Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Prevalence of and Factors Associated with Diabetic Retinopathy in Patients with Diabetes Mellitus at Siriraj Hospital – Thailand’s Largest National Tertiary Referral Center
Authors Boonsaen T , Choksakunwong S , Lertwattanarak R
Received 28 October 2021
Accepted for publication 14 December 2021
Published 29 December 2021 Volume 2021:14 Pages 4945—4957
DOI https://doi.org/10.2147/DMSO.S346719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Thirajit Boonsaen, Sawaraj Choksakunwong, Raweewan Lertwattanarak
Division of Endocrinology and Metabolism, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Raweewan Lertwattanarak
Division of Endocrinology and Metabolism, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Wanglang Road, Bangkoknoi, Bangkok, 10700, Thailand
Tel +66-2-419-7799
Fax +66-2-419-7792
Email [email protected]
Purpose: We aimed to determine the prevalence of and factors associated with diabetic retinopathy (DR) in patients with diabetes mellitus (DM) and to evaluate the relationship between significant factors and severity of DR.
Patients and Methods: A retrospective cross-sectional study of 1130 diabetic patients (mean age: 60 years, 62.7% female, 91% type 2 diabetes) was conducted in the diabetes clinic of Siriraj Hospital (Bangkok, Thailand) during January 2012 to June 2015. DR was graded as absent, mild, moderate, or severe non-proliferative DR, or proliferative DR. Multivariate logistic regression analysis was used to identify independent risk factors for DR in DM patients.
Results: The overall prevalence of DR was 34.78%. Multivariate analysis revealed duration of diabetes, glycated hemoglobin level (HbA1c), presence of albuminuria, and abnormal protective sensation to be independent risk factors for DR. The prevalence of DR increased with longer duration of diabetes (p < 0.001), deterioration of glucose control (p = 0.006 for HbA1c), presence of significant albuminuria (p = 0.010), and loss of protective sensation (p = 0.001).
Conclusion: In this study, one-third of DM were found to have DR. The independent predictors of DR were duration of diabetes, HbA1c level, presence of significant albuminuria, and impaired protective sensation. Heightened awareness of these risk factors will decrease the prevalence and severity of DR, and will improve early diagnosis and treatment of DR.
Keywords: prevalence, factors, diabetic retinopathy, diabetes mellitus, Siriraj Hospital, Thailand
Introduction
Diabetic retinopathy (DR) is one of the most common chronic complications of diabetes mellitus (DM). DR is characterized by gradually progressive alterations in the retinal microvasculature, leading to areas of retinal non-perfusion, increased vascular permeability, and pathologic intraocular proliferation of retinal vessels. The complications are associated with macular edema, and uncontrolled neovascularization, termed proliferative diabetic retinopathy (PDR), resulting in severe and permanent vision loss if not treated in a timely and appropriate manner. DR is the leading cause of blindness among working-aged adults worldwide. However, with appropriate medical and ophthalmologic care, more than 90% of vision loss from PDR can be prevented.1 Unfortunately, in many cases, presenting symptoms may go unnoticed or unheeded and the damage caused by the disease becomes irreparable.
By 2045, it is estimated that approximately 700 million people worldwide will have diabetes2 and that approximately 103 millions of those will have DR.3 In the USA, about 59% of the patients with type 1 diabetes with onset before the age of 30 had retinopathy after 4 years,4 and this figure increased to 89.3% and 95.9% after 10 and 14 years, respectively.5,6 Since type 2 diabetes accounts for 90% of the diabetic population,7 type 2 diabetes accounts for a higher proportion of patients with vision loss. The reported annual progression from DR to sight-threatening DR varied from 3.4% to 12.3%.8 The 2010 National Health and Nutrition Examination Survey revealed that only 44.7% of the Americans with diabetic macular edema (DME) were aware that diabetes had affected their eyes and that nearly 60% of those individuals did not have a dilated eye examination within the past year, which suggests both a lack of awareness among patients at risk for vision loss from diabetic eye complications and insufficient evaluation for many patients with vision-threatening retinopathy.9
In Thailand, the reported prevalence of DR varied among studies, and those studies ranged in size and scope from single-center studies to nationwide population-based studies.10–14 Several risk factors, including long duration of diabetes, higher levels of glycated hemoglobin (HbA1c), higher blood pressure, and the presence of proteinuria, were reported to be associated with DR progression.15 Reported findings specific to the influence of other factors, including body mass index (BMI), male gender, serum lipids, and smoking, were inconsistent.16,17 The aims of this study were to determine the prevalence and severity of DR in diabetic patients who attended the Diabetic Clinic of Siriraj Hospital (Bangkok, Thailand), to identify the risk factors significantly associated with DR, and to investigate the relationship between identified risk factors and the severity of DR.
Patients and Methods
Subjects
This study is part of the Diabetic Registry Project, which was conducted during January 2012 to June 2015 at the Diabetic Clinic of the Division of Endocrinology and Metabolism, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. Patients included in the registry who were aged 15 years or above and who were diagnosed as type 1 diabetes, type 2 diabetes, or other types of diabetes according to American Diabetes Association (ADA) classification were eligible for inclusion. Patients with gestational diabetes were excluded. The other types are specific types of diabetes due to other causes, eg, monogenic diabetes syndromes (such as neonatal diabetes and maturity-onset diabetes of the young [MODY]), diseases of the exocrine pancreas, and drug- or chemical-induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS or after organ transplantation).
A total of 1299 patients were registered in the registry. Of those, 1130 patients who underwent retinal examination were enrolled in this study.
Methods and Measurements
Collected data included demographic characteristics, relevant physical examination data, laboratory findings during the 12-month period prior to recruitment, diabetic complications, and use of medications including insulin, oral antidiabetic agents, antihypertensive agents, and lipid-lowering agents. Eye examination with full dilation of the pupils was performed by an ophthalmologist. If the eye examination was performed at another center, the official report of that examination had to be present in the patient’s medical record at our center. In this study, DR was classified as either non-proliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR). NPDR was characterized by an increase in vascular permeability or vascular closure, such as micro-aneurysms, dot and blot hemorrhage, and exudates. PDR was defined if proliferation of new vessels was observed on or within the retina, including complications, such as vitreous hemorrhage or preretinal hemorrhage. NPDR was further subcategorized into mild NPDR, moderate NPDR, or severe NPDR based on clinical findings and the modified Airlie House classification system. The final retinopathy grading for each participant was based on the diagnosis in the more severely affected eye.18
Definitions of Diabetes and Major Risk Factors
Duration of Diabetes
The duration of diabetes was determined by history taking and chart review. The diagnosis of diabetes mellitus was made according to 2015 ADA criteria.19 Diabetes mellitus can be diagnosed based on plasma glucose criteria, either fasting plasma glucose (FPG) level or the 2-hour plasma glucose (2-h PG) level after 75-g oral glucose tolerance test (OGTT), or HbA1c criteria.
Albuminuria and Renal Function
The urine albumin-to-creatinine ratio (UACR) used in this study reflecting the mean of the last three laboratory values appearing in the medical record, but not beyond 12 months after the patient entered the registry. Normal UACR is defined as <30 mg/g Cr, and increased urinary albumin excretion is defined as ≥30 mg/g Cr. Due to variability in urinary albumin excretion, two of three UACR values within a 3- to 6-month period should be abnormal before considering a diagnosis of albuminuria. Elevation of UACR due to other conditions, such as exercise within 24 hours, infection, fever, congestive heart failure, marked hyperglycemia, menstruation, or marked hypertension was excluded since an increased UACR value due to these causes is not due to kidney damage. Regarding renal function assessment, the estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.20
Diabetic Neuropathy
Diabetic neuropathy was defined as symptoms of neuropathy, including pain or burning sensation, electric shock-like pain, and/or numbness in diabetic patients.
Foot Ulcer and Amputation
Foot ulcer was defined as the presence of foot ulcers, abrasions, bruises, dry gangrene, or wet gangrene. Regarding bunions, if there was pain, swelling, and/or signs of inflammation, they were classified as a foot ulcer. Amputations were recorded if they were performed due to the presence of infection or dry gangrene.
Cardiovascular Disease
Angina pectoris was defined as tight pain or discomfort in the chest, ache/pain at the shoulder and jaw, or epigastric pain, and the observed pain was aggravated by exercise, and ameliorated by rest or nitroglycerin. Congestive heart failure (CHF) included both acute and chronic heart failure, as well as CHF with reduced left ventricular ejection fraction (LVEF) (LVEF ≤ 40%), preserved LVEF (LVEF ≥ 50%), or mildly reduced LVEF (LVEF 41–49%).21 Myocardial infarction (MI) included 1) acute coronary syndrome, including unstable angina, non-ST-segment elevation myocardial infarction (non-STEMI), and ST-segment elevation myocardial infarction (STEMI); and 2) chronic stable angina. A diagnosis of MI was reviewed from the medical records, including data specific to coronary angiography with coronary revascularization or coronary artery bypass graft (CABG) surgery.
Cerebrovascular Disease
Cerebrovascular disease was defined as ischemic stroke, hemorrhagic stroke, or transient ischemic attack (TIA). Data were obtained from medical record and imaging study.
Peripheral Vascular Disease (PVD)
PVD was defined as the presence of any one of the following: 1) claudication; 2) an Ankle-Brachial Index (ABI) of ≤0.9; 3) an angiography result showing narrowing of the blood vessels; or 4) record of the patient having been treated by percutaneous transluminal angioplasty, vascular bypass graft surgery, or endovascular intervention.
Hypertension
Hypertension was defined as systolic blood pressure (SBP) >140 mmHg, diastolic blood pressure (DBP) >90 mmHg, or prescribed use of antihypertensive medications.
Dyslipidemia
Dyslipidemia was defined as a serum total cholesterol level over 200 mg/dL, triglyceride level higher than 150 mg/dL, high-density lipoprotein cholesterol <40 mg/dL, or the prescribed use of lipid-lowering agents.
Family History of Cardiovascular or Cerebrovascular Disease
Family history of cardiovascular or cerebrovascular disease was defined as history of having one or more first-degree relatives, including parents, siblings, or children, diagnosed with MI or stroke.
Blood Pressure (BP)
Blood pressure was defined as the blood pressure reading or the mean of different blood pressure readings taken on the day of registration in the registry at the Diabetic Clinic.
Height and Weight
Height and weight were defined as the height and weight measurements taken on the day of registration in the registry at the Diabetic Clinic.
Waist Circumference and Hip Circumference
Waist circumference and hip circumference were defined as the waist circumference and hip circumference measurements taken on the day of registration in the registry at the Diabetic Clinic. Waist measurement was performed with the patient standing with his/her feet 10 cm apart and during the expiration phase. The waist was measured at the midpoint between the lowest rib and the top of the iliac crest as recommended by the International Diabetes Federation (IDF).22 Hip circumference was measured at the widest portion of the buttocks. Both the waist circumference and hip circumference were measured at a level parallel to the floor.
Foot Disorders
Abnormal foot examination was defined as the presence of any one of the following: 1) skin defect or callus; 2) foot deformity, including hammer toe, claw toe, hallux valgus/hallux varus, or Charcot joint; 3) ingrown toenail; or 4) impaired protective sensation by monofilament test.
Statistical Analysis
Descriptive statistics were used to summarize patient demographic and clinical data. Chi-square test or Fisher’s exact test was used to compare categorical variables, and those results are presented as number and percentage. Students t-test was used to compare continuous variables with normally distributed data, and those results are shown as mean plus/minus standard deviation. The results of univariate analysis to identify factors significantly associated with DR are shown as crude odds ratio (OR) and 95% confidence interval (CI). Significant factors from that analysis were then entered into multivariate analysis to identify independent predictors of DR. Whenever two variables were very similar and had multicollinearity, only one of them would be included in the model. The results of multivariate analysis are presented as adjusted OR (aOR) and 95% CI. All statistical analyses were performed using SPSS Statistics version 23 (SPSS, Inc. Chicago, IL, USA), and a p-value less than 0.05 was regarded as being statistically significant.
Results
In total, 1299 patients were recruited into the registry. Of those, 1130 patients (86.99%), mean age 60 years and 62.7% female, underwent retinal examination by direct ophthalmoscopy after full dilatation of the pupils. Sixty-seven (5.93%), 1028 (90.97%), and 35 (3.10%) cases were type 1 DM, type 2 DM, and other types of DM, respectively. As shown in Table 1, the prevalence of DR in this study was 34.78% (393 patients). Concerning the type of DR, NPDR was found in 76.6% (301 patients), and PDR was found in 23.4% (92 patients). Among diabetic patients, 143 (12.7%) had mild NPDR, 147 (13.0%) had moderate NPDR, and 11 (1.0%) had severe NPDR. As shown in Figure 1A, the prevalence of DR peaked during the age range of 50–59.9 years. An increasing frequency of DR was found with increasing age from less than 20 to 59.9 years of age, and then a decrease frequency was observed after age 60. Moreover, the prevalence of DR was increased with a longer duration of diabetes from less than 5 years to 20 years, as shown in Figure 1B. The highest prevalence of DR, 52.69%, was found in patients with a duration of diabetes longer than 20 years. The prevalence of DR in type 1 diabetes, type 2 diabetes, and other types of diabetes categorized by duration of diabetes is shown in Figure 2A. DR was detected since the first 5 years of diagnosis of type 1, type 2, and other types of DM. In patients with type 2 diabetes, the prevalence of DR was found to increase with increased duration of disease. Among those with other types of DM, the prevalence of DR markedly increased after a 20-year duration of diabetes.
 |
Table 1 Prevalence, Type, and Severity of DR Among Diabetic Patients Attending the DM Clinic of Siriraj Hospital |
 |
Figure 1 Prevalence of diabetic retinopathy stratified according to age group (A) and duration of diabetes (B). |
 |
Figure 2 Prevalence of diabetic retinopathy in different types of diabetes stratified according to duration of diabetes (A) and severity of diabetic retinopathy (B). |
Figure 2B shows the prevalence of DR among the different types of diabetes. The percentages of DR detected in patients with type 1 diabetes, type 2 diabetes, and other types of diabetes were 3.05% (12 patients), 95.42% (375 patients), and 1.53% (6 patients), respectively. Patients with type 2 diabetes had a higher prevalence of non-proliferative diabetic retinopathy (NPDR), including the mild, moderate, and severe forms. However, the prevalence of PDR was similar between patients with type 1 and type 2 diabetes.
The demographic and clinical characteristics of patients compared between those with and without DR are shown in Table 2. The demographic and clinical characteristics of diabetic patients compared between those with no DR and those with NPDR, and between those with no DR and those with PDR are shown in Table 3. Patients with DR had older age, longer duration of diabetes, higher systolic blood pressure, higher HbA1c, higher serum creatinine, lower eGFR, and a higher proportion of significant albuminuria compared to those without DR. The number of oral antidiabetic drugs used and fasting plasma glucose of patients with NPDR were both significantly higher than those in patients without DR. Patients with PDR had a significantly higher proportion of smokers compared to patients without DR. Diastolic blood pressure was not significantly different between patients with NPDR and patients without DR.
 |
Table 2 Demographic and Clinical Characteristics of Diabetic Patients Compared Between Those with and without DR |
 |
Table 3 Demographic and Clinical Characteristics of Diabetic Patients Compared Between Those with No DR and Those with NPDR, and Between Those with No DR and Those with PDR |
Metabolic and blood pressure control compared between those with no DR and those with DR, between those with no DR and those with NPDR, and between those with no DR and those with PDR are demonstrated in Table 4. This analysis was performed using the cut-off points recommended for adults with diabetes from the ADA 2015.23 Patients with glycosylated hemoglobin levels of more than 7% or systolic blood pressure (SBP) levels of more than 140 mmHg had stronger association with DR and NPDR. Other parameters of diabetic patients with and without DR were not different, including the percentage of patients with diastolic blood pressure (DBP) greater than 90 mmHg, fasting plasma glucose (FPG) above 130 mg/dL, total cholesterol (TC) above 200 mg/dL, serum triglyceride (TG) above 150 mg/dL, low-density lipoprotein cholesterol (LDL-C) above 100 mg/dL, or high-density lipoprotein cholesterol (HDL-C) less than 40 mg/dL.
 |
Table 4 Metabolic and Blood Pressure Control Compared Between Those with No DR and Those with DR, Between Those with No DR and Those with NPDR, and Between Those with No DR and Those with PDR |
Univariate logistic regression analysis revealed type of DM, duration of diabetes, presence of diabetic nephropathy, systolic blood pressure, diastolic blood pressure, HbA1c, eGFR, hypertension, history of neuropathy, foot ulcer, foot deformity and amputation, history of chest pain and coronary artery disease, history of heart failure, history of stroke, history of peripheral vascular disease, diminished leg pulse, and impaired monofilament test to be factors significantly associated with DR (Table 5). No significant association was found between DR and current smoker status, hypercholesterolemia, hypertriglyceridemia, or low level of HDL-C.
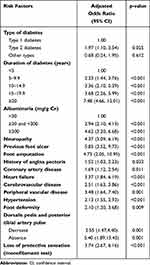 |
Table 5 Univariate Analysis for Factors Significantly Associated with DR in Patients with Diabetes |
In our multivariate logistic regression analysis (Table 6), model 1 included eGFR, presence of albuminuria, HbA1c, duration of diabetes, and abnormal protective sensation as variables since they were found to be closely associated with each other. The only difference between models was that model 2 did not include eGFR as a variable. Multivariate logistic regression analysis showed duration of diabetes (p < 0.001; model 1 and 2), HbA1c (p = 0.006; model 1), albuminuria (p = 0.010; model 2), and loss of protective sensation (p = 0.001; model 2) to be independent predictors of DR (Table 6).
 |
Table 6 Multivariate Analysis for Factors Independently Associated with DR in Patients with Diabetes |
The medications used among diabetic patients are shown in Table 7. Patients treated with insulin, either exclusive insulin use or insulin plus oral antidiabetic drug(s), were found to be significantly associated with DR. Treatment with antihypertensive drugs also showed association with DR. However, there was no significant difference in the use of lipid-lowering agents between patients with and without DR.
 |
Table 7 Medications Used Among Diabetic Patients Compared Between Those with and without DR |
Discussion
This study was a single-center registry-based study in DR among diabetes patients who attended the Diabetic Clinic of Siriraj Hospital – Thailand’s largest national tertiary referral center. Our results revealed a prevalence of DR of 34.78%. NPDR and PDR were found in 26.64% and 8.14% of DM patients, respectively. The prevalence of DR in the present study is similar to that reported by the Thailand Diabetes Registry Project, which was a 2006 multicenter hospital-based study.12,13 In that study, the prevalence of DR was 31.4% (NPDR 22%, PDR 9.4%). The prevalence of DR, especially PDR, in the present study is higher than the rates reported from two previous tertiary care-based studies in Thailand.10,11 The observed higher reported rates in our study may be partially explained by the fact that our center is the largest national tertiary referral center. A previous study14 conducted in the outpatient department of Siriraj Hospital among 722 diabetic patients treated by general practitioners reported a lower percentage of DR, including NPDR (25%) and PDR (6.2%). This can be explained by the fact that severe or difficult to control cases were usually transferred to the special diabetic clinic for management by an endocrinologist.
Similar to previous studies, our findings clearly demonstrate that DR is a common health problem among patients with DM. The Australian Diabetes, Obesity and Lifestyle study24 reported a prevalence of DR and PDR of 21.9% and 2.1%, respectively, in patients with type 2 diabetes, which is lower than our findings. This may be partly explained by the lower levels of glycosylated hemoglobin in their study population. A recent systematic review of 59 population-based studies published through 2020 reported a global prevalence of DR of 22.27%, and the prevalence was comparatively higher in Africa, North America, and the Caribbean, ranging from 33.3% to 35.9%.3 Among Thai patients with type 2 DM, the previously reported decrease in the overall prevalence of DR over a 5-year period may be explained by improved diabetic care due to Thai national health policy.25
In agreement with earlier reports,13,26 the current study found that the prevalence of DR increased with increasing duration of diabetes. The WESDR study16 reported an increased prevalence of any DR from 23% in people who had diabetes for less than 2 years to 57.7% in people with a duration of disease greater than 15 years. DR was found in 14.79% of our patients with a duration of diabetes less than 5 years, which suggests the importance of early detection and treatment of DR, even in type 2 diabetic patients with relatively short disease duration. Furthermore, the present study found DR to be detectable in type 1 DM patients during the first 5 years of diabetes; thus, a retinal examination may be required for type 1 diabetic patients with a duration of diabetes less than 5 years. We also demonstrated that the incidence of DR increased in patients with a history of diabetic complications, including history of neuropathy, previous foot ulcer, foot amputation, history of angina pectoris, coronary artery disease, heart failure, stroke, or peripheral vascular disease.
From previous studies,27–29 the prevalence of vision-threatening DR was higher in patients with younger-onset diabetes compared with patients with older-onset diabetes. Therefore, the patients with PDR may not have old age, and this may explain our finding that the patients without DR and the patients with PDR had similar mean age.
Our study found that the prevalence of DR was decreased in the old age groups (older than 59.9 years). Previous studies30,31 also demonstrated similar trend, and it may be explained by reduced survival rate in older patients with DR. However, our study found the increased prevalence of DR in patients who were older than 80 years. This finding may be explained by the limited number of patients among this age group in our study.
Poor glycemic control, including high glycemic variability, and high systolic BP were also common previously reported significant risk factors for DR.16,24,32–34 Regarding prevention of the development of DR, the United Kingdom Prospective Diabetes Study (UKPDS) reported the benefit of good glycemic control.35 Improved blood glucose control by reducing HbA1c levels from 7.9% to 7.0% could significantly decrease the overall rate of microvascular complications by 25% and could decrease the risk of retinal photocoagulation by 29%. Moreover, the UKPDS found that tight blood pressure control could decelerate DR progression. Therefore, improved blood pressure and glycemic control in those with type 2 diabetes can reduce the number of patients who develop retinopathy. However, our study did not find an independent association between hypertension and DR in multiple logistic regression analysis. This is in contrast to a recent study conducted in Iran36 that reported prehypertension and newly diagnosed hypertension to be independent risk factors for PDR.
The present study did not find association between serum lipid levels and DR. The association between DR and serum lipids was variously reported among previously published studies. Two studies, the ETDRS and WESDR studies,16,37 reported cholesterol level to be a significant factor for determining the severity of retinal hard exudates, but not the severity of DR in any group.
There were conflicting results regarding smoking to be the risk factor of DR.38 In the 10-year follow-up of WESDR study,39 there was no significant association between smoking and DR. However, in recent meta-analysis,40 the risk of DR in type 1 diabetic patients and in type 2 diabetic patients significantly increased and decreased in smokers compared with non-smokers, respectively. Cigarette smoking was found to be associated with PDR in our study. This is in agreement with the results from the recent study from Iran,36 which found that being smoker increased the risk of PDR.
Anemia was a factor previously reported41–44 to be associated with DR. Unfortunately, our study did not collect the hemoglobin or hematocrit level data of our study participants.
The strengths of our study include its large sample size compared to several previous studies, the fact that we included several types of diabetes (type 1 DM, type 2 DM, and other types of DM) and the fact that we determined the prevalence of and risk factors associated with DR. Due to the paucity of data regarding the association of other types of DM with DR, our results may fulfill this information. Moreover, the examination method for detecting DR in our study was direct eye exam by an ophthalmologist, not fundus photography by fundus camera. This method provides better accuracy for detection of DR.
Our study also has some limitations. First, since the study design was a cross-sectional, our study data could yield the prevalence and associated factors, but not causation. Second, our study was conducted in a single national tertiary referral care center, which means that our data may not be generalizable to other types of care settings.
Conclusion
DR was found to affect one-third of patients who attended the Diabetic Clinic of Siriraj Hospital. The prevalence of DR increased with longer duration of diabetes, high HbA1c level, presence of significant albuminuria, and impaired protective sensation. Diabetic patients should be encouraged to stringently control their diabetes and comorbidities. Appropriate management of associated risk factors can alleviate DR and other cardiovascular complications.
Abbreviations
ABI, Ankle-Brachial Index; ADA, American Diabetes Association; AIDS, acquired immunodeficiency syndrome; aOR, adjusted odds ratio; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft; CHF, congestive heart failure; CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DBP, diastolic blood pressure; DM, diabetes mellitus; DME, diabetic macular edema; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; ETDRS, Early Treatment Diabetic Retinopathy Study; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; IDF, International Diabetes Federation; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MODY, maturity-onset diabetes of the young; NPDR, non-proliferative diabetic retinopathy; OGTT, oral glucose tolerance test; OR, odds ratio; PDR, proliferative diabetic retinopathy; PG, plasma glucose; PVD, peripheral vascular disease; SD, standard deviation; SPSS, Statistical Package for Social Sciences; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TC, total cholesterol; TG, triglycerides; TIA, transient ischemic attack; UACR, urine albumin-to-creatinine ratio; UKPDS, United Kingdom Prospective Diabetes Study; WC, waist circumference; WESDR, Wisconsin Epidemiologic Study of Diabetic Retinopathy.
Data Sharing Statement
All relevant data are included in this submitted manuscript. All data are fully available upon reasonable request.
Ethics Approval
The protocol for this study was approved by the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (approval no. Si 625/2015) and was conducted under the ethical principles of the Declaration of Helsinki. This research study was conducted retrospectively from data obtained for clinical purposes. This research involves no more than minimal risk to subjects. The waiver of informed consent will not adversely affect the rights and welfare of the subjects, and the research could not practicably be carried out without the waiver of informed consent. Therefore, the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital approved that the informed consent from the patient was waived for this research.
Consent for Publication
Each author is aware of and is in agreement with this submission.
Acknowledgments
The authors gratefully acknowledge Professor Sutin Sriussadaporn for his expert advice, and Ms Khemajira Karaketklang of the Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, for assistance with statistical analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no personal or professional conflicts of interest relating to any aspect of this study.
References
1. Klein R, Klein BEK. Epidemiology of ocular functions and diseases in persons with diabetes. In: Cowie CC, Casagrande SS, editors. Diabetes in America. Bethesda (MD);2018.
2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
3. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi:10.1016/j.ophtha.2021.04.027
4. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237–243. doi:10.1001/archopht.1989.01070010243030
5. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112(9):1217–1228. doi:10.1001/archopht.1994.01090210105023
6. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801–1815. doi:10.1016/S0161-6420(98)91020-X
7. International Diabetes Federation. IDF Diabetes Atlas.
8. Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7(2):140–149. doi:10.1016/S2213-8587(18)30128-1
9. Bressler NM, Varma R, Doan QV, et al. Underuse of the health care system by persons with diabetes mellitus and diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(2):168–173. doi:10.1001/jamaophthalmol.2013.6426
10. Ausayakhun S, Jiraratsatit J. Prevalence of diabetic retinopathy in non-insulin dependent diabetes mellitus patients. Thai J Ophthalmol. 1991;5:133–138.
11. Bhuripanyo P, Graisopa S, Suwanwatana C, et al. Vascular complications in noninsulin dependent diabetes mellitus (NIDDM) in Srinagarind Hospital, Khon Kaen. J Med Assoc Thai. 1992;75(10):570–577.
12. Chetthakul T, Likitmaskul S, Plengvidhya N, et al. Thailand diabetes registry project: prevalence of diabetic retinopathy and associated factors in type 1 diabetes mellitus. J Med Assoc Thai. 2006;89(Suppl 1):S17–S26.
13. Chetthakul T, Deerochanawong C, Suwanwalaikorn S, et al. Thailand diabetes registry project: prevalence of diabetic retinopathy and associated factors in type 2 diabetes mellitus. J Med Assoc Thai. 2006;89(Suppl 1):S27–S36.
14. Sriwijitkamol A, Moungngern Y, Vannaseang S. Assessment and prevalences of diabetic complications in 722 Thai type 2 diabetes patients. J Med Assoc Thai. 2011;94(Suppl 1):S168–174.
15. Lin K-Y, Hsih W-H, Lin Y-B, Wen C-Y, Chang T-J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12(8):1322–1325. doi:10.1111/jdi.13480
16. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–532. doi:10.1001/archopht.1984.01040030405011
17. Yoshida Y, Hagura R, Hara Y, Sugasawa G, Akanuma Y. Risk factors for the development of diabetic retinopathy in Japanese type 2 diabetic patients. Diabetes Res Clin Pract. 2001;51(3):195–203. doi:10.1016/S0168-8227(00)00212-6
18. Fukuda M. Classification and treatment of diabetic retinopathy. Diabetes Res Clin Pract. 1994;24(Suppl):S171–S176. doi:10.1016/0168-8227(94)90246-1
19. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Supplement 1):S8–S16. doi:10.2337/dc15-S005
20. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
21. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726.
22. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi:10.1111/j.1464-5491.2006.01858.x
23. American Diabetes Association. 8. Cardiovascular disease and risk management. Diabetes Care. 2015;38(Supplement 1):S49–S57. doi:10.2337/dc15-S011
24. Tapp RJ, Shaw JE, Harper CA, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26(6):1731–1737. doi:10.2337/diacare.26.6.1731
25. Euswas N, Phonnopparat N, Morasert K, et al. National trends in the prevalence of diabetic retinopathy among Thai patients with type 2 diabetes and its associated factors from 2014 to 2018. PLoS One. 2021;16(1):e0245801. doi:10.1371/journal.pone.0245801
26. Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552–563.
27. Wong J, Molyneaux L, Constantino M, Twigg SM, Yue DK. Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care. 2008;31(10):1985–1990. doi:10.2337/dc08-0580
28. Hietala K, Harjutsalo V, Forsblom C, Summanen P, Groop PH; FinnDiane Study G. Age at onset and the risk of proliferative retinopathy in type 1 diabetes. Diabetes Care. 2010;33(6):1315–1319. doi:10.2337/dc09-2278
29. Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117(11):1487–1495. doi:10.1001/archopht.117.11.1487
30. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107(2):244–249. doi:10.1001/archopht.1989.01070010250031
31. The Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552–563. doi:10.1001/archopht.122.4.552
32. Foo V, Quah J, Cheung G, et al. HbA1c, systolic blood pressure variability and diabetic retinopathy in Asian type 2 diabetics. J Diabetes. 2017;9(2):200–207. doi:10.1111/1753-0407.12403
33. Hammes HP, Welp R, Kempe HP, et al. Risk factors for retinopathy and DME in type 2 diabetes-results from the German/Austrian DPV database. PLoS One. 2015;10(7):e0132492. doi:10.1371/journal.pone.0132492
34. Lu J, Ma X, Zhang L, et al. Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune diabetes of the adult and type 2 diabetes. J Diabetes Investig. 2019;10(3):753–759. doi:10.1111/jdi.12957
35. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. doi:10.1016/S0140-6736(98)07019-6
36. Sardarinia M, Asgari S, Hizomi Arani R, et al. Incidence and risk factors of severe non-proliferative/proliferative diabetic retinopathy: more than a decade follow up in the Tehran lipids and glucose study. J Diabetes Investig. 2021. doi:10.1111/jdi.13647
37. Chew EY, Klein ML, Ferris FL
38. Campagna D, Alamo A, Di Pino A, et al. Smoking and diabetes: dangerous liaisons and confusing relationships. Diabetol Metab Syndr. 2019;11(1):85. doi:10.1186/s13098-019-0482-2
39. Moss SE, Klein R, Klein BE. Cigarette smoking and ten-year progression of diabetic retinopathy. Ophthalmology. 1996;103(9):1438–1442. doi:10.1016/S0161-6420(96)30486-7
40. Cai X, Chen Y, Yang W, Gao X, Han X, Ji L. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: a meta-analysis. Endocrine. 2018;62(2):299–306. doi:10.1007/s12020-018-1697-y
41. Bahar A, Kashi Z, Ahmadzadeh Amiri A, Nabipour M. Association between diabetic retinopathy and hemoglobin level. Casp J Intern Med. 2013;4(4):759–762.
42. Ranil PK, Raman R, Rachepalli SR, et al. Anemia and diabetic retinopathy in type 2 diabetes mellitus. J Assoc Physicians India. 2010;58:91–94.
43. Mohan VKA, Nithyanandam S, Idiculla J. Microalbuminuria and low hemoglobin as risk factors for the occurrence and increasing severity of diabetic retinopathy. Indian J Ophthalmol. 2011;59(3):207.
44. Ito H, Takeuchi Y, Ishida H, et al. Mild anemia is frequent and associated with micro- and macroangiopathies in patients with type 2 diabetes mellitus. J Diabetes Investig. 2010;1(6):273–278. doi:10.1111/j.2040-1124.2010.00060.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
