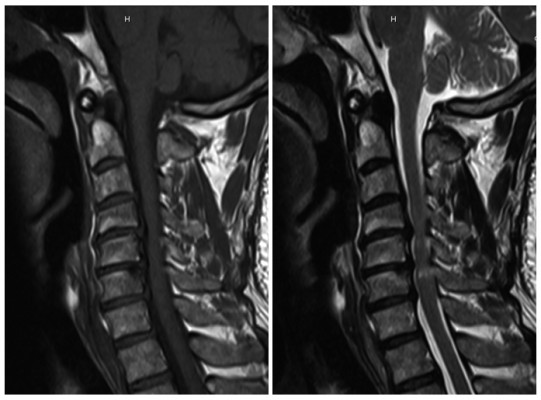
All patients underwent MRI to evaluate the presence of MC. MRIs were performed with a 3.0-T imager (Siemens Magnetom Symphony; Siemens, Berlin, Germany), and a body spine surface coil was used with the patients in the supine position. Images of sagittal T1-weighted turbo spin echo and sagittal T2-weighted turbo spin echo sequences were collected and analyzed. According to the definition by Modic et al,2,3 the MC was classified as Type 1, Type 2, and Type 3. The criteria of classification are listed in Table 1. An example of Type 2 MC at the C4–5 level in the cervical MRI is shown in Figure 1.
  | Table 1 Types of MC in vertebral body based on MRI Abbreviations: MC, Modic change; MRI, magnetic resonance imaging. |
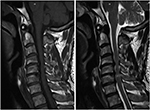  | Figure 1 An MC at the C4-5 level in the T1 sequence (left) and T2 sequence (right) of cervical MRIs. Abbreviations: MC, Modic change; MRI, magnetic resonance image. |
The classification of MC on MRI was completed by two independent reviewers who were blinded to patients’ information. Disagreements between the two reviewers were settled by discussion, and if no consensus could be reached, the third reviewer made the final decision.
Data collection
Data were collected on potential risk factors in the following four categories: demographic data, lifestyle variables, laboratory tests, and radiographic images. Demographic data and lifestyle variables were collected from the medical record, including age, gender, weight, body mass index (BMI), history of smoking or alcohol, history of cervical injury, participation in sports, and physical workload. Participation in sports was defined as regular performance of any kind of exercise routine with a minimum frequency of once per week. Occupation was categorized as sedentary, light, medium, heavy, or very heavy workload according to a classification of jobs scheme based on workload.13 Patients with sedentary, light, and medium workloads were defined as “nonheavy,” and those with heavy or very heavy workloads were defined as “heavy.” From laboratory tests, we retrieved the value of high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TRIG). Cervical alignment and range of motion (ROM) were measured in standing lateral radiographs of the cervical spine. Cervical alignment was defined as the Cobb angle from C2 to C7. The C2–7 ROM was defined as the sum of the C2–7 Cobb angle during flexion and extension on lateral radiographs. To perform multivariate analysis, continuous covariates were dichotomized. There is no generally accepted cutoff point for defining elder age, heavy weight, high BMI, large C2–7 Cobb angle, or large C2–7 ROM in patients with cervical spondylosis. The choice for their particular cutoff values (age ≥55 years, <55 years; weight ≥65 kg, weight <65 kg; BMI ≥25 kg/m2, <25 kg/m2; C2–7 Cobb angle ≥20°, <20°; C2–7 ROM ≥40°, <40°) were based on previous studies, clinical meaning, or the population distribution.
Statistical analysis
The statistical analyses were performed with the Statistical Package for the Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA). Variables were presented as a mean with standard deviation for continuous variables and with frequencies and percentages for categorical variables. The independent sample t-test or Mann–Whitney U- test was used for numerical data, and Fisher’s exact test was used to identify differences in the frequency of nominal variables between groups. After univariate analyses, variables found to be potentially predictive of the outcome variable from the univariate analyses (P<0.20) were included in the multivariate logistic regression models. P-values below 0.05 were regarded as statistically significant.
Results
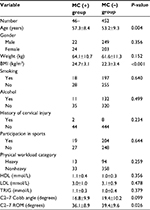
A total of 498 patients (271 males and 227 females) with cervical spondylosis were included in our study. The mean age of these patients was 53.6±8.9 years. Among them, 46 patients (9.24%) had MC, and the other 452 (90.76%) did not. The number of endplates with MC was 48 (1.93%), and the number of Type 1, Type 2, and Type 3 MC were 15 (31.2%), 31 (64.6%), and 2 (4.2%), respectively. The MC was most frequent at C5–6 (45.8%), followed by C6–7 (33.3%), C4–5 (16.7%), and C3–4 (4.2%; Figure 2). In patients with MC, 25 patients had symptoms of neck pain, and 21 did not. The proportion of Type 1 MC in patients with neck pain was 46.2%, which was significantly higher than that in patients without neck pain (13.6%), and the P-value was 0.027. The details of MC in patients with or without neck pain are listed in Table 2.
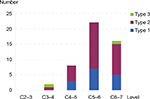  | Figure 2 The distribution of MC according to cervical disk level. Abbreviation: MC, Modic change. |
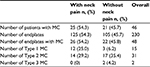  | Table 2 Details of MC in patients with or without neck pain Abbreviation: MC, Modic change. |
Patients’ variables from demographic data, lifestyle variables, laboratory tests, and radiographic images were summarized and compared (Table 3). The mean age in patients with MC was significantly higher than that in those without MC (57.3±8.4 vs 53.2±9.3, P=0.004), and the BMI was also significantly higher than that in patients without MC (24.7±3.1 vs 22.3±3.4, P<0.001). The C2–7 ROM was 36.1±8.9 in patients with MC, 39.4±9.6 in patients without MC, and the difference was statistically significant (P=0.026). In addition, the weight in patients with MC (64.1±10.7) was higher than that in patients without MC (61.6±11.3), although we cannot demonstrate a statistically significant difference (P=0.152), and the C2–7 Cobb angle in patients with MC was lower than that in patients without MC (16.8±9.9 vs 19.4±10.2, P=0.099). However, there was no significant difference in gender, history of smoking, alcohol, history of cervical injury, participation in sports, physical workload category, HDL, LDL, or TRIG (P>0.20).
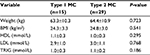
We further compared several variables about fat metabolism between patients with Type 1 MC and Type 2 MC. There were 15 patients with Type 1 MC, and 29 patients with Type 2 MC. However, none of these variables showed a significant difference between the two groups (Table 4).
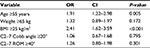
The multivariate analysis was also performed to investigate the impact of independent variables on the development of MC. The final results of multivariate logistic analysis showed that age ≥55 years (odds ratio [OR], 1.91; 95% confidence interval [CI], 1.22–2.98) and BMI ≥25 kg/m2 (OR, 2.41; 95% CI, 1.62–3.59) were two significant independent factors that are associated with MC in patients with cervical spondylosis (P<0.05, Table 5).
Discussion
MC is a common phenomenon on MRI of patients with cervical spondylosiss.14 In our study, we investigated the distribution of MC in cervical endplates and evaluated the relationships of MC with some potential risk factors. The MC was most frequent at C5–6, and followed by C6–7, C4–5, and C3–4. A multivariate logistic regression analysis confirmed that advanced age and high BMI were two factors that were responsible for MC. Type 1 MC is associated with the prevalence of neck pain; however, we cannot confirm that Type 2 MC is correlated with fat metabolism.
The prevalence of MC in the cervical spine has been reported by several previous studies. Sheng-yun et al15 reviewed 1,023 patients with neck pain and reported that MC was seen in 90 patients (8.8%). They also reviewed 497 asymptomatic patients and found 42 patients (8.5%) with MC.15 In our study, 46 (9.24%) of 498 patients had MC, and the lowest two cervical levels were most commonly seen. The subjects recruited in this study were patients with cervical spondylosis; therefore, the prevalence reported in this study was slightly higher than those based on neck pain or asymptomatic populations. However, the proportion of MC types showed various results in previous studies. Peterson et al16 reported that Type 1 MC was the most commonly seen, while Mann et al17 and Hayashi et al18 observed that Type 2 MC was the most common MC. We assumed that the inconsistent results between studies might be because of the differences in the study population. In addition, the natural history of MC suggests that they are reversible. The interconversion over time between Type 1 and Type 2 MC may be another factor that contributes to different proportions of MC types.5 Furthermore, differences in field strengths of the used MRI equipment may explain part of the differences as well.
In line with previous studies in which a significantly higher prevalence of MC was seen in patients with neck or back pain,15,19 we found that Type 1 MC was associated with the prevalence of neck pain. The spine is a nonlinear viscoelastic structure, and the vertebral endplates are its weak link. Axial compressive loading deforms the cartilage endplates during movement of the spine. The main cause of MC has been considered to be minor trauma of the endplate because of repetitive loading or injury, which causes the inflammatory reaction of nucleus pulposus, initiating endplate changes and leading to subsequent pain. Burke et al20 reported that levels of interleukin-6, interleukin-8, and prostaglandin E2 in the intervertebral disk are significantly higher when Type 1 MC is present in comparison with Type 2, suggesting that inflammatory changes in the disk may be involved in the initiation of Type 1 MC. Karppinen et al21 also reported that interleukin-1 cluster polymorphisms are significantly associated with Type 1 MC. Anti-inflammatory treatment may be a good choice for these patients with MC to relieve neck pain.
In a previous study by Mok et al12 investigating MC of the lumbar spine, the authors found the prevalence of MC increased with advancing age. Wang et al22 also reported that the presence of any type of MC in the lumbar region was statistically associated with greater age and that the prevalence of MC seems to be very low in patients at a young age. In our study, advanced age was demonstrated to be associated with the prevalence of MC in the cervical spine. This finding indicates that MC may be considered to be a cause of neck pain mainly in middle age or later.
High BMI value is another factor that may cause MC of the cervical spine. As previous studies reported, repeated mechanical loads could accelerate disk degeneration and lead to microfractures, which further encourage the nucleus pulposus to contact the circulatory system, induce autoimmune reactions, and result in MC.23 This process may explain why high BMI was positively related to MC in the lumbar spine. However, in contrast to the case of the lumber spine, obesity patients do not add significantly higher loads on the cervical spine than other patients. We hypothesized that the interrelationship between fat metabolism and marrow composition may play an important role in the development of MC. Although the etiopathogenesis is unclear yet, some insights may be gained from the studies of bone marrow lesion in osteoarthritis. The MC adjacent to a degenerated disc shares many characteristics with bone marrow lesion in the femur/tibia of an osteoarthritis knee joint. High serum lipids increase the risk of developing bone marrow lesion in the knee.24 The two disorders may share the same pathological variables at a systemic level.8 Therefore, knowledge from bone marrow lesion research at peripheral skeletal sites may also help clarify the etiopathogenesis of MC. In addition, Type 2 MC is histologically characterized by fatty replacement;2,3 it is possible that this type of pathological change is strongly linked to obesity. However, our further comparison of variable about fat metabolism did not reveal any significant difference between Type 1 and Type 2 MC, and studies are still needed on this aspect in the future.
There are several limitations in our study. First, because of the low prevalence of MC in the cervical spine, the sample of patients was relatively small, limiting our ability to conclude which factors cause specific types of MC. Secondly, we did not take the size of the MC in MRI into consideration; thus, we cannot determine whether the size of MC affects the clinical symptoms of patients. Quantitative measurement of MC in further studies may provide more valuable information. Finally, this study analyzed only patients with neurological symptoms. The results of this study should be interpreted with caution in the general population.
Conclusion
In spite of the aforementioned limitations, this study is clinically valuable to some extent. In summary, the prevalence of MC in patients with cervical spondylosis was 9.24%. It appears that advanced age and high BMI are two factors that may be responsible for MC. Type 1 MC is associated with the prevalence of neck pain; however, we cannot confirm that Type 2 MC is correlated with fat metabolism.
Acknowledgment
The authors thank Mr Yanbin Zhu and Mr Hengrui Chang for their help in collecting data.
Disclosure
The authors report no conflicts of interest in this work.
References
de Roos A, Kressel H, Spritzer C, Dalinka M. MR imaging of marrow changes adjacent to end plates in degenerative lumbar disk disease. AJR Am J Roentgenol. 1987;149(3):531–534. | ||
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. | ||
Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168(1):177–186. | ||
Teichtahl AJ, Finnin MA, Wang Y, et al. The natural history of Modic changes in a community-based cohort. Joint Bone Spine. 2017;84(2):197–202. | ||
Mann E, Peterson CK, Hodler J, Pfirrmann CW. The evolution of degenerative marrow (Modic) changes in the cervical spine in neck pain patients. Eur Spine J. 2014;23(3):584–589. | ||
Kerttula L, Luoma K, Vehmas T, Gronblad M, Kaapa E. Modic type I change may predict rapid progressive, deforming disc degeneration: a prospective 1-year follow-up study. Eur Spine J. 2012;21(6):1135–1142. | ||
Park MS, Moon SH, Kim TH, Lee SY, Jo YG, Riew KD. Relationship between modic changes and facet joint degeneration in the cervical spine. Eur Spine J. 2015;24(12):2999–3004. | ||
Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–3734. | ||
Nguyen C, Poiraudeau S, Rannou F. From Modic 1 vertebral-endplate subchondral bone signal changes detected by MRI to the concept of ‘active discopathy’. Ann Rheum Dis. 2015;74(8):1488–1494. | ||
Frymoyer JW, Pope MH, Clements JH, Wilder DG, MacPherson B, Ashikaga T. Risk factors in low-back pain. An epidemiological survey. J Bone Joint Surg Am. 1983;65(2):213–218. | ||
Leboeuf-Yde C, Kjaer P, Bendix T, Manniche C. Self-reported hard physical work combined with heavy smoking or overweight may result in so-called Modic changes. BMC Musculoskelet Disord. 2008;9:5. | ||
Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, Cheung KM. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016;16(1):32–41. | ||
Field JE, Field TF. The Classification of Jobs. 4th ed. Athens: Elliott& Fitzpatrick; 1992. | ||
Matsumoto M, Okada E, Ichihara D, et al. Modic changes in the cervical spine: prospective 10-year follow-up study in asymptomatic subjects. J Bone Joint Surg Br. 2012;94(5):678–683. | ||
Sheng-yun L, Letu S, Jian C, et al. Comparison of modic changes in the lumbar and cervical spine, in 3167 patients with and without spinal pain. PLoS One. 2014;9(12):e114993. | ||
Peterson CK, Humphreys BK, Pringle TC. Prevalence of modic degenerative marrow changes in the cervical spine. J Manipulative Physiol Ther. 2007;30(1):5–10. | ||
Mann E, Peterson CK, Hodler J. Degenerative marrow (modic) changes on cervical spine magnetic resonance imaging scans: prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine (Phila Pa 1976). 2011;36(14):1081–1085. | ||
Hayashi T, Daubs MD, Suzuki A, Phan K, Shiba K, Wang JC. Effect of Modic changes on spinal canal stenosis and segmental motion in cervical spine. Eur Spine J. 2014;23(8):1737–1742. | ||
Jensen TS, Karppinen J, Sorensen JS, Niinimaki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17(11):1407–1422. | ||
Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196–201. | ||
Karppinen J, Solovieva S, Luoma K, Raininko R, Leino-Arjas P, Riihimaki H. Modic changes and interleukin 1 gene locus polymorphisms in occupational cohort of middle-aged men. Eur Spine J. 2009;18(12):1963–1970. | ||
Wang Y, Videman T, Battie MC. Modic changes: prevalence, distribution patterns, and association with age in white men. Spine J. 2012;12(5):411–416. | ||
Han C, Kuang MJ, Ma JX, Ma XL. Prevalence of Modic changes in the lumbar vertebrae and their associations with workload, smoking and weight in northern China. Sci Rep. 2017;7:46341. | ||
Lim YZ, Wang Y, Wluka AE, et al. Association of obesity and systemic factors with bone marrow lesions at the knee: a systematic review. Semin Arthritis Rheum. 2014;43(5):600–612. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.