Back to Journals » Journal of Asthma and Allergy » Volume 16
Prevalence, Management, and Risk Factors of Asthma Among School-Age Children in Yogyakarta, Indonesia
Authors Triasih R , Setyowireni D, Nurani N, Setyati A
Received 16 October 2022
Accepted for publication 15 December 2022
Published 5 January 2023 Volume 2023:16 Pages 23—32
DOI https://doi.org/10.2147/JAA.S392733
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Rina Triasih,* Dwikisworo Setyowireni,* Neti Nurani, Amalia Setyati*
Department of Pediatrics, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada /Dr. Sardjito Hospital, Yogyakarta, Indonesia
*These authors contributed equally to this work
Correspondence: Rina Triasih, Department of Pediatrics, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/ Dr. Sardjito Hospital, Jl. Kesehatan 1, Yogyakarta, 55284, Indonesia, Tel +62 81392764269, Fax +6274 583745, Email [email protected]
Purpose: Childhood asthma in developing countries has been increasing, but underdiagnosed and undertreated. We reported prevalence, management, and risk factors of asthma among school-age children in Yogyakarta, Indonesia.
Patients and Methods: We recruited children aged 6– 7 years and 13– 14 years attending schools in all districts in Yogyakarta, Indonesia. The schools were randomly selected via cluster random sampling. We used the Indonesian version of the Global Asthma Network (GAN) questionnaire, and the methodology employed by this study was in accordance with the GAN’s protocol.
Results: A total of 2106 children aged 6– 7 years and 3142 adolescents aged 13– 14 years were eligible for analysis. The prevalence of current wheeze in children and adolescents was similar, which was 4.6%. Inhalation therapy was reported in < 30% of those with asthma. Risk factors for current wheeze in children were wheezing in infancy period, ever had pneumonia, the house was passed by trucks every day, and fast-food consumption in the previous 12 months; whereas exclusive breastfeeding for more than 6 months decreased the risk of current wheeze. In adolescence, obesity, consumption of fast food once or twice a week, and paracetamol in the previous 12 months increased the risk of current wheeze.
Conclusion: The prevalence of current wheeze in children and adolescents in Indonesia was quite low. The use of inhalation therapy was limited. Respiratory problems during infancy, environmental, and nutritional factors play a role in the development of asthma.
Keywords: asthma, child, adolescent, prevalence, risk factors
A Letter to the Editor has been published for this article.
Introduction
Based on morbidity and mortality, asthma is not ranked high as a cause of death and disability in children. However, its prevalence, impact upon quality of life, and health care expenditure provide compelling reasons to consider asthma a major health problem in children.1 The recent Global Asthma Study (GAN) phase reported that the global prevalence of asthma was 11% in children aged 6–7 years and 9.1% among children aged 13–14 years.2 Asthma has accounted for missed school days among children in Asia-Pacific (16–61%), Europe (34–68%) and the United States (43%) and association with lower academic achievement has been postulated.3,4
Studies have shown different epidemiological trends of asthma prevalence between developed and developing countries. The prevalence of asthma in developed countries tends to increase in children aged 6–7 years but stabilizes or decreases in older children. Contrary, the prevalence of asthma is increasing in developing countries regardless of age, and maybe continuing.2,5 Although asthma in children in low- to middle-income countries is not as common as that in developed countries, they suffered more significantly as a result of being underdiagnosed and undertreated.6
The causes of increasing prevalence of childhood asthma in developing countries are not clearly understood, but the wide variations in asthma prevalence even in genetically similar groups suggest that environmental factors play a role. Changes in air pollution, the infectious burden in early childhood, family history of asthma or allergy, obesity, and exposure to tobacco smoke have been postulated as some possible causes of and risk factors for asthma development, as well as disease control.7,8
Data on asthma and related factors in children in Indonesia is limited; meanwhile, there has been a trend of increasing cases of asthma in children, including in Yogyakarta province. This study aimed to describe the prevalence, management and risk factors of asthma in school-age children in Yogyakarta province, which information can be used to improve the management of asthma in children.
Materials and Methods
Participants
We conducted a cross-sectional study in all five districts of Yogyakarta province, Indonesia, from May 2016 until December 2016, which involved students aged 6–7 years (children) and 13–14 years (adolescents). We selected schools randomly via cluster sampling from all primary (1845) and secondary (421) schools. We first selected 20 primary schools in each district, which we expected to have a minimum of 30 eligible children in each school; and 8 secondary schools in each district, which we expected to have 75 eligible adolescents in each school. All of the students (aged 6–7 years and 13–14 years) who attended the school during the study day were invited to complete a standardized questionnaire. Additional primary schools were selected because, with the first selection, the number of children did not reach the expected number of 3000.
Data Collection
We followed protocol and used a questionnaire which was developed by Global Asthma Network (GAN) to evaluate asthma and related risk factors.9 The questionnaire was translated into Indonesian. For the children, the questionnaire was sent to and completed by their parents at home. Meanwhile, the adolescents completed the questionnaire in their classroom under the supervision of the study team. Demographic data collected in this study included date of birth, gender, and school district.
Prevalence of wheezing and asthma were evaluated based on the following questions: ever had wheezing at any time (ever wheezing), ever had wheezing in the last 12 months (current wheeze), ever had asthma (ever asthma), and had been diagnosed with asthma by a doctor (doctor-diagnosed asthma). We also reported other conditions that may represent asthma, included wheezing with exercise and dry night cough in the past 12 months. Asthma severity was assessed among those who had wheezing in the past 12 months, which classification followed the criteria used in the GAN protocol: moderate to severe wheezing were defined as one or more of these conditions in the past 12 months: (i) four or more attacks of wheezing, (ii) woken by wheezing on one or more nights per week or (iii) wheezing severe enough to limit speech to only one or two words at a time between breaths.9
Anthropometric measurements for the students were performed at school by trained study team personnel. Weight was measured by using a calibrated weighing scale; whereas height was measured by using a portable calibrated stadiometer. We used WHO Growth Reference to determine nutritional status based on body mass index (BMI) according to age, as follows: obese (BMI greater than +2 standard deviations (SDs), overweight (BMI between +1 SD and +2 SD), normal (BMI between −2 SD and −1 SD), undernourished (BMI between −3SD and −2 SD), and severely malnourished (BMI under – 3 SD).
Sample Size
We invited all students (aged 6–7 years and 13–14 years) in the selected schools to fulfil the sample size of 3000 children per age group. This number followed the GAN sample size calculation, which provided more than 99% ability to detect differences in the prevalence of wheeze in one center and 25% in another center.9
Statistical Analysis
All data were double entered into EpiData (EpiData Software, RRID: SCR_008485) version 3.1 to reduce the risk of entry error. We excluded subjects with incomplete questionnaire data related to the prevalence of asthma or wheezing. Categorical variables are presented as proportion and continuous variables as mean or median, as appropriate. Comparison of proportion between two groups were tested using chi-square tests. Univariate association of factors with wheezing in the past 12 months was calculated using logistic regression and was presented as odds ratio (OR) with 95% confidence interval. Statistically significant factors in the univariate analysis (p <0.05) were included in multiple logistic regression analysis and reported as adjusted odds ratio (aOR). All data were analyzed using Stata (Stata, RRID: SCR_012763) version 14.1.
Ethical Consideration
Ethical approval for the study was obtained from the Ethics Committee, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia. Informed consent was obtained from the parents/guardians of the eligible children and adolescents. We also asked for informed consent from the adolescents. The study followed the Declaration of Helsinki.
Results
A total of 2350 parents of children aged 6–7 years from 111 primary schools and 3560 adolescents aged 13–14 years from 39 secondary schools throughout Yogyakarta province completed the questionnaire. Among these, 2106 (91.8%) questionnaires from the children and 3142 (88.3%) questionnaires from the adolescents were deemed valid for analysis. The proportions of males and females in both groups were similar (49.6% vs 50.4% in the children and 49.1% vs 50.9% in the adolescents).
The prevalences of wheezing ever and wheezing in the past 12 months were not different between two groups; however, the adolescents had significantly higher prevalence of asthma ever and physician-diagnosed asthma (Table 1). The prevalence of wheezing ever was higher than asthma ever in the children (9.7% vs 5.4%, p = 0.01), whereas in the adolescents it was similar (9.3% vs 8.4%, p = 0.23). Dry night cough in the past 12 months, which was widely accepted as alternative presentation of asthma, was more commonly reported than wheezing in the past 12 months in both groups (12.7% vs 4.6%, p = 0.00 in the children and 12.9% vs 4.6%, p = 0.00 in the adolescents). Among the students who had wheeze in the past 12 months, moderate to severe wheeze (yes answer to any of the questions in Table 1) was identified in 35 (35/97; 36.1%) children and 50 (50/144, 34.7%) adolescents. The prevalence of wheeze ever varied between districts, ranging from 8.9% to 12.1% in children and 4.7% to 12.4% in adolescents.
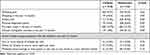 |
Table 1 Prevalence of Wheeze, Asthma, Severe Wheeze, and Other Conditions That May Represent Asthma |
Of 204 children aged 6–7 years with wheezing ever, 197 (96.6%) reported the age of first wheezing episode. The majority (73.5%) of these children started to experience wheeze before the age of 5 years old, which mostly (31.4%) were in the age of 3–4 years. Forty-eight (23.5%) children were reported to start having wheeze before one year of age and only 15 (7.4%) children at age more than 6 years.
Asthma management and utilization of health facilities were assessed among those who ever had asthma, which included 113 children and 265 adolescents (Table 2). Inhalation therapy was uncommon in this study. Inhaled steroid was used in less than 5% in both groups, while inhaled short-acting beta-agonist (SABA) was more commonly used in children than in adolescents (29.2% vs 14%, p = 0.00). Oral asthma medication, emergency room and doctor visit were more common in the children, whereas missing school because of breathing problems was similar in both age groups.
 |
Table 2 Management and Utilization of Health Facilities Among Participants with Asthma Ever |
The children who ever had wheezing in the first 12 months of life, had history of pneumonia, consumed fast food in the previous 12 months, and living in truck traffic area were more likely to have current wheeze; whereas those who were exclusively breastfed for more than 6 months were less likely to have current wheeze (Table 3).
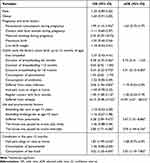 |
Table 3 Factors Related to the Risk of Wheezing in the Past 12 Months in Children |
In the adolescents, obesity, consumption of fast food once or twice a week, and paracetamol in the past 12 months increased the risk of current wheeze; whereas consumption of nuts once or twice a week decreased the risk of current wheeze (Table 4).
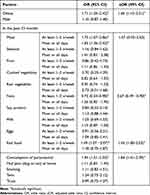 |
Table 4 Factors Related to the Risk of Wheezing in the Past 12 Months in Adolescents |
Discussion
The prevalences of current wheeze in children and adolescents in our setting were similar, which was 4.6%; whereas the prevalence of asthma ever in children in our study was lower than in adolescents (5.4% vs 8.4%). In a previous study in Bandung, Indonesia, which was part of the ISAAC study Phase One (1996) and Phase Three (2002), the prevalence of current wheeze in children was 4.1% and 2.8%; whereas in adolescents it was 2.1% and 5.2%. The prevalence of asthma ever in children was 6.6% and 4.8%; whereas in adolescents it was 1.6% and 12.4%, respectively.10,11 The findings showed that the prevalence of asthma in children in Indonesia was relatively stable and low, which may indicate the real condition but may also be because of underdiagnosis or under-reporting as found in a current survey of asthma among pediatricians in Indonesia (unpublished data).
A more recent multicenter asthma study in children using the GAN protocol has been conducted, and some countries have published a single-center report. The prevalence of current wheeze in Thailand was 14.6% in children and 12.5% in adolescents, in Brazil was 15.8% in adolescents, in Mexico was 10.2% in children and 11.6% in adolescents, and in Spain was 14.7% in adolescents.12–15 The prevalence of asthma ever in Thailand was 6.1% in children and 8.8% in adolescents, in Mexico was 26.1% in children and 23.9% in adolescents, and in Spain was 19.7% in adolescents.12,14,15 The most recent report from a multicenter GAN study reported that the prevalence of current wheeze and asthma ever in adolescents was 11.1% and 10.5%, respectively; whereas in children they were 9.1% and 7.6%, respectively.2 Wide variation in childhood asthma prevalence globally has been acknowledged. ISAAC Phase One and Phase Three documented that asthma was more commonly reported in English language countries, Latin America and Western Europe, but relatively low in Africa and Asia.5,16 Several possible reasons for the wide variation have been proposed such as different level of awareness of asthma among community and healthcare providers, as well as different environmental exposures and diet.7,17,18 The higher prevalence of asthma in adolescents compared to that in children may be caused by a variety of factors, including more comprehensive medical histories, which enable doctors to make diagnosis with greater assurance; and higher exposure to smoking or tobacco smoke which increase the risk of asthma.
Despite lower prevalence compared to industrialized countries, the prevalence of childhood asthma in most developing countries has increased over the last few decades.5 The prevalence of current wheeze in Indonesia in adolescents increased from 2.1% in 1996 to 5.2% in 2002 and relatively stabilized to 4.6% in 2016, which is in line with the results of our current study.10,11 In the younger children, the prevalence decreased from 4.1% in 1996 to 2.8% in 2002 and increased to 4.6% in 2016.10,11 The increasing burden of childhood asthma in developing countries is often associated with severe disease, which can be due to sub-optimal management or the disease may be clinically more severe in the less affluent countries.19 Our study documented severe wheeze of 34.7% in adolescents and 36.1% in children with current wheeze.
Sub-optimal management of asthma in children has been a global concern. Poorer management in developing countries is more common because of some challenges including poor access to care, low awareness and lack of ability of healthcare workers to diagnose and manage asthma, unavailability and high cost of inhaled therapy, poor environmental control of potential triggers, lack of education among healthcare providers and affected population, and cultural or language issues.6,20,21 While the use of nebulizers for children with coughs and other respiratory symptoms has been increasing in Indonesia over the last decade, our findings documented that less than 30% of children and adolescents with asthma ever reported to have inhaled SABA and less than 5% had inhaled steroids. This may indicate that the disease is less severe in Indonesia, but may also be because of the lack of knowledge and experience of the healthcare providers in the appropriate management of asthma in children. The use of oral drugs for asthma is common. The low usage of inhaled steroids for children with asthma in Indonesia may also be because of lack of availability of the drugs as has been reported in other low- and middle-income countries, in which the availability of beclomethasone metered-dose inhaler (MDI) ranged from 0% to 98% of the health facilities surveyed.22 Furthermore, the high cost of the drugs and limited availability of spacer devices hampered the use of the inhaled therapy.
Besides genetic background, environmental factors have been shown to be an important factor that is associated with the risk of asthma. Studies have been conducted to identify the environmental factors because modification of the factors in early life becomes essential intervention considering that a cure for asthma has not been found. Nevertheless, the findings among studies were varied, which is as expected. Besides various methodologies, most studies evaluating the link between environment and asthma were observational, by which cause and effect cannot be straightforwardly interpreted.23 In addition, most studies were retrospective in design, in which recall bias as well as incorrect and incomplete reporting by parents or the adolescent were more likely. A systematic review of 135 studies documented a consistent association between asthma and second-hand smoking, inhaled chemicals, mold, respiratory infections, air pollutants, maternal diet and inconsistent association with exposure to pets, breastfeeding and infant diet.24 The prevalence of asthma in our study varied between districts, but we could not correlate the various prevalences with the level of air pollution in each district.
Our findings documented that wheezing in the first 12 months (aOR 10.94; 95% CI 4.27 −28.01) and ever had pneumonia (aOR 3.67; 95% CI 1.51–8.88) increased the risk of current wheeze at the age of 6–7 years. Evidence on the role of respiratory infections in early life and subsequent development of asthma was conflicting.25,26 The protective effect of the infections on asthma is postulated to relate to the hygiene hypothesis, which suggested that exposure to microbes in infancy could invoke immune response via the conversion of neonatal Th2 biased pattern (known to promote IgE and eosinophilic responses seen in atopy) to Th1 dominant pattern.26 Meanwhile, recurrent lower respiratory tract infections might cause damage of the airway, which leads to variable airway obstruction and wheezing. The causative association between viral illnesses and asthma could also be reversed, whereby children genetically predisposed to asthma are more likely to develop symptoms in the lower respiratory tract when infected.27
The “hygiene hypothesis” has also been the underlying explanation to the lower prevalence of asthma among children who were exposed to pets in early life, by which home bacterial microbiota is altered.28,29 With similar hypotheses, having many siblings, attending day care at an early age, and growing up on a farm are related to the lower risk of asthma.30,31 Nevertheless, our findings were not in line with this hypothesis (OR 1.59; 95% CI 0.78–3.23). Differences in sensitization rates of the exposures might explain this discrepancy. In addition, the development of asthma is not from a single exposure, but involves interactions between different environmental exposures and/or environmental exposures and atopic exposures.24
Several studies revealed a critical role of breastfeeding in protecting children from asthma and asthma-related symptoms.32,33 Breast milk contains live microbes that help the development of infant oral and gut micro biota, which play an important role in the development of immune systems and reduce the risk of asthma in later childhood. The immune system development may also be affected by the components of breast milk such as secretory IgA, cytokine, long-chain fatty acids, and oligosaccharides. Stimulation of lung growth and epigenetic effects has also been proposed as a protective mechanism of breastfeeding in the development of asthma. However, no association or even increased risk of asthma in breastfed children has also been postulated. Similarly, the effect of duration and exclusiveness of breastfeeding on asthma in later childhood remains unclear. The conflicting findings may be due to methodological limitations or related to the tremendous biological variability in human milk.34 Our study found no association between breastfeeding and the risk of asthma (OR 1.37; 95% CI 0.42–4.41), but children who were exclusively breastfed for more than 6 months were less likely to develop asthma (aOR 0.31; 95% CI 0.12–0.80).
Our study found that consumption of paracetamol in the past 12 months increased the risk of current wheeze in adolescents (aOR 1.84; 95% CI 1.41–2.39). On the other hand, the use of paracetamol in infancy periods (OR 1.04; 95% CI 0.62 −1.74) and during pregnancy (aOR 1.62; 95% CI 0.75–3.47) did not associate with the risk of current wheeze in children. The association between paracetamol consumption and the development of asthma in children has also been controversial for decades. It has been postulated that paracetamol metabolite disrupts the glutathione pathway, which results in the increase of oxidative stress in the airways, causing epithelial damage and more inflammation in the airway.35 Other proposed mechanisms include cytotoxic effect of pneumocytes, modulator effect on the activity of myeloperoxidase, and depletion of Th1 cytokine release during febrile episodes, which leads to a predominance of Th2 cytokines. Considerable evidence from epidemiological studies supports consistent findings that paracetamol use during pregnancy and paracetamol consumption in early childhood periods increase the risk of asthma in later childhood.36,37 However, the causative effect of paracetamol on asthma is doubted and the association can be confounded by the indication of paracetamol use, i.e the frequent use of paracetamol may be for recurrent respiratory infections, which increased the risk of asthma. The confounding may also be because of reverse causation, in which children who are at risk for developing asthma have more respiratory infections, which results in increased use of paracetamol.38 In a number of prospective studies, the association of paracetamol use and the development of asthma was significantly weakened, or even disappeared, after adjustment for respiratory infections, and stratification by the indication for paracetamol use.39–41
Our study also documented that obesity increased the risk of current wheeze in adolescents (aOR 1.60; 95% CI 1.10–2.31). A large retrospective cohort study found that the relative risk for asthma incident and spirometer-confirmed asthma in obese children was 1.26 (95% CI 1.18–1.34) and 1.29 (95% CI 1.16–1.42).42 Other studies documented higher prevalence of current wheeze and more frequent emergency department visits as well as medications used in obese children.43,44 Asthma in obese patients was more difficult to control and more severe, probably due to airway dysanapsis and skewing of CD4 towards Th1, increasing eosinophil in sub-mucosal layer, altered response of innate lymphoid cells, metabolic dysregulation, oxidative stress, adipokines or other cytokines which may result in airway hyper-reactivity.45 Based on the previous studies, it is completely recognized that obesity is a risk factor for asthma; however, in some patients, asthma precedes obesity. A longitudinal study of relationship between obesity and asthma in more than 5000 children found there was 37% higher odds of becoming obese in non-obese asthmatic children compared to non-asthmatic children in the next annual visit.46
In this study, there was an association between current wheeze and fast-food consumption once or twice per week (aOR 1.43; 95% CI 1.00–2.03); however, it was not found in the more frequent consumption (OR 1.18; 95% CI 0.75–1.87). A systematic review of 16 studies revealed that fast-food consumption was significantly associated with current/severe/ever asthma and current/ever had wheezing. After an adjustment for BMI, the association between current/ever asthma and current wheeze dissipated, probably due to the confounding effect of BMI.47 Fast-food consumption is usually accompanied by antioxidant deficiency, which might increase airway sensitivity to oxidant damage and induce airway inflammation. Furthermore, higher intake of polyunsaturated fatty acids such as linoleic acid might increase the production of several pro-inflammatory mediators such as 2-series prostaglandins, thromboxanes, and 4-series leukotrienes which might provoke TH2 associated with asthma.48
The relationship between nut consumption and asthma has been conflicting. Our study found a negative association between current wheeze and nut consumption once or twice per week in the previous 12 months in adolescents (aOR 0.67; 95% CI 0.49 −0.90). However, it was not found in more frequent consumption. An analysis of the ISAAC study involving more than 200,000 adolescents revealed an association between nut consumption three or more times a week with lower BMI value (0.2743kg/m2) compared to adolescents who never or only occasionally consumed nuts in the previous 12 months.49 Another study suggested that limiting the amount of fat intake (oils and nuts) to approximately 10% of daily energy requirements might ameliorate symptoms of asthma.50
Self-reported and parent-reported asthma and asthma-related symptoms in ISAAC study have been widely accepted in epidemiological studies to indicate the incidence of asthma and asthma-related symptoms in children. Nevertheless, misclassification of asthma and asthma-related symptoms is more likely as response to the questions will be influenced by the understanding and familiarity of the respondent to asthma, which may differ between countries, urban versus rural area and depends on the age and educational background of the respondents. There has not been a survey among adolescents and parents in Indonesia, but the understanding of and familiarity with asthma and asthma-related symptoms may not be as good as that of infectious diseases (tuberculosis, diarrhea, pneumonia, malaria, dengue), which is more common. This should be taken into account as one of the limitations in our study. We used the local term of “wheeze” for better understanding of the respondents. However, the use of wheeze as the diagnostic marker of asthma is problematic because wheezing can be caused by infections, which are not always related to or develop into asthma. Furthermore, some confounding factors and recall bias as are common in observational studies have likely contributed to different findings compared to established mechanisms or association of risk factors and asthma.
Conclusion
In conclusion, the prevalence of current wheeze in children and adolescents in Indonesia is quite low. The use of inhalation therapy was limited. Respiratory problems during infancy, environmental and nutritional factors play a role in the development of asthma. National asthma registry and a multicenter study in Indonesia which can reflect the situation of the prevalence and management of asthma in children is required.
Acknowledgments
We acknowledge the Province and Districts Education, Youth and Sport Offices of Yogyakarta for the support to involve schools in this study.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bateman ED, Jithoo A. Asthma and allergy - A global perspective. Allergy Eur J Allergy Clin Immunol. 2007;62:213–215. doi:10.1111/j.1398-9995.2007.01324
2. García-Marcos L, Asher MI, Pearce N, et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur Respir J. 2022;60:2102866. doi:10.1183/13993003.02866-2021
3. Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114:40–47. doi:10.1016/j.jaci.2004.04.042
4. Koinis-Mitchell D, Kopel SJ, Farrow ML, McQuaid EL, Nassau JH. Asthma and academic performance in urban children. Ann Allergy Asthma Immunol. 2019;122:471–477. doi:10.1016/j.anai.2019.02.030
5. Asher M, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood. Lancet. 2006;368:733–743. doi:10.1016/s0140-6736(06)69283-0
6. Ardura-Garcia C, Kuehni CE. Reducing childhood respiratory morbidity and mortality in low and middle income countries: a current challenge. Eur Respir J. 2019;54:1900987. doi:10.1183/13993003.00987-2019
7. Holst GJ, Pedersen CB, Thygesen M, et al. Air pollution and family related determinants of asthma onset and persistent wheezing in children: nationwide case-control study. BMJ. 2020;370:1–9. doi:10.1136/bmj.m2791
8. Thomson JA, Widjaja C, Darmaputra AAP, et al. Early childhood infections and immunisation and the development of allergic disease in particular asthma in a high-risk cohort: a prospective study of allergy-prone children from birth to six years. Pediatr Allergy Immunol. 2010;21:1076–1085. doi:10.1111/j.1399-3038.2010.01018
9. Ellwood P, Asher MI, Ellwood E; Global Asthma Network Steering Group. Global Asthma Network Phase I Manual. Global Surveillance: Prevalence, Severity, Management and Risk Factors. Auckland: Global Asthma Network; 2015:1–228.
10. ISAAC Phase One Study Group. ISAAC Phase One Data; 1996. Available from: http://isaac.auckland.ac.nz/phases/phaseone/results/results.php.
11. ISAAC Phase Three Study Group. ISAAC Phase Three Data; 2002. Available from: http://isaac.auckland.ac.nz/phases/phasethree/results/results.php.
12. Chinratanapisit S, Suratannon N, Pacharn P, Sritipsukho P, Vichyanond P. Prevalence and severity of asthma, rhinoconjunctivitis and eczema in children from the Bangkok area: the global asthma network (GAN) Phase I. Asian Pacific J Allergy Immunol. 2019;37:226–231. doi:10.12932/AP-120618-0336
13. Urrutia-Pereira M, Chong-Neto H, Mocellin LP, et al. Prevalence of asthma symptoms and associated factors in adolescents and adults in southern Brazil: a global asthma network phase I study. World Allergy Organ J. 2021;14:100529. doi:10.1016/j.waojou.2021.100529
14. Del-Río-Navarro BE, Berber A, Reyes-Noriega N, et al. Global asthma network phase I study in Mexico: prevalence of asthma symptoms, risk factors and altitude associations-a cross-sectional study. BMJ Open Respir Res. 2020;7:e000658. doi:10.1136/bmjresp-2020-000658
15. Marín-Cassinello A, Vega-Hernández MC, Lumbreras-Lacarra B, De Arriba-Méndez S, Pellegrini-Belinchón J. Prevalence of symptoms, severity and diagnosis of asthma in adolescents in the Province of Salamanca, Spain: Global Asthma Network (GAN) phase I. Allergol Immunopathol. 2021;49:106–112. doi:10.15586/aei.v49i5.438
16. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J. 1998;12:315–335. doi:10.1183/09031936.98.12020315
17. Yeatts K, Davis KJ, Sotir M, Herget C, Shy C. Who gets diagnosed with asthma? Frequent wheeze among adolescents with and without a diagnosis of asthma. Pediatrics. 2003;111:1046–1054. doi:10.1542/peds.111.5.1046
18. Wong GWK, Ko FWS, Hui DSC, et al. Factors associated with difference in prevalence of asthma in children from three cities in China: multicentre epidemiological survey. BMJ. 2004;329:486. doi:10.1136/bmj.329.7464.486
19. Lai CKW, Beasley R, Crane J, et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009;64:476–483. doi:10.1136/thx.2008.106609
20. Lalloo UG, Walters RD, Adachi M, et al. Asthma programmes in diverse regions of the world: challenges, successes and lessons learnt. Int J Tuberc Lung Dis. 2011;15:1574–1587. doi:10.5588/ijtld.11.0289
21. Zar HJ, Levin ME. Challenges in treating pediatric asthma in developing countries. Paediatr Drugs. 2012;14:353–359. doi:10.2165/11597420-000000000-00000
22. Mendis S, Fukino K, Cameron A, et al. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ. 2007;85:279–288. doi:10.2471/blt.06.033647
23. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi:10.1056/NEJMra054308
24. Dick S, Friend A, Dynes K, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4:e006554. doi:10.1136/bmjopen-2014-006554
25. Illi S, von Mutius E, Lau S, et al. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ. 2001;322:390–395. doi:10.1136/bmj.322.7283.390
26. Henderson AJ, Warner JO. Fetal origins of asthma. Semin Fetal Neonatal Med. 2012;17:82–91. doi:10.1016/j.siny.2012.01.006
27. Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi:10.1586/eri.11.92
28. O’Connor GT, Lynch SV, Bloomberg GR, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol. 2018;141:1468–1475. doi:10.1016/j.jaci.2017.06.040
29. Fujimura KE, Johnson CC, Ownby DR, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126:410–412.e4123. doi:10.1016/j.jaci.2010.05.042
30. Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–543. doi:10.1056/NEJM200008243430803
31. Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Böhm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30:187–193. doi:10.1046/j.1365-2222.2000.00801
32. Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179:1153–1167. doi:10.1093/aje/kwu072
33. Sonnenschein-van der Voort AMM, Jaddoe VWV, van der Valk RJP, et al. Duration and exclusiveness of breastfeeding and childhood asthma-related symptoms. Eur Respir J. 2012;39:81–89. doi:10.1183/09031936.00178110
34. Miliku K, Azad MB. Breastfeeding and the developmental origins of asthma: current evidence, possible mechanisms, and future research priorities. Nutrients. 2018;10:995. doi:10.3390/nu10080995
35. Shaheen SO, Newson RB, Sherriff A, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57:958–963. doi:10.1136/thorax.57.11.958
36. Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, FitzGerald JM. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest. 2009;136:1316–1323. doi:10.1378/chest.09-0865
37. Shaheen S, Potts J, Gnatiuc L, et al. The relation between paracetamol use and asthma: a GA2LEN European case-control study. Eur Respir J. 2008;32:1231–1236. doi:10.1183/09031936.00039208
38. Bjerg A. Acetaminophen and asthma, a bitter pill to swallow? J Allergy Clin Immunol. 2020;146:1008–1009. doi:10.1016/j.jaci.2020.09.015
39. Amberbir A, Medhin G, Alem A, Britton J, Davey G, Venn A. The role of Acetaminophen and geohelminth infection on the incidence of wheeze and eczema: a longitudinal birth-cohort study. Am J Respir Crit Care Med. 2011;183:165–170. doi:10.1164/rccm.201006-0989OC
40. Lowe AJ, Carlin JB, Bennett CM, et al. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ. 2010;341:c4616. doi:10.1136/bmj.c4616
41. Kreiner-Møller E, Sevelsted A, Vissing NH, Schoos A-M-M, Bisgaard H. Infant acetaminophen use associates with early asthmatic symptoms independently of respiratory tract infections: the Copenhagen prospective study on asthma in childhood 2000 (COPSAC 2000) cohort. J Allergy Clin Immunol. 2012;130:1434–1436. doi:10.1016/j.jaci.2012.09.017
42. Lang JE, Bunnell HT, Hossain MJ, et al. Being overweight or obese and the development of asthma. Pediatrics. 2018;142:e20182119. doi:10.1542/peds.2018-2119
43. Kajbaf TZ, Asar S, Alipoor MR. Relationship between obesity and asthma symptoms among children in Ahvaz, Iran: a cross sectional study. Ital J Pediatr. 2011;37:1–5. doi:10.1186/1824-7288-37-1
44. Belamarich PF, Luder E, Kattan M, et al. Do obese inner-city children with asthma have more symptoms than nonobese children with asthma? Pediatrics. 2000;106:1436–1441. doi:10.1542/peds.106.6.1436
45. Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–1179. doi:10.1016/j.jaci.2018.02.004
46. Zhang Y, Chen Z, Berhane K, et al. The dynamic relationship between asthma and obesity in schoolchildren. Am J Epidemiol. 2020;189:583–591. doi:10.1093/aje/kwz257
47. Wang CS, Wang J, Zhang X, et al. Is the consumption of fast foods associated with asthma or other allergic diseases? Respirology. 2018;23:901–913. doi:10.1111/resp.13339
48. Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–1117. doi:10.1016/j.jaci.2004.12.1139
49. Wall CR, Stewart AW, Hancox RJ, et al. Association between frequency of consumption of fruit, vegetables, nuts and pulses and BMI: analyses of the International Study of Asthma and Allergies in Childhood (ISAAC). Nutrients. 2018;10:316. doi:10.3390/nu10030316
50. Alwarith J, Kahleova H, Crosby L, et al. The role of nutrition in asthma prevention and treatment. Nutr Rev. 2020;78:928–938. doi:10.1093/nutrit/nuaa005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
