Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Prevalence, Influencing Factors, and Cognitive Characteristics of Depressive Symptoms in Elderly Patients with Schizophrenia
Received 25 September 2021
Accepted for publication 2 December 2021
Published 14 December 2021 Volume 2021:17 Pages 3645—3654
DOI https://doi.org/10.2147/NDT.S341297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Yaopian Chen, 1 Wei Li 2, 3
1Department of Sleep Medicine, Wenzhou Seventh People’s Hospital, Wenzhou, People’s Republic of China; 2Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Alzheimer’s Disease and Related Disorders Center, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
Correspondence: Wei Li
Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 200030, People’s Republic of China
Tel +86 021 64387250
Email [email protected]
Purpose: To investigate the prevalence, influencing factors, and cognitive characteristics of depressive symptoms in elderly patients with chronic schizophrenia.
Patients and Methods: A total of 241 elderly patients with chronic schizophrenia and 156 healthy controls were enrolled in this study. The Geriatric Depression Scale (GDS) was used to assess depressive symptoms; the Positive and Negative Syndrome Scale was used to assess psychotic symptoms; and both the Mini-Mental State Examination and Montreal Cognitive Assessment were used to assess overall cognitive function, while the Activity of Daily Living Scale was used to assess daily living ability.
Results: The prevalence of depressive symptoms was 48.5% (117/241) in elderly patients with chronic schizophrenia, which was substantially higher than that of normal controls (17.3%, 27/156). Using a stepwise binary logistic regression analysis, we found that high education (p=0.006, odds ratio [OR]=1.122, 95% confidence interval [CI]:1.034– 1.218) and hypertension (p=0.019, OR=0.519, 95% CI: 0.300– 0.898) were influencing factors for the comorbidity of depressive symptoms. Compared with individuals without depressive symptoms, individuals with depressive symptoms usually display worse overall cognitive function and more severe impairment of activities of daily living, but fewer psychotic symptoms. Interestingly, the GDS score was negatively correlated with the course of the disease (r=− 0.157, p=0.016), suggesting that patients who had recently been admitted to the hospital were more likely to develop depression.
Conclusion: Elderly patients with chronic schizophrenia are often associated with higher levels of depression. Therefore, their overall cognitive function is worse, and their activities of daily living are more seriously impaired. Therefore, these patients should be provided with appropriate psychological comfort, especially those who have recently been admitted to the hospital.
Keywords: elderly, chronic schizophrenia, depressive symptoms, hypertension, education
Introduction
Clinical and subclinical depression are common complications of schizophrenia,1 which affects 44–75% of the elderly.2 Elderly patients with both schizophrenia and depressive symptoms tend to experience increased physical illness, increased functional impairment, and poorer medication management, causing a reduction in life expectancy by 10–25 years.3 In addition to difficulties in treatment, depressive symptoms can affect cognitive function, activities of daily living, and quality of life in elderly patients with schizophrenia.4 Moreover, depressive symptoms are also associated with impaired functional remission and suicide in patients with schizophrenia, independent of psychotic remission.5 Therefore, it is essential that depressive symptoms are identified and managed properly to improve clinical outcomes in elderly patients with schizophrenia.
Accumulated evidence suggests that people with schizophrenia are more likely to suffer from depression than the general population;6 however, data often varies widely. For example, one community study found that 47.5% of older patients with schizophrenia had experienced a depressive episode,7 while another cross-sectional study found that 78.1% of older patients with schizophrenia had either syndromal (47.5%) or subsyndromal (30.6%) depressive symptoms.8 We speculate that the primary reason for the differing results in the above studies may be differences in survey tools and methods. In addition, because the negative symptoms and depressive symptoms of schizophrenia are very similar and often overlap, it is also quite difficult to distinguish them effectively.9
There are several reasons to explain the association between chronic schizophrenia and depressive symptoms. First, depressive symptoms are associated with both insight and negative evaluations of schizophrenia, suggesting that the way a person thinks about the illness may influence the occurrence of depressive responses.10 Second, patients with chronic schizophrenia often develop secondary depressive symptoms within the first year after discontinuing medication.11 Third, inflammatory stimuli can decrease connectivity in reward-relevant neural circuitry and decrease neural activity in the ventral striatum, which ultimately leads to depression;12 however, this mechanism has not yet been widely recognized.
Recently, researchers have focused on identifying biomarkers, such as illness severity13 and marital status, that might be associated with depressive symptoms in patients with schizophrenia.14 Similarly, other general demographic data such as sex, education, hypertension, and diabetes mellitus, may also play a role in the incidence of depressive symptoms in schizophrenia patients.15–17 In China, elderly patients with chronic schizophrenia tend to experience lengthy hospitalizations, and the closed environment often aggravates depression in patients. However, previous studies only focused on elderly individuals with schizophrenia in the community, while ignoring the long-term hospitalized population.1,7 We hypothesize that older patients with schizophrenia who are hospitalized for an extended time period tend to have more severe depressive symptoms and more impaired social functioning.
To test the research hypothesis for the current study, we described clinical characteristics of long-term hospitalized elderly patients with schizophrenia based on four dimensions: depressive symptoms, psychotic symptoms, cognitive symptoms, and daily living ability. Concomitantly, we also explored risk factors that may affect depressive symptoms, such as sex, age, education, smoking, drinking, and lipid metabolism.
Materials and Methods
Participants
This cross-sectional study was conducted from January 1, 2020 to January 1, 2021, and a total of 241 elderly chronic patients (men/women=133/108, average age: 66.38±5.601) with schizophrenia were recruited from the Shanghai Mental Health Center. All participants were required to meet the following requirements: 1) aged 65 years and above; 2) hospitalized long-term (at least one year); 3) diagnosed with schizophrenia according to the International Classification of Diseases 10 diagnostic standards; 4) not diagnosed with any severe medical conditions, such as infections or cancer; and 5) willing to participate in the project. Exclusion criteria were: 1) aged < 65 years; 2) suffering from other mental illnesses or cognition-related disorders, such as Alzheimer’s disease (dementia was diagnosed according to Clinical Dementia Rating (CDR) and cognitive scores), bipolar disorder, or depression; and 3) refusing to cooperate; 4) having data defects in their patient history. All eligible participants’ information, such as general demographic information (age, sex, education, BMI), daily life habit information (such as smoking and drinking), disease-related information (diabetes, hypertension, and hyperlipidemia), and currently prescribed medicines (typical antipsychotics or atypical antipsychotics, antidepressants, and benzodiazepines), were collected using standardized questionnaires. Additional information, such as blood lipids, high-density lipoprotein, low-density lipoprotein, albumin, and fasting glucose was gathered from medical records and collateral resources. Simultaneously, we also recruited 156 control patients who had: 1) no subjective memory complaints; 2) a global CDR score of 0, rated by the clinician; and 3) objective cognitive score in the normal range. General demographic data for the control patients are shown in Schedule 1.
The Research Ethical Committee of the Affiliated Mental Health Center of the Shanghai Jiaotong University School of Medicine approved the study protocol. Written informed consent was obtained from all participants prior to the study. All research procedures were carried out according to the principles of the Declaration of Helsinki.
Neuropsychological Assessment
Depressive Symptoms
The Geriatric Depression Scale (GDS)18 was used to evaluate depressive symptoms in all elderly patients with schizophrenia. The GDS consists of 30 items, assessing a wide range of areas, from emotion (eg apathy, sadness, and crying) to cognition (eg guilt, helplessness, and worthlessness).19 Its scores range from 0 to 30, with a score of 0 to 10 indicating no depression, and a score greater than 11 indicating possible depression.20,21 Previous studies have shown that GDS can effectively assess depressive symptoms in patients with schizophrenia.22–24 In our current study, we used a score of 11 or above as the basis for classifying elderly patients with schizophrenia with depressive symptoms.
Cognitive Function
The Mini Mental State Examination (MMSE) and Montreal Cognitive Assessment Test (MoCA) are the most commonly used assessment tools in the field of geriatric cognition.25,26 The scores of both tests range from 0 to 30, with lower scores representing cognitive impairment. In general, the sensitivity of MoCA is higher than that of MMSE, but MMSE helps to classify the severity of dementia.27,28 Previous studies have suggested that the MMSE and MoCA may be used successfully in elderly patients with schizophrenia.29,30
Psychobehavioral Symptoms
The Positive and Negative Syndrome Scale (PANSS) is considered an operable, drug-sensitive tool that provides a balanced representation of positive and negative symptoms and measures relationships between symptoms as well as with global psychopathology.31 The scale consists of 30 items that can be used to measure positive (7 items) and negative syndromes (7 items), as well as their differences, and general severity (16 items) of the disease.32 The PANSS has been widely used in the assessment of psychiatric symptoms in schizophrenia and other psychiatric disorders.33,34
Daily Living Abilities
The Activity of Daily Living (ADL) Scale assesses 14 activities of daily living and is administered to the patient caregiver as an interview. The scale contains two components: basic ADL, such as self-feeding, dressing, and personal hygiene, and instrumental ADL, such as managing finances, leisure activities, and household chores. The ADL scores range from 14 to 64. A higher score indicates greater impairment.35,36
Blood Biochemistry and Apolipoprotein E genotype Detection
All study subjects ceased eating after 9 p.m. the day before testing, and their peripheral blood was collected the next day between 7:00 a.m. and 9:00 a.m. Anticoagulant tubes and clot activating gel-containing serum separator tubes were used to assay blood indices such as fasting plasma glucose, low-density lipoprotein, high-density lipoprotein, cholesterol, and triglycerides. Apolipoprotein E (APOE) has three different forms: E2, E3, and E4; of which E3 is the most common. The APOE E3 allele is differentiated by cysteine at 112 and arginine at 158 in the APOE receptor binding region, while the binding ability of APOE E2 alleles (cys-112 and cys-158) decreases significantly. For this study, genomic DNA was extracted from blood cells after high-speed centrifugation using a blood genomic DNA extraction kit (Qiagen NV, Venlo, the Netherlands), and multiplex amplification refractory mutation system polymerase chain reaction was used to determine APOE genotype. According to a previously described method,37 APOE E2 included ε2/ε2, ε2/ε3, and APOE E3 included ε3/ε3, while APOE E4 types included ε3/ε4, and ε4/ε4.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables as frequencies (%). An analysis of variance (ANOVA) was performed for continuous variables between the two groups (the schizophrenia with depressive symptoms group and the schizophrenia without depressive symptoms group), while the chi-square test was used to analyze the categorical variables. Next, we used different binary regression models to examine the association between depressive symptoms and the differential variables between the two groups. Model a) controlled some variables, such as level of education, hypertension, and APOE genotypes; Model b) further controlled for other variables, such as the total score of MMSE, MoCA, ADL, and PANSS. Correlation analysis was used to investigate the association between GDS, general demographic data, and neuropsychological tests. Two-tailed tests were performed at a significance level of P<0.05, and all statistical analyses were performed using SPSS (version 22.0; IBM Corporation, Armonk, NY, USA).
Results
Prevalence of Depressive Symptoms
The prevalence of depressive symptoms in elderly patients with schizophrenia was 48.5% (117/241), whereas that in elderly control patients was 17.3% (27/156). Using the chi-square test, we found that the prevalence of depressive symptoms in elderly patients with schizophrenia was significantly higher (X2=52.964, p<0.001) than that in normal controls. Figure 1 shows the results.
Comparison of General Demographic Data and Neuropsychological Tests Between the Two Groups
Patients with depressive symptoms had lower average education; lower proportion of hypertension; lower MMSE, MoCA, and PANSS scores; higher GDS and ADL scores (p<0.05); and a higher proportion of APOE E2 and APOE E4 genotypes than patients without depressive symptoms. There was no statistical difference in age, albumin, triglycerides, total cholesterol, high-density lipoprotein, low-density lipoprotein, fasting plasma glucose, body mass index, course of disease, age of onset, male sex, diabetes, hyperlipidemia, smoking, drinking, or atypical antipsychotics between the two groups. The results are shown in Table 1.
 |
Table 1 Demographic, Clinical, and Cognitive Characteristics in Chronic Elderly Schizophrenia with or Without Depressive Symptoms |
Results of the Binary Regression Analysis
Using binary regression analysis, treating the presence or absence of depressive symptoms as the dependent variable, we found that high education (p=0.006, OR=1.122, 95% CI:1.034–1.218) may be a risk factor for depressive symptoms, while hypertension (p=0.019, OR=0.519, 95% CI:0.300–0.898) was a protective factor (model); this relationship remained statistically significant after adjusting for total scores of MMSE, MoCA, ADL, and PANSS (model b). The results are shown in Table 2.
 |
Table 2 Binary Logistic Regression Analyses for Factors Related to Depressive Symptoms in Chronic Elderly Schizophrenia |
Factors Possibly Related to GDS
Next, we explored the factors that may be related to GDS, such as age, sex, and neuropsychological tests, and found that GDS was negatively correlated (r=−0.157, p=0.006) with the course of disease. Figure 2 shows the results.
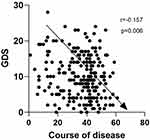 |
Figure 2 Correlation between GDS and course of disease. Note: The GDS scale was negatively correlated with the course of disease (r=−0.157, p=0.006). |
Discussion
In the current study, we investigated the prevalence and influencing factors of depressive symptoms in elderly patients with schizophrenia and found that 1) the prevalence of depressive symptoms in elderly patients with schizophrenia was 48.5%, which was substantially higher than that in normal controls (17.3% [27/156]); 2) high education (p=0.006, OR=1.122, 95% CI:1.034–1.218) and hypertension (p=0.019, OR=0.519, 95% CI:0.300–0.898) were important factors of depressive symptoms; 3) elderly patients with schizophrenia and depressive symptoms tended to have worse overall cognitive function and activities of daily living, but also had fewer psychiatric symptoms; and 4) the total score of GDS was negatively correlated (r=−0.157, p=0.006) with the course of disease.
Long-term hospitalization is a primary issue in the treatment of schizophrenia. As the development of community-based services is limited, Chinese inpatients with schizophrenia tend to experience prolonged hospital stays.38 According to a survey, the average length of stay for these patients in mental hospitals in 2011 was about 300 days, and the number of patients hospitalized for more than 1 year accounted for approximately 60% of the total capacity of the hospital.39 Studies have shown that loneliness and poor social support have negative effects on depression and mortality,40 so it is necessary to investigate the prevalence of depressive symptoms and their influencing factors in long-term hospitalized elderly patients with schizophrenia.
Using the geriatric depression scale (GDS), our study found that the prevalence of depressive symptoms in elderly patients with schizophrenia was 48.5%, which was much higher than that in normal controls (Table 3). In a study by Xu et al,5 the prevalence of depressive symptoms in male schizophrenia inpatients was found to be 41.8%, while in the study by Bener et al, the prevalence of major depressive disorder in inpatients with schizophrenia was 36.8%.41 Yet another study by Baynes et al found that the prevalence of depressive symptoms in chronic schizophrenia was 13.3%.42 Therefore, our research was not consistent with previous studies; however, those previous studies have not been consistent with each other. We suggest that the differences could be attributed to different evaluation tools and diagnostic methods, with some studies using clinical diagnosis and some scale threshold diagnosis.
 |
Table 3 Demographic, Clinical, and Cognitive Characteristics in Normal Controls with or Without Depressive Symptoms |
Using binary logistic regression analysis, we found that high education and hypertension were the main influencing factors of depressive symptoms in elderly patients with schizophrenia. A meta-analysis showed that high education is one of the main risk factors of suicide in patients with schizophrenia;15 another study also pointed out that being well-educated was a risk factor for both depression and suicide in patients with schizophrenia.39 The relationship between hypertension and depressive symptoms is very complex, and the relevant conclusions are inconsistent; for example, one study showed that the risk of depressive symptoms increased significantly within one year of the diagnosis of hypertension but decreased after one year.43 A separate study showed that hypertension was a chronic stressor of depression,44 while another study showed a negative correlation between hypertension and depressive symptoms.45 There are several explanations for why hypertension helps prevent depressive symptoms in elderly patients with schizophrenia. First, the combination of some blood pressure medications, for example beta blockers such as thiazide, and an angiotensin converting enzyme inhibitor at half the standard dose, can help prevent depressive symptoms and Alzheimer’s disease.46 Second, other blood pressure-lowering treatments, such as exercise, diet, weight control, and smoking control, have been shown to have a protective effect on subsequent depressive symptoms.47 Third, high blood pressure can increase the levels of neurotrophic factors in the brain, which is thought to be a protective factor for depressive symptoms.48 However, hypertension can also play an important role in the development of depressive symptoms; for example, sympathetic activation is a specific feature of essential hypertension, and may play a pathogenic role in depression. Therefore, the relationship between hypertension and depressive symptoms needs to be further studied.
Additionally, some depressive symptoms in elderly patients with schizophrenia can be a side effect of medication. For example, previous studies have shown that an increased blockade of dopamine D2 receptors by antipsychotics is associated with depressive symptoms and/or poorer subjective well-being, especially in high doses.49,50 In our current study, some patients received higher doses of antipsychotics, so we could not rule out the effect of antipsychotics on depressive symptoms, which might also be a limitation of our study.
We also explored the influence of depressive symptoms on cognitive symptoms, activities of daily living, and psychotic symptoms in elderly patients with schizophrenia. By using an ANOVA and controlling for education and hypertension, we found that patients with schizophrenia and depressive symptoms tended to have lower MMSE, MOCA, and PANSS scores and higher ADL scores, suggesting that they have more severe cognitive and ADL impairment. There have been many studies on the effect of depression on cognition and daily life ability.51,52 Interestingly, our study found that schizophrenic patients with depressive symptoms had significantly lower psychiatric symptoms. Because the depressive symptoms and negative symptoms of patients with schizophrenia can often overlap,53 we are unable to explain whether the above conclusion is an objective truth or an accidental phenomenon. Several mechanisms can explain why individuals with depressive symptoms may have fewer psychotic symptoms. First, patients with schizophrenia with depressive symptoms are more likely to have predominantly negative symptoms,54 which could explain why patients with depressive symptoms have fewer psychotic symptoms. In addition, depressive symptoms may be a core symptom of a particular type of schizophrenia and may be more prominent than other positive or negative symptoms.55 In addition, we also found that the total GDS score was negatively correlated with the course of disease, suggesting that patients with schizophrenia were more likely to have depressive symptoms in the early stage of onset.
We acknowledge that there are several limitations to our study. First, this was a cross-sectional study, and it was impossible to establish causality between depressive symptoms and activities of daily living or cognition; second, using the scale to diagnose depressive symptoms is likely to result in some deviation; and third, some depressive symptoms may have resulted from side effects of antipsychotics, but we were unable to rule out the effects of antipsychotics. Furthermore, the relatively small sample size also reduces the reliability of the study.
Conclusion
Depressive symptoms are very common in elderly patients with schizophrenia, especially those who are newly diagnosed and have been hospitalized for a long time. High education and hypertension are significantly associated with depressive symptoms in elderly patients with chronic schizophrenia, and elderly patients with schizophrenia and depressive symptoms tend to have worse overall cognitive function and activities of daily living, but also had fewer psychiatric symptoms. Therefore, early recognition and control of these depressive symptoms will help to improve cognitive function and activities of daily living in elderly patients with schizophrenia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Clinical Research Center Project of Shanghai Mental Health Center (CRC2017ZD02), the Cultivation of Multidisciplinary Interdisciplinary Project in Shanghai Jiaotong University (YG2019QNA10), curriculum reform of Medical College of Shanghai Jiaotong University, the Feixiang Program of Shanghai Mental Health Center (2020-FX-03 and Clinical study on the treatment of senile depression by tiaoqi jieyu acupuncture (18401970602)), and Wenzhou Public Welfare Science and Technology Project, No. : Y 20170373.
Disclosure
The authors declare that they have no competing interests.
References
1. Amore M, Murri MB, Calcagno P, et al. The association between insight and depressive symptoms in schizophrenia: undirected and Bayesian network analyses. Eur Psychiatr. 2020;63(1):1–21. doi:10.1192/j.eurpsy.2020.45
2. Cohen CI, Ryu HH. A longitudinal study of the outcome and associated factors of subsyndromal and syndromal depression in community-dwelling older adults with schizophrenia spectrum disorder. Am J Geriatr Psychiatr. 2015;23(9):925–933. doi:10.1016/j.jagp.2014.06.011
3. Brooks JM, Blake J, Sánchez J, et al. Self-reported pain intensity and depressive symptoms among community-dwelling older adults with schizophrenia spectrum disorders. Community Ment Health J. 2019;55(8):1298–1304. doi:10.1007/s10597-019-00403-x
4. Akinsulore A, Aloba OO, Mapayi BM, Oloniniyi IO, Fatoye FO, Makanjuola RO. Relationship between depressive symptoms and quality of life in Nigerian patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2014;49(8):1191–1198. doi:10.1007/s00127-014-0838-8
5. Fond G, Faugere M, Richieri R, et al. Depressive symptoms and chronic peripheral inflammation are associated with impaired functional remission in schizophrenia independently of psychotic remission. J Affect Disord. 2021;280(PtA):267–271. doi:10.1016/j.jad.2020.11.046
6. Xu YM, Li F, Liu XB, Zhong BL. Depressive symptoms in Chinese male inpatients with schizophrenia: prevalence and clinical correlates. Psychiatry Res. 2018;264:380–384. doi:10.1016/j.psychres.2018.04.016
7. Meesters PD, Comijs HC, Sonnenberg CM, et al. Prevalence and correlates of depressive symptoms in a catchment-area based cohort of older community-living schizophrenia patients. Schizophr Res. 2014;157(1–3):285–291. doi:10.1016/j.schres.2014.05.002
8. Hoertel N, Jaffré C, Pascal de Raykeer R, et al. Subsyndromal and syndromal depressive symptoms among older adults with schizophrenia spectrum disorder: prevalence and associated factors in a multicenter study. J Affect Disord. 2019;251:60–70. doi:10.1016/j.jad.2019.03.007
9. Felmet K, Zisook S, Kasckow JW. Elderly patients with schizophrenia and depression: diagnosis and treatment. Clin Schizophr Relat Psychoses. 2011;4(4):239–250. doi:10.3371/CSRP.4.4.4
10. Thomas N, Ribaux D, Phillips LJ. Rumination, depressive symptoms and awareness of illness in schizophrenia. Behav Cogn Psychother. 2014;42(2):143–155. doi:10.1017/S1352465812000884
11. Hirsch SR, Jolley AG, Barnes TR, et al. Dysphoric and depressive symptoms in chronic schizophrenia. Schizophr Res. 1989;2(3):259–264. doi:10.1016/0920-9964(89)90002-9
12. Goldsmith DR, Rapaport MH. Inflammation and negative symptoms of schizophrenia: implications for reward processing and motivational deficits. Front Psychiatry. 2020;11:46. doi:10.3389/fpsyt.2020.00046
13. Kilzieh N, Wood AE, Erdmann J, Raskind M, Tapp A. Depression in Kraepelinian schizophrenia. Compr Psychiatry. 2003;44(1):1–6. doi:10.1053/comp.2003.50002
14. Nyer M, Kasckow J, Fellows I, et al. The relationship of marital status and clinical characteristics in middle-aged and older patients with schizophrenia and depressive symptoms. Ann Clin Psychiatr. 2010;22(3):172–179.
15. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 Suppl):81–90. doi:10.1177/1359786810385490
16. Kumar P, Kraal AZ, Prawdzik AM, Ringold AE, Ellingrod V. Dietary glutamic acid, obesity, and depressive symptoms in patients with schizophrenia. Front Psychiatr. 2020;11:620097. doi:10.3389/fpsyt.2020.620097
17. Khan A, Fahl Mar K, Gokul S, Brown WA. Mortality during US FDA clinical trials in patients with diabetes, hypertension, depression and schizophrenia. World J Biol Psychiatr. 2020;21(1):64–71. doi:10.1080/15622975.2018.1514465
18. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi:10.1001/archpsyc.1961.01710120031004
19. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res. 2011;63(Suppl 11):S454–466.
20. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi:10.1016/0022-3956(82)90033-4
21. D’Antonio E, Serper MR. Depression and cognitive deficits in geriatric schizophrenia. Schizophr Res. 2012;134(1):65–69. doi:10.1016/j.schres.2011.10.006
22. Gupta S, Steinmeyer C, Frank B, Lockwood K, Lentz B, Schultz K. Older patients with schizophrenia: nature of dwelling status and symptom severity. Am J Psychiatry. 2003;160(2):383–384. doi:10.1176/appi.ajp.160.2.383
23. Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr. 2019;31(4):491–504. doi:10.1017/S1041610218001370
24. Lim MYL, Loo JHY. Screening an elderly hearing impaired population for mild cognitive impairment using Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Int J Geriatr Psychiatry. 2018;33(7):972–979. doi:10.1002/gps.4880
25. Vissoci JRN, de Oliveira LP, Gafaar T, et al. Cross-cultural adaptation and psychometric properties of the MMSE and MoCA questionnaires in Tanzanian Swahili for a traumatic brain injury population. BMC Neurol. 2019;19(1):57. doi:10.1186/s12883-019-1283-9
26. Rambeau A, Beauplet B, Laviec H, et al. Prospective comparison of the Montreal Cognitive Assessment (MoCA) and the Mini Mental State Examination (MMSE) in geriatric oncology. J Geriatr Oncol. 2019;10(2):235–240. doi:10.1016/j.jgo.2018.08.003
27. Fisekovic S, Memic A, Pasalic A. Correlation between moca and mmse for the assessment of cognition in schizophrenia. Acta informatica medica. 2012;20(3):186–189. doi:10.5455/aim.2012.20.186-189
28. Yang Z, Abdul Rashid NA, Quek YF, et al. Montreal cognitive assessment as a screening instrument for cognitive impairments in schizophrenia. Schizophr Res. 2018;199:58–63. doi:10.1016/j.schres.2018.03.008
29. Rosca EC, Cornea A, Simu M. Montreal cognitive assessment for evaluating the cognitive impairment in patients with schizophrenia: a systematic review. Gen Hosp Psychiatry. 2020;65:64–73. doi:10.1016/j.genhosppsych.2020.05.011
30. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261
31. Fleischhacker W, Galderisi S, Laszlovszky I, et al. The efficacy of cariprazine in negative symptoms of schizophrenia: post hoc analyses of PANSS individual items and PANSS-derived factors. Eur Psychiatr. 2019;58:1–9. doi:10.1016/j.eurpsy.2019.01.015
32. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137(1–3):246–250. doi:10.1016/j.schres.2012.01.031
33. Freitas R, Dos Santos B, Altamura C, et al. Can the Positive and Negative Syndrome scale (PANSS) differentiate treatment-resistant from non-treatment-resistant schizophrenia? A factor analytic investigation based on data from the Pattern cohort study. Psychiatry Res. 2019;276:210–217. doi:10.1016/j.psychres.2019.05.002
34. Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172. doi:10.1093/annonc/mdr587
35. Barberger-Gateau P, Commenges D, Gagnon M, Letenneur L, Sauvel C, Dartigues JF. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc. 1992;40(11):1129–1134. doi:10.1111/j.1532-5415.1992.tb01802.x
36. Donohoe GG, Salomäki A, Lehtimäki T, Pulkki K, Kairisto V. Rapid identification of apolipoprotein E genotypes by multiplex amplification refractory mutation system PCR and capillary gel electrophoresis. Clin Chem. 1999;45(1):143–146. doi:10.1093/clinchem/45.1.143
37. Wu G, Ouyang X, Yang B, et al. Long- and short-term inpatients with schizophrenia in China: implications for community-based service development. Asia-Pacific Psychiatr. 2013;5(1):E39–46. doi:10.1111/j.1758-5872.2012.00229.x
38. Yanagida N, Uchino T, Uchimura N. The effects of psychoeducation on long-term inpatients with schizophrenia and schizoaffective disorder. Kurume Med J. 2017;63(3.4):61–67. doi:10.2739/kurumemedj.MS00011
39. Wang J, Mann F, Lloyd-Evans B, Ma R, Johnson S. Associations between loneliness and perceived social support and outcomes of mental health problems: a systematic review. BMC Psychiatr. 2018;18(1):156. doi:10.1186/s12888-018-1736-5
40. Bener A, Dafeeah EE, Abou-Saleh MT, Bhugra D, Ventriglio A. Co-morbidity between major depression and schizophrenia: prevalence and clinical characteristics. Psychiatr Danub. 2020;32(1):78–83. doi:10.24869/psyd.2020.78
41. Baynes D, Mulholland C, Cooper SJ, et al. Depressive symptoms in stable chronic schizophrenia: prevalence and relationship to psychopathology and treatment. Schizophr Res. 2000;45(1–2):47–56. doi:10.1016/S0920-9964(99)00205-4
42. Sher L, Kahn RS. Suicide in schizophrenia: an educational overview. Medicina (Kaunas, Lithuania). 2019;55(7). doi:10.3390/medicina55070361
43. Villarreal-Zegarra D, Bernabe-Ortiz A. Association between arterial hypertension and depressive symptoms: results from population-based surveys in Peru. Asia-Pacific Psychiatr. 2020;12(2):e12385. doi:10.1111/appy.12385
44. Jin Y, Luo Y, He P. Hypertension, socioeconomic status and depressive symptoms in Chinese middle-aged and older adults: findings from the China health and retirement longitudinal study. J Affect Disord. 2019;252:237–244. doi:10.1016/j.jad.2019.04.002
45. Vallée A, Wiernik E, Kab S, et al. Association of depressive symptoms and socioeconomic status in determination of blood pressure levels and hypertension: the CONSTANCES population based study. J Affect Disord. 2021;279:282–291. doi:10.1016/j.jad.2020.10.018
46. Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008;7:37. doi:10.1186/1476-511X-7-37
47. Jin Y, He P. Antihypertensive treatment and depressive symptoms in Chinese middle-aged and older hypertensive adults: a population-based longitudinal study. Int J Geriatr Psychiatry. 2020;35(3):312–320. doi:10.1002/gps.5250
48. Nemcsik J, László A, Lénárt L, et al. Hyperthymic affective temperament and hypertension are independent determinants of serum brain-derived neurotrophic factor level. Ann Gen Psychiatry. 2016;15:17. doi:10.1186/s12991-016-0104-4
49. de Haan L, van Bruggen M, Lavalaye J, Booij J, Dingemans PM, Linszen D. Subjective experience and D2 receptor occupancy in patients with recent-onset schizophrenia treated with low-dose olanzapine or haloperidol: a randomized, double-blind study. Am J Psychiatry. 2003;160(2):303–309. doi:10.1176/appi.ajp.160.2.303
50. Mizrahi R, Rusjan P, Agid O, et al. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry. 2007;164(4):630–637. doi:10.1176/ajp.2007.164.4.630
51. McCall WV, Dunn AG. Cognitive deficits are associated with functional impairment in severely depressed patients. Psychiatry Res. 2003;121(2):179–184. doi:10.1016/j.psychres.2003.09.003
52. Bartfay E, Bartfay WJ, Gorey KM. Prevalence and correlates of potentially undetected dementia among residents of institutional care facilities in Ontario, Canada, 2009–2011. Int J Geriatr Psychiatry. 2013;28(10):1086–1094. doi:10.1002/gps.3934
53. Rahim T, Rashid R. Comparison of depression symptoms between primary depression and secondary-to-schizophrenia depression. Int J Psychiatry Clin Pract. 2017;21(4):314–317. doi:10.1080/13651501.2017.1324036
54. An der Heiden W, Leber A, Häfner H. Negative symptoms and their association with depressive symptoms in the long-term course of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2016;266(5):387–396. doi:10.1007/s00406-016-0697-2
55. Huang J, Zhuo C, Song X, et al. Does depressive-type schizophrenia exist? Do we prove it?: an updated review and overview. J Nerv Ment Dis. 2019;207(7):555–560. doi:10.1097/NMD.0000000000001004
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

