Back to Journals » Journal of Blood Medicine » Volume 14
Prevalence of ABO and Rh Blood Group Among Volunteer Blood Donors at the Blood and Tissue Bank Service in Addis Ababa, Ethiopia
Authors Debele GJ, Fita FU , Tibebu M
Received 4 October 2022
Accepted for publication 12 January 2023
Published 18 January 2023 Volume 2023:14 Pages 19—24
DOI https://doi.org/10.2147/JBM.S392211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Getu Jenbere Debele,1 Fekadu Urgessa Fita,2 Melatwork Tibebu2
1Ethiopian Blood and Tissue Bank Service, Addis Ababa, Ethiopia; 2College of Health Science, Department of Medical Laboratory Science, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Melatwork Tibebu, Email [email protected]
Background: The discovery of the ABO blood group system and testing of blood donors highly reduced the fatalities associated with blood transfusion reactions and improved the safety of blood transfusion. Blood group antigens are found on the surface of red blood cells that are inherited biological characteristics that do not change throughout life in healthy individuals.
Objective: To determine the prevalence ABO and Rh blood groups Among Volunteer Blood Donors at Ethiopian blood and tissue bank service (EBTBS), Addis Ababa.
Methods: A cross-sectional study was carried out from January 2022 to May 2022, on 1700 volunteer blood donors to assess prevalence of ABO and Rh blood groups among volunteer blood donors at the Ethiopian blood and tissue bank service. All tests were performed using fully automated immunohematology analyzer (Galileo Neo Immucor). Data processing and analysis were undertaken by using Statistical Package for the Social Sciences (SPSS) version 26. An ethical clearance letter was obtained from Addis Ababa University and informed consent was also obtained from the participants of the study.
Results: A total of 1700 donors were included, of which 57% of donors were males. The majority of the donors belonged to the age group between 18 and 25 years old (53%). The antigen frequencies of ABO and Rh(D) blood group system showed that O was the most prevalent blood group 44.65% followed by A (28.41%), B (21.24%), and AB (5.71%). The Rh-positive donors were more prevalent (94.82%) as oppose to the Rh-negative (5.18%).
Conclusion: The knowledge of the distribution of blood groups is very important for blood banks and transfusion services which play an important role in the patient’s health care. The findings of the ABO blood group in this study were comparable to other studies conducted in Ethiopia.
Keywords: ABO blood group, Rh factor
Introduction
The discovery of the ABO blood group system and testing of blood donors highly reduced the fatalities associated with blood transfusion reactions and improved the safety of blood transfusion.1 Blood group antigens are inherited biological characteristics that do not change throughout life in healthy people and represent antigens found on the surface of red blood cells (RBCs).2 The International Society of Blood Transfusion (ISBT) currently recognized 43 blood group systems containing 345 red cell antigens (June 2021). The ISBT also maintains three categories of antigens that have not yet been associated with blood group systems. “Collections” (the 200 series) were designed to group antigens that are biochemically, genetically, or serologically similar where the genetic basis has not yet been discovered. The “700 Series” contains antigens that do not fit into any system or collection and have an incidence of less than 1% across all human ethnic populations, and the “901 Series” contains antigens that have a frequency of greater than 99% across populations of different ethnic ancestry.3
The ISBT acknowledged 9 blood group systems that are clinically significant and associated with hemolytic transfusion reactions (HTR), and hemolytic disease in fetuses and newborns (HDFN).4 These blood group systems are ABO, Rhesus, Kell, Duffy, Kidd, MNS, P, Lewis, and Lutheran. Out of these blood group systems, antigens of the three blood group systems are important in clinical transfusion practices, which are the ABO, Rh, and Kell blood group systems are highly immunogenic5 The ABO and Rhesus (Rh) blood group system is most important for blood transfusion purposes, parental testing, legal medicine, and in population genetic study.6
WHO blood safety initiative described that the quality and safety of blood and blood products must be assured throughout the process from the selection of blood donors to the administration of blood to the patient.7 Safe blood transfusion is not realized only by testing of blood for transfusion transmittable Infections (TTIS), but also thorough protection from HTR resulting from Alloimmunization against red cell antigens,8 which commonly occurs following a transfusion or in pregnancy.9 The primary responsibility of all blood bank services was to ensure safe blood and blood products for the transfusing facilities and recipients for routine use. Laboratory testing of the donor’s blood is important to ensure that the recipient receives the safest possible blood. Transfusing phenotype-matched blood can prevent the development of antibodies against clinically significant antigens. However, the problem is greater in developing countries, especially in resource-limited countries like Ethiopia, as the lack of awareness, and typing antisera as well as the associated costs and logistics were a serious concern. The primary outcome of this study is to detect the prevalence of ABO and Rh-D blood groups among voluntary blood donors at EBTBS, Addis Ababa.
The results of this study will be beneficial for health personnel, planners, policymakers, Non-Governmental Organizations, and others who are engaged in Blood transfusion activities. Hence, the findings of this study will be disseminated to the relevant bodies, stakeholders, and others who are involved in improving the safety of blood donation. This study can also be used to establish guidelines for blood group distribution throughout the country, used as a baseline contribution for the researchers, and additional input for EBTBS and MoH.
Methods
This study was carried out between January and May, 2022 at the Ethiopian Blood and Tissue Bank (EBTBS), located in Addis Ababa, the capital city of Ethiopia. The blood bank service in Ethiopia was established in 1969 by the Ethiopian Red Cross Society (ERCS). The Government made a policy decision to revert the responsibility from ERCS to a Government-owned service under the Federal Ministry of Health (FMoH) as an autonomous agency as of January 8, 2015. Currently, since January 25, 2022, the blood bank has been re-established as an EBTBS under Regulation Number No. 1263/2021, tasked to coordinate, give logistic and technical support as well as oversee all the 43 regional blood bank services by managing and controlling tissue and organ transplantation in the country.
A cross-sectional study was carried out to determine the frequency of ABO and Rh blood groups in volunteer blood donors. Blood donors who were available during the data collection period were selected after proper history taking and complete examination for fulfilling the eligibility criteria to donate blood. A convenient sampling method was used for sample collection. All blood samples that were collected at EBTBS during the study period were the source population and those blood samples that were used for the study during the study period were the study population.
The sample size was calculated using the single population proportion formula with a proportion of 50%. The 2.5% margin of error was used to increase accuracy. A 95% of a Confidence interval and 10% for an inconvenient sample were used to determine the required sample size for the study.
Where: - n: Estimated sample size. 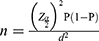
Z: Z statistic for a level of confidence, Z=1.96 (from normal distribution table for CI of 95%)
p: Expected prevalence or proportion, 50%, because there is no previous study in Addis Ababa (in a proportion of one; if 50%, P = 0.5), d: precision 2.5%, which is a tolerable error between the sample and the true population. (In proportion of one; if 2.5%, d = 0.025). Then 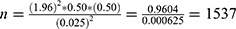
Considering a 10% inconvenient sample (1537 ×10% = 153) The estimated sample size was 1537 + 153 = 1690. However, additional 10 samples were added to make a sample size of 1700.
A total of 1700 blood samples, including 970 (57.1%) males and 730 (42.9%) females from volunteer healthy blood donors between the ages of 18 and 65, weighing more than 45 kilograms with normal hemoglobin values were included in the study. The Blood donors were selected strictly according to the nationally standardized selection criteria established by the blood bank service. A questionnaire was given to each donor, that includes details regarding their basic Socio-demographic profile, age, sex, address, any history of the previous transfusion, drug intake, jaundice, and any clinically significant disease related to autoimmune disorders. The general health conditions of blood donors were assessed through a medical examination.
Laboratory Procedure
Blood specimens were collected using a standard phlebotomy technique, by well-trained nurses, from volunteer non-remunerated blood donors, through a mobile campaign, and at the center clinic. A 5 mL of blood sample was collected at the time of donation into a tube containing ethylenediaminetetraacetic acid (EDTA). From the mobile collection site, blood specimens were transported to the blood bank by maintaining the cold chain at 2–10°C with a cold box. After arrival at the blood bank, the blood samples were centrifuged at 3000 rpm for 5 min.
Blood samples were analyzed to determine ABO and Rh-D blood groups; using the Galileo fully automated immunohematology analyzer (Galileo Neo Immucor Gamma automated system). The ABO forward and, reverse grouping, as well as Rh-D tests, were performed based on the agglutination principle with microplates. Forward grouping was done using anti-A, anti-B, anti-AB, and anti-D antisera. Reverse grouping was done using A1 and B red blood cells. Donors typed as Rh-D negative were confirmed using an antiglobulin weak D test in an automated solid-phase test using Novaclone anti-D (Immunocor Med. Diagnostik GmbH, Germany) IgG and IgM antisera. The sample was considered Rh (D) negative only after the “weak D” testing was confirmed to be negative.
Quality Assurance
Blood samples were collected by well-trained nurses, after obtaining written or oral informed consent from the volunteer blood donors. Universal precautions were taken during sample handling, processing, and testing. Reagent storage and labeling of samples were managed properly according to the kit manufacturer (Immucor, Inc, Germany) and EBTBS standards. All data recordings were checked for completeness as well as the results were recorded with the donor identification number.
Daily quality control of selected red blood cells and antiserum was performed to confirm the reactivity and specificity of the reagent. These reagents were tested with the corresponding antigen-positive and antigen-negative red blood cells. The reagents were considered appropriate for use if only antigen-positive red blood cells demonstrate a positive result. To establish the validity of the results’ of the automated system, the initial 30 samples were typed manually using the tube method in parallel to the study using reagents from Immucor Inc. The manual testing was done according to the manufacturers’ (Immucor, Inc, Germany) instructions. No discrepancies were found in any of these tests.
The collected data was cleaned, coded, summarized, entered into an Excel spreadsheet, and imported to the statistical package for social science version 26 (SPSS Inc. Chicago, IL, USA) for analysis and interpretation. The Data were analyzed using descriptive statistical analysis utilizing absolute frequencies and percentages, and the results were organized in Tables 1 and 2. P-value of <0.05 was considered statistically significant with a 95% confidence level.
 |
Table 1 Blood Group and Rh(D) Prevalence Among Voluntary Blood Donors and the Correlation with Gender from January 2022 to May 2022 at EBTBS (n=1700) |
 |
Table 2 ABO and Rh (D) Blood Group Distribution in Ethiopian Blood Donors |
The research proposal was ethically cleared by the Department Research and Ethical Review Committee at the Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University. Official permission from EBTBS was obtained as well. We confirm that our study complies with the Declaration of Helsinki. During data collection, all blood donors gave their consent that their blood sample can be used for research purposes and no donor name was recorded. Blood samples were labeled only with the donor identification number used as a unique study code. Confidentiality of the result was maintained, and the results were shared only with the medical staff of authorized members of the study. Computerized files were password protected and paper files were also locked safely and accessible only to authorized personnel.
Result
A total of 1700 volunteer donor samples were analyzed to determine the prevalence blood groups. Male predominance in blood donation accounted for 57.1% (970/1700). The younger age group (18–25 years) contributed about 53.1% (903/1700) of the total blood donations followed by 26–35, 36–45, 46–55, and 56–65 years of age groups accounting for 25.9% (441/1700), 14.3% (243/1700), 5.2% (88/1700) and 1.5% (25/1700) respectively. The mean age of the donors was 28 years, the median age was 24 years and the most frequently donating age group was among 19-year-olds.
The distribution of ABO and Rh blood groups showed that, O was the most prevalent blood group 559(44.65%) followed by A 483(28.41%), B 361(21.24%), and AB 97(5.71%) of the total volunteer donors in the study. The distributions of O positive blood group were the highest (41.88%), followed by A positive (27.41%), B positive (20.12%), AB positive (5.47%), O negative (2.76%), B negative (1.18%), A negative (0.94%), and AB negative (0.24%). Rh-positive donors were more prevalent (94.82%) as opposed to Rh-negative (5.18%). ABO and Rh frequency were not significantly different between male and female donors (P = 0.3) (Table 1). There was also no statistically significant correlation between age and blood group (P =0.2).
The most dominant blood group was found to be O (44.5%) and the least common AB (5.7%). The Rh-positive blood group was also the most predominant which covered 94.8% and the rest (5.2%) were Rh-negative (Table 2).
Discussion
To the best of our knowledge, our study is the first study to be done in Addis Ababa, not to mention the larger sample size and the use of fully automated immunohematology machine. In our study, we found the majority of volunteer donors to be male donors. The studies conducted in other countries also show a predominance of male donors; for instance; a study done by El-Faramawy et al reported that 65% of the donors were male and only 35% were female.17 Another study by Luhar et al reported in their study that 95.27% were male.18 The study done in South India, by Sachan et al,19 also found 96% of donors to be male. In our study, however, the percentage of female donors is relatively high in comparison to other studies, which may be because females have better awareness on blood donation service at the EBTBS, Addis Ababa center.
This study was comparable with other studies in Ethiopia and most regions of Africa.10–16 However, very good improvement was seen in volunteer turnouts and overall awareness about the importance of blood donation. The predominance of male donors is observed might be because female donors are more likely to be excluded due to medical backgrounds, such as low hemoglobin levels, low body weight, pregnancy, or breast feeding. The younger age group (18–25 years) were the most common blood donors, contributing about 53.1% of the total blood donations, this might be due to the younger population may have better awareness, good physical health, easy understanding, and be easily convinced group.
In this study, the most predominant blood group was O (44.5%) and the least common AB (5.7%) which is consistent with studies in other regions of Ethiopia (Table 2) which also showed the predominant group to be O and the least common to be AB.20–23 In Kenya, Tanzania, and Uganda, similar studies also showed the predominant blood group to be O and the least prevalent to be AB.12–14 On contrary, some studies in India showed that it was blood group B which was the dominant one,24,25 while studies in Iran and Europe indicated that blood group A was the predominant blood group.26 The Rh-positive blood group was also the most predominant which covered 94.8%, similarly, a study conducted in Jimma, Bahir Dar, Gondar, Arba Minch, Jigjiga, and Debre Tabor regions of Ethiopia, indicated that Rh positive blood group was the most prevalent with 92.8%, 91.5%, 94.2%, 92.8%, and 95.6% and 91.5% frequency respectively.10,11,20–23 Although, a study conducted in Gambela region of Ethiopia reported a slightly higher frequency of 19.37% for the Rh-negative blood group.27 Consistent with the other studies, a study conducted in Tanzania, and Uganda showed that Rh D negative frequency to be 2% for both countries.13,14
This difference in the distribution of the ABO blood group might be due to geographic, regional, and ethnic differences as well as genetic variations in the study groups.
Limitation
The study was conducted on volunteer blood donors who came to the center located downtown Addis Ababa and some mobile sites which may have inadvertently led to conducting the study on somewhat a homogenous population.
Conclusion
The knowledge of the distribution of blood groups is very important for blood banks and transfusion services which play an important role in the patient’s health care. We recommend a larger nation-based study involving blood donors from different regions and backgrounds in Ethiopia to confirm these findings and to assess for any region-specific variations in the ABO blood group and Rh-D antigens among blood donors considering the heterogeneous geography, ethnicity, and genetic variation of the country.
Acknowledgments
First of all, our great respect and gratitude goes to volunteer blood donors, nurses, and laboratory technologists of the EBTBS, who had taken full responsibility for interviewing participants and collecting blood samples despite having the usual heavy workload. Last but not least, our deepest gratitude also goes to EBTBS for covering the cost of reagents and giving us chance to use laboratory facilities to perform the test analysis.
Disclosure
The authors report no conflicts of interest regarding the publication of this paper.
References
1. Garratty G, van Rhenen DJ. Red blood cell alloimmunization after blood transfusion. Leiden Univ Press. 2008;870:11–12.
2. Milanović MK, Bujandrić N, Knezević NM. PROBLEM DAVALACA KRVI RETKIH KRVNIH GRUPA SA IREGULARNIM ANTITELIMA [Rare blood donors with irregular antibodies]. Med Pregl. 2013;66(7–8):331–334. Serbian. doi:10.2298/MPNS1308331K
3. Smart E, Armstrong B. Blood group systems. ISBT Sci Series. 2020;15(S1):123–150. doi:10.1111/voxs.12593
4. Tobian AAR, Heddle NM, Wiegmann TL, Carson JL. Red blood cell transfusion: 2016 clinical practice guidelines from AABB. Transfusion. 2016;56(10):2627–2630. doi:10.1111/trf.13735
5. Sharma S, Sharma DC, Rai S, et al. Prevalence of ABO, RhD and other clinically significant Blood Group Antigens among blood donors at tertiary care center, Gwalior. Bali Med J. 2020;9(2):437. doi:10.15562/bmj.v9i2.1779
6. Singh A, Srivastava R, Deogharia K, Singh K. Distribution of ABO and Rh types in voluntary Blood donors in Jharkhand area as a study conducted by RIMS, Ranchi. J Family Med Prim Care. 2016;5(3):631. doi:10.4103/2249-4863.197319
7. Chaurasia R, Zaman S, Das B, Chatterjee K. Screening donated blood for transfusion transmitted infections by serology along with NAT and response rate to notification of reactive results: an Indian experience. J Blood Transfus. 2014;2014:1–6. doi:10.1155/2014/412105
8. Rath G, Mitra R, Mishra N. Blood groups systems. Indian J Anaesth. 2019;58(5):524–528. doi:10.4103/0019-5049.144645
9. Chauhan DL, Premkumar DR. Significance of antibody screening and identification in pretransfusion testing-a retrospective study. Semantic Scholar. 2017;6(5):2319–7064.
10. Alemu G, Mama M. Assessing ABO/Rh Blood Group frequency and association with asymptomatic malaria among blood donors attending Arba Minch Blood Bank, South Ethiopia. Malar Res Treat. 2016;2016(1):1–7. doi:10.1155/2016/8043768
11. Sheikaden A. Somali Regional Blood Bank. Am J Lab Med. 2019;4(2):48–52.
12. Lyko J, Gaertner H, Kaviti JN, Kariithi MW, Akoto B. Grupy krwi układu ABO i Rh u ludności Kenii [Blood-group systems ABO and RH in the Kenyan population]. Folia Med Cracov. 1992;33(1–4):85–92. Polish.
13. Apecu RO, Mulogo EM, Bagenda F, Byamungu A. ABO, and Rhesus (D) blood group distribution among blood donors in rural south-western Uganda: a retrospective study. BMC Res Notes. 2016;9(1). doi:10.1186/s13104-016-2299-5
14. Jahanpour O, Pyuza JJ, Ntiyakunze EO, Mremi A, Shao ER. ABO and Rhesus blood group distribution and frequency among blood donors at Kilimanjaro Christian Medical Center, Moshi, Tanzania. BMC Res Notes. 2017;10(1). doi:10.1186/s13104-017-3037-3
15. Enawgaw B, Aynalem M, Melku M. Distribution of ABO and Rh-D blood group antigens among blood donors in the Amhara Regional State, Ethiopia. J Blood Med. 2022;13(1):97–104. doi:10.2147/JBM.S356425
16. Amsalu WF, Daniel GD. Distribution of ABO and Rh (D) blood groups among students attending secondary and preparatory schools in Bote town, Oromia national regional state, Ethiopia. Int J Sci Technol Educ Res. 2019;10(1):1–8. doi:10.5897/IJSTER2019.0448
17. Jungbauer C, Hobel CM, Schwartz DWM, Mayr WR. High-throughput multiplex PCR genotyping for 35 red blood cell antigens in blood donors. Vox Sang. 2012;102(3):234–242. doi:10.1111/j.1423-0410.2011.01542.x
18. Luhar RK, Shah RJ, Harimoorthy V. Antibody screening and identification in voluntary blood donors – need of the hour. Global J Transfus Med. 2022;7(1):47. doi:10.4103/gjtm.gjtm_1_22
19. Sachan D, Krishna GD, Saha S, Prasath R. Prevalence of irregular red blood cell antibodies among healthy blood donors in South India. Glob J Transfus Med. 2019;4:219–223. doi:10.4103/GJTM.GJTM_22_19
20. Zerihun T, Bekele S. Pattern of ABO and Rhesus Blood Groups distribution of five years survey in Jimma Town Blood Bank, South West Ethiopia. J Health Educ Res Dev. 2016;4(3). doi:10.4172/2380-5439.1000177
21. Legese B, Shiferaw M, Tamir W, Tiruneh T. Distribution of ABO and Rhesus Blood Group phenotypes among blood donors at Bahir Dar Blood Bank, Amhara, Northwest Ethiopia: a retrospective cross-sectional study. J Blood Med. 2021;12(1):849–854. doi:10.2147/JBM.S329360
22. Woldu B, Melku M, Shiferaw E, Biadgo B, Abebe M, Gelaw Y. Phenotype, allele, and genotype frequency of ABO and Rhesus D blood groups of blood donors at the North Gondar District Blood Bank, Northwest Ethiopia. J Blood Med. 2022;13(1):11–19. doi:10.2147/JBM.S346904
23. Tiruneh A, Yetneberk T, Gelaw M, Eshetie D. Frequency of ABO and Rh blood group distribution at Debre Tabor blood bank, Amhara Region, North-Central Ethiopia. A six-year retrospective survey. J Blood Med. 2020;11(1):357–361. doi:10.2147/JBM.S266624
24. Chandra T, Gupta A. Frequency of ABO and rhesus blood groups in blood donors. Asian J Transfus Sci. 2012;6(1):52. doi:10.4103/0973-6247.95057
25. Barot T, Patel D, Shah R. Distribution of ABO and Rhesus (Rh) blood groups among voluntary blood donors in Central Gujarat, India. Int J Contemp Med Res. 2020;7(7):114.
26. Babaei K, Esmaeilzadeh A, Asadi S, Sohrabi R. Prevalence of red blood cell alloantibodies in blood donors of Zanjan Province; the preliminary report of the North West of Iran. Biosci Biotechnol Res Asia. 2016;13(4):2207–2210. doi:10.13005/bbra/2385
27. Golassa L, Tsegaye A, Erko B, Mamo H. Correction to: high rhesus (Rh(D)) negative frequency and ethnic-group based ABO blood group distribution in Ethiopia. BMC Res Notes. 2018;11(1). doi:10.1186/s13104-018-3281-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
