Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Prevalence of alcohol use disorders and associated factors among people with epilepsy attending Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia
Authors Waja T, Ebrahim J , Yohannis Z, Bedaso A
Received 13 September 2016
Accepted for publication 8 October 2016
Published 22 November 2016 Volume 2016:12 Pages 2989—2996
DOI https://doi.org/10.2147/NDT.S122296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Tsegereda Waja,1 Jemal Ebrahim,2 Zegeye Yohannis,1 Asres Bedaso2
1Department of Psychiatry, Amanuel Mental Specialized Hospital, Addis Ababa, 2School of Nursing and Midwifery, College of Medicine and Health Sciences, Hawassa University, Hawassa, SNNPR, Ethiopia
Introduction: Alcohol use disorders represent one of the leading causes of preventable death, illness, and injury in many societies throughout the world. Heavy alcohol consumption has multiple negative consequences for people with epilepsy such as precipitation of seizure, exacerbation of seizure, poor seizure control, increased side effects of antiepileptic drugs, noncompliance to antiepileptic drugs, alcohol withdrawal seizures, long-term hospital admission, status epilepticus, sudden unexpected death, and premature mortality.
Methods: An institution-based cross sectional study was conducted from April 15, 2014 to May 15, 2014 with the aim of assessing prevalence of alcohol use disorders and associated factors among people with epilepsy attending Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia. A total of 413 randomly selected epileptic patients were included in this study. Data were structured using the 10-item Alcohol Use Disorders Identification questionnaire. Data were analyzed using SPSS Version 20. Bivariate and multivariate logistic regression analyses were performed to study the association, and variables with P-value <0.05 were considered as having a statistically significant association at 95% confidence interval.
Results: A total of 423 study participants were selected, of whom 413 completely filled the questionnaire making the response rate 97.6%. The mean age of the respondents was 31.9 years with standard deviation of ±10.97, and 248 (60%) were males. The prevalence of alcohol use disorder was 17.4%. Educational status (grade 9–12) (adjusted odds ratio [AOR] =3.25, [1.21, 8.69]), not living with family members (AOR =1.89, [1.06, 3.39]), availability of house (AOR =2.04, [1.10, 3.78]), taking carbamazepine (AOR =2.38, [1.13, 5.01]), and drinking alcohol to find relief from stress (AOR =4.28, [1.89, 9.67]) were significantly associated with alcohol use disorder among people with epilepsy.
Conclusion and recommendation: The findings of this study revealed that the prevalence of alcohol use disorder among people with epilepsy was high. Routine screening of epileptic patients with the Alcohol Use Disorders Identification Test is recommended.
Keywords: alcohol use disorder, epilepsy, Amanuel Mental Specialized Hospital, sub-Saharan Africa, stress relief
Introduction
Epilepsy is a common chronic neurological disorder that affects nearly 70 million people worldwide.1 More than 85% of people with epilepsy are living in low- and middle-income countries including sub-Saharan Africa.2,3 Meta-analysis studies on the estimation of burden of epilepsy showed that the median lifetime prevalence for epilepsy in developed countries was 5.8 per 1,000 population (2.7–12.4) compared with the 15.4 per 1,000 (4.8–49.6) for rural and 10.3 per 1,000 (2.8–37.7) for the urban population in developing countries.3,4
A systematic analysis study on the prevalence of epilepsy in sub-Saharan Africa showed that active epilepsy was estimated to affect 4.4 million people, while lifetime epilepsy was estimated to affect 5.4 million.5 Another meta-analysis showed that the median incidence of epilepsy was 50.4/100,000/year (33.6–75.6), while it was 45.0 (30.3–66.7) for high-income countries and 81.7 (28.0–239.5) for low- and middle-income countries.6 A huge treatment gap is suspected for epilepsy, and data suggest that nearly 80%–85% of people with epilepsy have never been diagnosed and treated. The existing studies suggest that there is a three- to fourfold increased risk of dying from epilepsy.7
A community-based study in 61,686 populations in a rural area of central Ethiopia showed that 139 incident case of epilepsy, corresponding to an annual incidence of 64 in 100,000 people. The study showed that incidence of epilepsy is high in Ethiopia.8 Alcohol use disorders represent one of the leading causes of preventable death, illness, and injury in many societies throughout the world.9 Alcohol is a causal factor for 60 types of diseases and injuries and a component cause in 200 others. Almost 4% of all deaths worldwide are attributed to alcohol, greater than deaths caused by HIV/AIDS, violence, or tuberculosis. Alcohol is also associated with many serious social issues, including violence, child neglect and abuse, and absenteeism in the workplace.10 Overall, 4% of global burden of disease is attributable to alcohol.10,11 Alcohol abuse contributes to 4.6% global disability adjusted life years.12
The most dangerous complication of alcohol intoxication is respiratory depression.13 A blood level of 500 mg/dL is lethal in around 50% of patients, but when used with other central nervous system depressants like phenobarbital (antiepileptic drug), much lower blood levels can be fatal.13,14
Heavy alcohol consumption has multiple negative consequences for people with epilepsy such as precipitation of seizure, exacerbation of seizure, worsening of seizure control, increased side effects of antiepileptic drugs, noncompliance to antiepileptic drugs, alcohol withdrawal seizures, long-term hospital admission, status epilepticus (9%–25%), sudden unexpected death, and premature mortality.12,15–17
Alcohol and epilepsy are completely interrelated. The seizure threshold is raised by alcohol drinking and declines on cessation of drinking. As a result, during withdrawal from alcohol, usually 6–8 hours after the cessation of drinking, seizures may occur.16 Although it is commonly perceived that patients with epilepsy experience problems with seizure control if they use alcohol, this is not confirmed by the few experimental studies that have tested the hypothesis. The mechanisms by which the long-term neurotoxic effects of alcohol lead to chronic epilepsy also need further studies.17,18
Alcohol-related withdrawal contributes to 26% and 9% of status epilepticus and refractory status epilepticus in adults, respectively.19 The relationship of alcoholism to epilepsy has been recognized for many years, but the role of alcohol in the exacerbation of primary epilepsy and in triggering seizures in epileptic patients is often not well recognized. Control of alcohol ingestion is an important factor in the management of epilepsy.20
Despite alcohol use disorders having severe, chronic, and fatal consequences in people with epilepsy, little is known about its prevalence and associated factors among people with epilepsy in Ethiopia. Therefore, this study was performed to determine prevalence of alcohol use disorders and associated factors among people with epilepsy attending Amanuel Mental Specialized Hospital. Also, this study was intended to fill this gap, and the findings of this study help to give special attention for those patients with epilepsy and alcohol use disorder.
Methods and materials
An institution-based cross sectional study was conducted at Amanuel Mental Specialized Hospital from April 15, 2014 to May 15, 2014. Amanuel Mental Specialized Hospital is one of the oldest hospitals in Ethiopia. It was established in 1930 by Italians to serve the nation of Ethiopia. It is located in western part of Addis Ababa in Addis ketema subcity kebele 08. The hospital has 259 beds including 11 private wing beds and 23 emergency beds. The hospital has 13 outpatient departments. An average of 26,400 follow-up and new patients receive services annually, and most of the patients attend with family members. A total of 2,200 epileptic patients are seen by neuropsychiatry case team each month, and it ranks second in outpatient department patient volume in the hospital.
All adult people with epilepsy attending Amanuel Mental Specialized Hospital were the source population, and those people with epilepsy who visited Amanuel Mental Specialized Hospital during the study period and fulfilled the inclusion criteria were considered as study population. Participants aged 18 years and above were included in the study, and those people who were unable to communicate and were seriously disturbed were excluded from the study.
The sample size was determined by using a single proportion formula considering the following the assumption: standard normal distribution with confidence interval of 95% (Z=1.96), absolute precision or tolerable margin of error (d=0.05), since proportion of alcohol use disorder among people with epilepsy is unknown in Ethiopia (P=50%), and so taking 10% nonresponse rate, the final calculated sample size was 423.
Systematic random sampling method was used to select the study subjects. A total of 2,200 patients are seen at outpatient department of Amanuel Mental Specialized Hospital per month, and the sampling interval was calculated by using the formula K=N/n=2,200/423=~5.2. The first patient was selected by lottery method and every fifth patient was selected until the sample number was reached.
Data were collected through face-to-face interview technique by using a structured questionnaire. The English questionnaire was translated to Amharic by an expert and adopted with minor modifications and was back-translated to English. Alcohol Use Disorders Identification Test (AUDIT), a 10 item tool, was used for assessing alcohol use. A structured questionnaire was used to assess sociodemographic and other independent variables.
A pretest was conducted on 21 subjects at Zewditu Memorial Hospital 1 week prior to the actual data collection and the appropriateness of the questionnaire checked by the investigator. Training was given for data collectors and supervisors before the data collection period. Completeness of the questionnaire was checked daily by supervisors.
Data were coded, entered, and cleaned by using EPI Info Version 7 and analyzed by using SPSS version 20 (IBM Corp., Armonk, NY, USA). Descriptions of variables were presented as frequencies, percentage, tables, and graphs. Bivariate and multivariate analyses were done to study the association, which was explained by P-value, odds ratio, and 95% confidence interval. A P-value <0.05 was considered as being statistically significant.
According to AUDIT, an individual whose score is 16 or more is said to have alcohol use disorder. Poor social support is defined as those who score 3–8 on the Oslo-3 Social Support Scale (OSSS-3). Moderate social support is defined as those who score 9–11 on OSSS-3. Strong social support includes those who score 12–14 on the OSSS-3. A patient is defined as “a current substance user” if he/she has used any of the following specified substances: cigarette, khat, cannabis, and cocaine in the past 3 months.
Ethical statement
Ethical clearance was obtained from the ethical review board of Institute of Public Health, College of Medicine and Health Sciences, University of Gondar and Amanuel Mental Specialized Hospital. The Institute of Public Health ethical review board approved the methods of data collection and forms of consent. Written informed consent was obtained from each participant. The objective of the study was explained to study subjects and the right was given for the study subjects to discontinue the interview at any time. Those who were considered as alcohol dependent by data collectors were referred to addiction case team for further evaluation and management. Confidentiality was maintained by not writing identification and name of the participants.
Results
A total of 423 study participants were selected, of whom 413 completely filled the questionnaire, resulting in a response rate 97.6%.
Sociodemographic and related characteristics of the respondents
The age of respondents ranged from 18 to 78 years with a mean age of 31.9 (±SD 10.97) years. From the total respondents, 137 (33.2%) were between 25 and 31 years of age. Of the participants, 248 (60%) were males, 272 (65.9%) were Orthodox Christians by religion, and 171 (41.4%) were Amhara by ethnicity. With regard to marital status, 223 (54%) of the participants were single. One hundred thirty seven (33.2%) of study participants had been educated to between grade 9 and 12. Majority (257 [62.2%]) of the participants were living with their family members (Table 1).
  | Table 1 Distribution of sociodemographic characteristics of study subjects at Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia (n=413) |
Clinical factors of the respondents
Concerning the clinical factors, duration of illness ranges from less than 1 to 37 years with a mean duration of 9.91 (±standard deviation [SD] 6.94) years. One hundred forty eight (35.8%) of the respondents had been ill for 6–10 years. Duration of treatment with antiepileptic drug also varied from treatment for less than 1 year to having been treated for 36 years, and the mean duration of treatment was 7.02 (SD ±5.90) years. Of the total study subjects, 218 (52.8%) were treated with antiepileptic drug for less than 5 years. Three hundred forty eight (84%) of the respondents took phenobarbital and 319 (77.2%) took only one antiepileptic drug. For 90 (21.8%) patients, tella/tej was most preferred alcohol, and 107 (25.9%) patients stated that they drank for relaxation purpose (Table 2).
  | Table 2 Clinical factors of people with epilepsy attending Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia (n=413) |
Social support characteristics of the respondents
One hundred ninety five (47.2%) study participants stated that they have one or two persons who share their problems. Only 127 (30.8%) of participants reported that other people had a lot of concern about what they were feeling/doing. On the basis of the results of the OSSS-3, 260 (63%) had poor social support (score <9 out of 14) (Table 3).
  | Table 3 Social support characteristics of people with epilepsy attending Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia (n=413) |
History of substance use other than alcohol in the past 3 months
Among the respondents, 343 (83.1%) of them did not use any substance other than alcohol in the past 3 months. Sixty seven (16.2%) and 31 (7.5%) of the respondents reported the use of Khat and cannabis, respectively, in the past 3 months (Table 4).
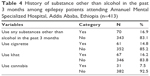  | Table 4 History of substance other than alcohol in the past 3 months among epilepsy patients attending Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia (n=413) |
Prevalence of alcohol use disorder
In this study, the prevalence of alcohol use disorder among people with epilepsy was 72 (17.4%). On the AUDIT, 72 (17.4%) of the respondents scored above the cutoff point of 16. Male and female respondents who scored above the cutoff point of AUDIT were 46 (18.5%) and 26 (15.8%), respectively.
Factors associated with alcohol use disorder among people with epilepsy
During bivariate analysis, educational status, living conditions, availability of own house, duration of illness, duration of treatment with antiepileptic drug, taking phenobarbital, taking carbamazepine, history of other substance use in the past 3 months, history of Khat chewing, type of alcohol-containing beverage, reason for drinking alcohol, and family history of alcohol use were associated (P-value <0.2) with alcohol use disorders among people with epilepsy.
After adjusted multiple logistic regression, educational status, living condition, availability of own house, taking carbamazepine, and reason for drinking alcohol were significantly associated with alcohol use disorder among people with epilepsy.
Those participants who had studied to between grade 9 and 12 were three times more likely to have alcohol use disorder (adjusted odds ratio [AOR] =3.25, [1.21, 8.69]) compared with those who had studied beyond grade 12. Those who did not live with family members were 1.89 times more likely to have alcohol use disorder (AOR =1.89, [1.06, 3.39]) compared with those living with family members. Those participants who reported not having their own house were also two times more likely to have alcohol use disorder (AOR =2.04, [1.10, 3.78]) compared with those who have an own house.
Those respondents taking carbamazepine were two times more likely to have alcohol use disorder (AOR =2.38, [1.13, 5.01]) compared with those who were not taking carbamazepine. Those participants who drank alcohol to find relief from stress were four times more likely to have alcohol use disorder (AOR =4.28, [1.89, 9.67]) compared with those patients who had no specific reason for drinking alcohol (Table 5).
Discussion
Alcohol use disorder has severe health consequences, especially in those people with epilepsy. The prevalence of alcohol use disorder was 17.4% among people with epilepsy. In this study, the prevalence of alcohol use disorder among people with epilepsy was higher than that observed in the study done on epileptic outpatients (12%) but lower than that of inpatients (35%) in Norway.21,22 The difference might be due to AUDIT cutoff point and severity of illness. In the current study, a cutoff point of ≥16 on the AUDIT was used, whereas the Norwegian study used a cutoff point of ≥8.
Also, those epileptic patients treated in outpatient department of Amanuel Mental Specialized Hospital have relatively less severe illness compared with those patients in Norway. The reason for admission of epileptic patients might be alcohol-related complication, status epilepticus, alcohol withdrawal, or alcohol intoxication. This may be the reason for high prevalence of alcohol use disorder in admitted patients in Norway.
The prevalence of alcohol use disorder was lower compared with the study conducted on admitted epileptic patients in United States of America (41%).23 This variation might be due to cultural acceptance of drinking alcohol, accessibility of alcohol containing beverages, and severity of illness.
The proportions of unemployed, cannabis users, and those treated with a single antiepileptic drug were lower than that observed in the study done in South Africa.24 In this study, 17.2% were unemployed, 7.5% used cannabis, and 77.2% were treated with a single antiepileptic drug. A similar study done on 101 epileptic patients who were readmitted to Komani Hospital, South Africa, showed that 63% were unemployed, 24% used cannabis, and 97% were on a single antiepileptic drug.24 This difference might be due to variation in inclusion criteria, severity of illness, accessibility of cannabis, and practice of rational use of antiepileptic drugs.
In South Africa, they assessed readmitted epileptic patients, whereas in our study only patients who were treated at outpatient department were included. Severity of illness may influence employment status of the patients in South Africa. A higher percentage of patients were on a single antiepileptic drug when compared with this study. These might be due to availability of highly qualified professionals and the application of standard treatment recommendations (monotherapy) for epileptic patients in Komani hospital, South Africa.24
Those participants whose educational status ranged from grade 9 to 12 were three times more likely to have alcohol use disorder (AOR =3.25, [1.21, 8.69]) compared with those who had studied above grade 12. This might be because grade 9–12 is a critical time that determines the academic success and the future career of the students, and so, this is associated with increased academic stress. The students might also drink alcohol to find relief from stress.
Those who are not living with family members were 1.89 times more likely to have alcohol use disorder (AOR =1.89, [1.06, 3.39]) compared with those living with family members. This may be due to freedom from parental control and also ability to make independent decisions in all aspect of his/her life.
Those participants reported not having their own house were also two times more likely to have alcohol use disorder (AOR =2.04, [1.10, 3.78]) compared with those who have reported having their own house. This may be because they may suffer more from different kinds of life stressors that lead to alcohol use as reliving factor for this stress.
Those respondents taking carbamazepine were two times more likely to have alcohol use disorder (AOR =2.38, [1.13, 5.01]) compared with those who were not taking carbamazepine. Alcohol may lower carbamazepine levels because of microsomal enzyme induction. Due to this factor, patients who take carbamazepine may drink alcohol more than other patients.
Those participants who drink alcohol to find relief from stress were four times more likely to have alcohol use disorder (AOR =4.28, [1.89, 9.67]) compared with those patients who drink alcohol for no specific reasons. This might be because people drink alcohol for relaxant and euphoric effects for a moment and to reduce stress, but this may worsen the stress in the long term.
Limitations of the study
Alcohol may cause epilepsy, and epileptic patients are also more vulnerable to use alcohol than the general population. But, it is difficult to distinguish the cause and effect relationship using a cross-sectional study design. The type of epilepsy is not studied because clinicians do not clearly state the type of epilepsy during diagnosis in the patient chart.
Conclusion and recommendations
The prevalence of alcohol use disorder among people with epilepsy was high, which is a public health problem. Educational status, living condition, availability of own house, taking carbamazepine, and drinking alcohol to find relief from stress were associated with alcohol use disorder among people with epilepsy.
Amanuel Mental Specialized Hospital has to work to improve assessment and management of alcohol use disorder among people with epilepsy. It will be better if clinicians do routine screening of epileptic patients with the AUDIT. During assessment of alcohol use disorder among epileptic patients, clinicians need to better focus on educational status, living conditions, availability of own house, consumption of carbamazepine, and reason of drinking alcohol (eg, to find relief from stress). Also, it would be better to make a note of the type of epilepsy in the patient chart. Finally, we recommend other researchers to conduct better prospective studies to investigate potential risk factors of alcohol use disorder among patients with epilepsy.
Acknowledgments
We thank University of Gondar, College of Medicine and Health sciences, and Amanuel Mental Specialized Hospital for providing fund to conduct the research. Also our deepest gratitude goes to Amanuel Mental Specialized Hospital neuropsychiatry case team members, plan and program office for providing us valuable information and for their keen cooperation. Finally we would like to forward our appreciation to study participants, data collectors, and supervisors who honestly shared their time to generate the data required for the study.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Yemadje LP, Houinato D, Quet F, Druet-Cabanac M, Preux PM. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. 2011;52(8):1376–1381. | ||
Kariuki SM, Matuja W, Akpalu A, et al. Clinical features, proximate causes and consequences of active convulsive epilepsy in Africa. Epilepsia. 2014;55(1):76–85. | ||
Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–890. | ||
Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4(1):21–23. | ||
Paul A, Adeloye D, George-Carey R, Kolčić I, Grant L, Chan KY. An estimate of the prevalence of epilepsy in sub-Saharan Africa: a systematic analysis. J Glob Health. 2012;2(2):020405. | ||
Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology. 2011;77(10):1005–1012. | ||
Diop AG, Hesdorffer DC, Logroscino G, Hauser WA. Epilepsy and mortality in Africa: a review of literature. Epilepsia. 2005;46 (Suppl 11):33–35. | ||
Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia. 1997;38(5):541–546. | ||
World Health Organization. Global Status Report on Alcohol and Health. Geneva: WHO; 2011. Available from: http://www.who.int/iris/handle/10665/44499. Accessed March 20, 2014. | ||
Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. | ||
Rehm J, Baliunas D, Borges GL, et al. The relation between different dimension of alcohol consumption and burden of diseases: an overview. Addiction. 2010;105(5):817–843. | ||
Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of diseases and injury and economic cost attributable to alcohol use and alcohol use disorders. Lancet. 2009;373:2223–2233. | ||
Schwarzmann V, Berthaux N. The action of barbiturates upon liver alcohol dehydrogenase: their interference with alcohol metabolism. Biomedicine. 1974;21:26–29. | ||
Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266–1272. | ||
Gheorghiev C, De Montleau F, Defuentes G. Alcohol and epilepsy: a case report between alcohol withdrawal seizures and neuroborreliosis. Encephale. 2011;37(3):231–237. | ||
Hauser WA, Ng SK, Brust JCM. Alcohol, seizures, and epilepsy. Epilepsy. 2007;29(2):S66–S78. | ||
Barclay GA, Barbour J, Stewart S, Day CP, Gilvarry E. Adverse physical effects of alcohol misuse. Adv Psychiatr Treat. 2008;14:139–151. | ||
Hillbom M, Pieninkeroinen I, Leone M. Seizure in alcohol dependent patients. CNS Drugs. 2003;17(14):1013–1030. | ||
Bleck TP. Less common etiologies of status epilepticus. Epilepsy Curr. 2010;10(2):31–33. | ||
Heckmatt J, Shaikh AA, Swash M, Scott DF. Seizure induction by alcohol in patients with epilepsy experience in two hospital clinics. J R Soc Med. 1990;83(1):6–9. | ||
Brathen G, Brodtkorb E, Sand T, Helde G, Bovim G. Weekday distribution of alcohol consumption in Norway: influence on the occurrence of epileptic seizures and stroke? Eur J Neurol. 2008;7(4):413–421. | ||
Bråthen G, Brodtkorb E, Helde G, Sand T, Bovim G. The diversity of seizures related to alcohol use. A study of consecutive patients. Eur J Neurol. 2003;6(6):697–703. | ||
Earnest MP, Yarnell PR. Seizure admissions to a city hospital: the role of alcohol. Epilepsy. 2007;17(4):387–393. | ||
Saha SK, Nel M, Prinsloo EA. Profile and associated factors for re-admitted epileptic patients with complications in a South African hospital. Cent Afr J Med. 2006;52(3–4):35–38. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

