Back to Journals » Research and Reports in Tropical Medicine » Volume 12
Prevalence, Infection Intensity and Associated Factors of Soil-Transmitted Helminthiasis Among School-Aged Children from Selected Districts in Northwest Ethiopia
Authors Zeleke AJ , Derso A , Bayih AG , Gilleard JS, Eshetu T
Received 1 November 2020
Accepted for publication 3 February 2021
Published 15 February 2021 Volume 2021:12 Pages 15—23
DOI https://doi.org/10.2147/RRTM.S289895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Ayalew Jejaw Zeleke,1 Adane Derso,1 Abebe Genetu Bayih,1,2 John S Gilleard,3 Tegegne Eshetu1
1Department of Medical Parasitology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Armauer Hansen Research Institute, Addis Ababa, Ethiopia; 3Host-Parasite Interactions Program, Department of Comparative Biology and Experimental Medicine, Faculty of Veterinary Medicine, University of Calgary, Calgary, Canada
Correspondence: Tegegne Eshetu Tel +251921738483
Email [email protected]
Background: Globally, soil-transmitted helminths affect beyond a billion people and cause 1.9 million disability-adjusted life years worldwide. It affects children disproportionately due to their unaware activities like walking barefoot, playing with dirty objects that might be contaminated with feces. The control of soil-transmitted helminths principally relies on periodic deworming using either a single dose of albendazole/mebendazole. To assure the effectiveness of this measure, performing continuous parasitological survey is necessary. Herein, the prevalence, intensity and associated factors of soil-transmitted helminth infections were assessed among school-aged children in northwest Ethiopia.
Methods: A cross-sectional study design was conducted among school-aged children (6– 14 years old) from January 21st to February 21st/2019. Multistage sampling technique was employed. A Kato-Katz concentration technique was utilized to detect STHs in stool samples. Moreover, risk factors for STH infections were assessed using well-structured questionnaire. Bivariate and multivariate analyses were used to assess the association between explanatory and the outcome variables. The magnitude of the association was measured using the adjusted odds ratio (AOR) and 95% confidence interval (CI). A P-value < 0.05 was considered statistically significant.
Results: The overall STHs prevalence in this study was 32.3% (95% CI: 29– 35.6%) with Ascaris lumbricoides being the predominant species (24.3%) followed by hookworm (8.9%) and Trichuris trichiura (1%). Most (80.3%) of the infected school-aged children had light-intensity infections. Age of 11 years and above (AOR, 12.9, 95% CI, 1.6– 103.6, P=0.004), being residing in Chuahit district (AOR, 3.9, 95% CI, 2.3– 6.5, P< 0.001), and untreated water supply (AOR, 1.7, 95% CI, 1.1– 2.7, P=0.018) were identified as predictors for the overall STH prevalence.
Conclusion: Our findings revealed STH infections are considerable health problems in the study areas. Thus, public health interventions such as provision of safe water supply, health education, and de-worming programs should be regularly implemented in the study areas.
Keywords: STH, helminths, prevalence, northwest Ethiopia
Background
Globally, the burden of Soil-Transmitted Helminths (STHs) ie Ascaris lumbricoides, Trichuris trichiura, and hookworm (Ancylostoma duodenale and Necator americanus) infections remained high, and continues to have a devastating impact on people’s health especially among individuals in low- and middle-income countries.1 In 2017, the global burden of STHs was estimated at 1.9 million disable-adjusted life years (DALYs).2 Moreover, currently more than one billion people are affected by STHs worldwide.1 Tropical and subtropical regions are the most hotspot areas for STH infections, the greatest numbers of the case occur in sub-Saharan Africa (SSA), the Americas, China, and East Asia.3
In SSA regions over one quarter of the population is affected with one or more STH infections, especially children, disproportionately affected.4 The disproportionate effect of STHs in children might be due to unaware activities like walking barefoot, playing with dirty objects that might be contaminated with feces, and fetching of unclean water for drinking and to not fully developed immune systems.5 Factors such as low household income, poor personal and environmental sanitation, over-crowding, limited access to clean water, tropical climate and low altitude are significantly associated with the occurrence of high intestinal parasitic infections especially in tropical and sub-tropical areas.6–8
The burden of the disease is mainly attributed to their chronic and insidious impact on the health and quality of life of infected individuals rather than to the mortality they cause.3,9 Such adverse effects of STH infections among children are so diverse and alarming. They have detrimental effects on their survival, as well as mental and physical development. Moreover, they are associated with poor school performance and absenteeism in children.10,11
The current global strategy for the control of STH infections is implemented through regular mass drug administration using regular anti-helminthic treatment, health education, sanitation and personal hygiene, and other means of prevention with vaccines and remote sensing.3,12,13 According to the World Health Organization (WHO) reports, an estimate of around 267.5 million preschool-aged children and 568.8 million school-aged children requires treatment across 103 countries endemic to STH.14 The major objective of control approach using mass drug administration as a preventive chemotherapy is to eliminate morbidity in the target population by reducing the prevalence of moderate- and heavy-intensity infections to <2%.15,16
Therefore, regular parasitological surveys are essential to produce up to date data on the prevalence and infection intensity to evaluate the implementing prevention and control measures. Moreover, continuous evaluation and monitoring of the prevalence status of STH are crucial to map additional or alternative control strategies according to the finding. Despite the existence of many recent epidemiological studies documented with a high burden of intestinal parasites in Ethiopia,17–20 there has been no recently published report on the magnitude of STHs among elementary school-aged children in the current study districts, Northwest Ethiopia. Therefore, in this study, we were initiated to determine the current prevalence, infection intensity of STHs and associated factors of infection among school-aged children of four selected districts in Northwest Ethiopia.
Materials and Methods
Study Area
The study was conducted in four different districts of primary schools in the northwest part of Ethiopia. Study participants were from Sanja (13°20′N/36°45′E), Maksegnit (12°40′N/37°20′E, one of the kebeles in Gondar Zuria), Debark (13°08′N/37°54′E), and Chuahit (one of the kebeles from Dembia) (12°40′N/37°10′E) districts. According to previously published reports and current unpublished data available in the health centers indicating all of the study areas included in this study are among the predominant sites where intestinal parasitosis are predominant in northwest Ethiopia.
Study Design and Period
A school-based cross-sectional study was conducted to determine the prevalence, infection intensity, and associated factors of STHs among school-aged children whose age ranged from 6 to 14 years old from January 21st to February 21st/2019.
Sample Size and Sampling Technique
The required sample size was determined using a single population proportion formula21 and considering 37.1%22 prevalence rate in the population, 95% confidence level, a 5% expected margin of error and a 10% non-response rate. Accordingly, the calculated sample size was 395. The design effect of two (to minimize error arising from sampling procedure) was used to allow for multistage sampling techniques; and hence, the final sample size was calculated to be 790. The source of population for the study was elementary school-aged children.
Prior to the study initiation, based on the accessibility of transportation, only four districts were selected purposefully from Northwest Ethiopia. Then, the sample size was proportionally allocated to each district, school and class section based on the total number of students who were attending primary school during the study period. Students’ enrollment lists were taken as the sampling frame. Finally, study participants were selected using systematic random sampling technique (see Figure 1).
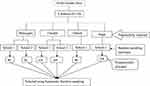 |
Figure 1 Diagramatical presentation of sampling techniques. |
Inclusion and Exclusion Criteria
Participants who had a written informed consent and all randomly selected school-aged children in the selected schools who were agreed to provide all required socio-demographic data and stool sample were included.
Exclusion Criteria
Participants who were on anti-helminthic drug within the past two months prior to data collection and participant who were unable to provide sufficient stool sample were excluded.
Data Collection and Laboratory Procedures
Socio-Demographic Data and Risk Assessment of the Study Participants
Prior to the laboratory investigation, a pre-tested and well-structured questionnaire was prepared in English and translated to Amharic, the local language, was used to collect socio-demographic data and associated factors of the study participants. Trained data collectors were recruited for data collection and supervision was made by the investigators. At each of the data collection spot, sufficient explanation about the aim of the study was given to the students, school teachers and parents. A unique identification number was given to each study participant.
Stool Sample Collection and Laboratory Procedures
After the collection of essential participant related data, each participant received a sterile stool container labeled with his/her unique identification number and was asked to provide approximately 3g of fresh stool samples. The children were instructed on how to provide stool specimen and remained to avoiding contamination with urine. The specimens were collected at each school and immediately (within four hours of collection) transported to University of Gondar, department of medical parasitology laboratory. Each stool sample was examined by direct wet mount microscopy and two slides Kato-Katz technique. Hookworm ova detection was performed by examination of the Kato-Katz slides within one hour of its preparation. For identification of the other two helminths ova, the prepared Kato-Katz slides were left for 24 h for better clearing and easy visualization of eggs. Infection intensity of the STHs was estimated by multiplying the total number of eggs counted by 24, which gives as the eggs per gram (epg) of stool. Besides, the species-specific classes of infection intensity were classified as light, moderate and heavy as per the threshold set by WHO and classified as A. lumbricoides: light infection (1–4999epg), moderate (5000–49999epg) and heavy (greater than 50,000epg). Intensity of T. trichiura: light infection (1–999epg), moderate (1000–9999epg) and heavy (greater than 10,000epg). Classification of Hookworm: light infection (1–1999epg), moderate (2000–3999 epg) and heavy (greater than 4000epg).23
Data Management and Analysis
Data was entered to Epi-data software to assure the data completeness and clearance, and transferred to SPSS version 20 software package for further statistical analysis. Descriptive statistics (mean, standard deviation, and percentages) were used to summarize the demographic profile of the study participants. Levene’s and Kolmogorov–Simonov's statistical tests were carried out to check the homogeneity and the normality of data, respectively. Bivariate and multivariate analyses were used to assess the association between explanatory and the outcome variables. Variables with p-value<0.25 in the bivariate analysis were subjected to further multivariate logistic regression analysis model to identify predictor variables and to control cofounders. Then, the study findings were explained using tables. The magnitude of the association was measured using adjusted odds ratio (AOR) and 95% confidence interval (CI). A P-value<0.05 was considered statistically significant.
Result
Socio-Demographic Characteristics of Study Participants
A total of 786 school-aged children participated in this study with a response rate of 99.5%. Out of these, 452 (57.5%) were female. The mean age of the participants was 10.64 ±1.8 years and the majority (68.3%) was within the age group of 8–11 years. The study participants were from four different districts. 21.9%, 10.6%, 32.4%, and 35.1% were from Maksegnit, Debark, Sanja, and Chuahit, respectively. Moreover, most of the study participants used untreated source of drinking water (54.2%) and did not have a latrine at home (66.7%).
Prevalence, Intensity and Risk Factors of Soil-Transmitted Helminths
The overall STH prevalence was 32.3%. The prevalence of A.lumbricoides, Hookworm, and T.trichuira was found to be 24.3%, 8%, and 1%, respectively. The overall and species-wise prevalence showed statistically significant differences across districts. The overall prevalence of STH was 26.2%, 32.5%, 25.5%, and 42.4% in Maksegnit, Debark, Sanja, and Chuahit, respectively. On the other hand, the prevalence of A.lumbricoides and Hookworm was higher in Chuahit (41.3) and Sanja (24.3%), respectively, than any other districts (Table 1). Almost all (95%) of the infections were due to mono-infection.
 |
Table 1 Overall and Species-Wise Prevalence of STHs Among School-Aged Children Across Districts in Northwest Ethiopia, 2019 |
Out of 254 school children who had STH infections, 204 (80.3%), and 47 (18.6%) were due to light and moderate intensity of infection, respectively. From the total of 191 study participants who were positive for A. lumbricoides, 138 (72.3%), 50 (26.2%), and 3(0.5%) were grouped as light, moderate and heavy infections, respectively. All Hookworm and T. trichiura infected children were due to light infections (Table 2).
 |
Table 2 Intensity of STHs Infections Among School-Aged Children in Northwest Ethiopia, 2019 |
In this study, participants whose age greater than 11 years (AOR, 12.9, 95% CI, 1.6–103.6), residing in Chuahit District (AOR, 3.9, 95% CI, 2.3–6.5), and using untreated water supply (AOR, 1.7, 95% CI, 1.1–2.7) were identified as potential risk factors for the transmission of STHs (Tables 3 and 4).
 |
Table 3 Binary Logistic Regression Analysis of Determinant Factors of STHs Infections Among School-Aged Children (n = 786) in Northwest Ethiopia, 2019 |
 |
Table 4 Multivariate Logistic Regression Analysis of Determinant Factors of STHs Infections Among School-Aged Children (n = 786) in Northwest Ethiopia, 2019 |
Discussion
The present study showed a high prevalence (32.3%) of STH infections among school-aged children in northwest Ethiopia regardless of the implementing school-based periodic anti-helminthic treatment. Despite there is significant reduction in the overall prevalence of STH infections revealed from other previous reports (Jimma (45.6%),24 Chincha (63%),25 Tilili (44.2%),26 and around Gilgel Gibe Dam (52.1%)),27 our finding showed STH continues to afflict school children at a rate higher than the threshold recommended by the WHO (20%)28 for implementation of mass deworming of school children in endemic areas.
Moreover, our finding is higher than other similar studies done in Ambo (12.6%),29 Babile (0.47%),30 and Dera district (20.9%).31 This result discrepancy might be due to difference in the sample size of enrolled study participants, the laboratory techniques carried out, specimen preparation, environmental and socio-economic factors that may account for the variation in the prevalence of these parasites. For instance, a study conducted in Babile applied the McMaster diagnostic technique, whereas the study in Ambo and Dera district used the formol-ether concentration technique. Thus, variation in the diagnostic technique’s sensitivity might be the possible source of result discrepancies. Moreover, the transmission of STHs highly depends on a number of environmental factors (including the nature of soil type and climate) that influence the parasite survival, the extent to which the environment is contaminated with infectious egg/larvae and the amount of contact between susceptible host and contaminated soil.32 So it might be the other possible source for the inconsistence of findings across study areas.
In this study, 80.3%, 18.6%, and 1.1% of the infected schoolchildren were within light, moderate, and heavy intensity of STHs infections. All infections caused by Hookworm and T.trichiura were under light infection category, whereas, 72.3%, 26.2%, and 0.5% of the infected children by A.lumbricoides were due to light, moderate, and heavy infection, respectively. Although the proportion of heavy and moderate infection intensity is relatively low in this study area, the existence of a significant number of children harboring light infections could serve as a potential source of re-infection for those who are already infected or new infections for other healthy children. Thus, this study calls for regular de-worming and other interventions in order to further decrease the prevalence until the targeted minimum threshold level of parasite intensity is determined for their elimination.
As evidenced from previously reported studies in many parts of the world, the prevalence of A.lumbricoides was also found to be the most prevalent STHs encountered in this study. This is supported with studies done in Ambo,29 Chincha,25 Tilili,26 and Gondar.33 The predominance of A. lumbricoides could be related with the extreme resistance of the eggs for harsh environments than the other STHs.34 The second most prevalent was hookworm (8.9%). This data is lower than the studies conducted in Jimma, Wolaita, and Dera districts.27,31,35 However, it is higher than the previous reports from Ambo, Babile, and Gondar Towns.29,30,33 The prevalence of T.trichiura in the current study was 1% and similar results were reported from Wolaita and Ambo towns.29,35 However, it is lower than previous studies reported from Gondar, Chincha, and Tilili towns.25,26,32 Climatic variability, environmental factors, method and type of control measure implementation might have contributed for the infection rate differences across different regions. For instance, in the case of hookworm species climatic variability might ultimately favor the occurrence of the parasite due to its unique ability to undergo developmental arrest as dauer larvae in human tissues for the means of surviving the environmental extremes.32,36,37 Moreover, the type of implementing control measures might affect the dominance of a certain worm within STHs due to worms having a unique susceptibility status for the applied anthelmintic drug as a preventive chemotherapy.
Moreover, the current study has identified potential risk factors for STHs infection. Accordingly, age, study sites (districts), and source of treated water were found to be significant predicators of STHs. Study participants, who had11 years and older were 13 times more likely to be infected by STHs than those who were <7 years old. This is probably related to prolonged exposure time as age increases. The prevalence of STH was significantly higher in Chuahit than any other districts. Students residing in Chuahit district were four times more likely to be infected by STH than those who were living in Sanja, Debark and Maksegnit. Debark and Maksegnit took the second and third ranks in terms of STH prevalence, while Sanja is the least. Variation of infection rates across the districts might be due to the difference in the weather condition of the study sites. Chuahit is located closer to a lake than other sites with more humid and warm climate. This may favor helminths ova for embryonation and ultimately enhance their transmission. Moreover, despite Sanja is a very dry hot area among our study sites and such weather condition may not be favorable for STH transmission; hookworm is relatively higher than other STHs may be due to the loamy soil type present in the district. The use of untreated water as a source of drinking and other domestic supply was almost two times more likely to be infected by STHs than their counterparts. This is due to the fact that untreated water might have been contaminated by human excreta which are potential for intestinal helminthiasis infection.
Conclusions
Approximately, one third of the study participants were infected with at least one STH species and this implies that they are still a health problem among school-aged children in Northwest Ethiopia. However, just over three-fourth of the infection intensity of STH was light. Age, residential district, and source of drinking water were associated with STHs infections. Thus, public health interventions such as provision of safe water supply, health education and de-worming programs should be regularly implemented in the study areas.
Limitation
The infection intensity of the common intestinal helminthiasis was determined by the examination of a single stool sample of each participant. Thus, intermittent egg excretion might have affected the sensitivity and accuracy of the egg count of the STHs. Thus, the findings should be interpreted with that limitation in mind.
Abbreviations
IPIs, intestinal parasitic infections; STH, soil-transmitted helminths; WHO, World Health Organization; SSA, Sub Saharan Africa.
Data Sharing Statement
All data generated or analyzed during this study are included in this manuscript. Other required data will be available from the corresponding author upon request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. Ethical clearance was obtained from the ethical committee of School of Biomedical and Laboratory Sciences, University of Gondar (Ref. No. O/V/P/RCS/05/118/2017). Permissions were also obtained from North Gondar Zone and the district Health Departments. Moreover, parents/participant guardians who agreed that their child should be enrolled in the study were asked to provide a written informed consent, since the study participants were under the age of 18 years. Parents/legal guardians who were unable to read and write were asked to provide a thumbprint following the informed consent have been read clearly. Verbal assent was also sought from each study participant. Individuals who were found to be positive for STHs and other intestinal parasites were treated using anti-helminthic drug based on the standard treatment guideline.
Acknowledgments
We would like to thank the Bill and Melinda Gates Foundation (grant number OPP1172974) and the University of Calgary for financial support for this research. We are grateful to all study participants, parents, school teachers, and data collectors.
Author Contributions
All authors made a significant contribution to the conception, design and execution, acquisition of data, analysis and interpretation; took part in drafting the initial manuscript, revising it critically for final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Funding
The research was funded by the University of Calgary, Canada.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Montresor A, Mupfasoni D, Mikhailov A, et al. The global progress of soil-transmitted helminthiases control in 2020 and World Health Organization targets for 2030. PLoS Negl Trop Dis. 2020;14(8):e0008505. doi:10.1371/journal.pntd.0008505.
2. Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1859–1922. doi:10.1016/S0140-6736(18)32335-3.
3. World Health Organization. Soil-transmitted helminth infections; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections.
4. Hotez PJ, Kamath A, Cappello M. Neglected tropical diseases in sub-saharan africa: review of their prevalence, distribution, and disease burden. Cappello M, ed. PLoS Negl Trop Dis. 2009;3(8):e412. doi:10.1371/journal.pntd.0000412
5. Rao V, Sugunan A, Murhekar M, Sehgal S. Malnutrition and high childhood mortality among the Onge tribe of the Andaman and Nicobar Islands. Public Health Nutr. 2006;9(1):19–25. doi:10.1079/PHN2005761
6. WHO/Department of Communicable Disease Prevention, Control and Eradication. Prevention and control of intestinal parasitic infections; 1987. Available from: who.int/intestinal_worms/resources/who_trs_749/en/.
7. Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC Infect Dis. 2017;17(1):362. doi:10.1186/s12879-017-2466-x
8. Erismann S, Diagbouga S, Odermatt P, et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the plateau central and centre-ouest regions of Burkina Faso. Parasit Vectors. 2016;9(1):554. doi:10.1186/s13071-016-1835-4
9. Molyneux DH, Hotez PJ, Fenwick A, Newman RD, Greenwood B, Sachs J. Neglected tropical diseases and the global fund. Lancet. 2009;373(9660):296–297. doi:10.1016/S0140-6736(09)60089-1
10. Blouin B, Casapía M, Joseph L, Kaufman JS, Larson C, Gyorkos TW. The effect of cumulative soil-transmitted helminth infections over time on child development: a 4-year longitudinal cohort study in preschool children using Bayesian methods to adjust for exposure misclassification. Int J Epidemiol. 2018;47(4):1180–1194. doi:10.1093/ije/dyy142
11. Pabalan N, Singian E, Tabangay L, Jarjanazi H, Boivin MJ, Ezeamama AE. Soil-transmitted helminth infection, loss of education and cognitive impairment in school-aged children: a systematic review and meta-analysis. Budke CM, ed. PLoS Negl Trop Dis. 2018;12(1):e0005523. doi:10.1371/journal.pntd.0005523
12. Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P. Hookworm: the great infection of mankind. PLoS Med. 2005;2(3):e67. doi:10.1371/journal.pmed.0020067
13. Mascarini-Serra L. Prevention of soil-transmitted helminth infection. J Glob Infect Dis. 2011;3(2):175. doi:10.4103/0974-777X.81696
14. World health organization. Soil transmitted helminths. Neglected tropical diseases program. Available from: https://www.neglecteddiseases.gov/usaid-targeted-diseases/soil-transmitted-helminths/.
15. Mupfasoni D, Bangert M, Mikhailov A, Marocco C, Montresor A. Sustained preventive chemotherapy for soil-transmitted helminthiases leads to reduction in prevalence and anthelminthic tablets required. Infect Dis Poverty. 2019;8(1):82. doi:10.1186/s40249-019-0589-6.
16. World Health Organization. Preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups guideline; 2017. Available from: https://www.who.int/nutrition/publications/guidelines/deworming/en/.
17. Tiruneh T, Geshere G, Ketema T. Prevalence and determinants of soil-transmitted helminthic infections among school children at goro primary school, South West Shewa, Ethiopia. Int J Pediatr. 2020;2020:1–7. doi:10.1155/2020/8612054
18. Molla E, Mamo H. Soil-transmitted helminth infections, anemia and undernutrition among school children in Yirgacheffee, South Ethiopia. BMC Res Notes. 2018;11(1):585. doi:10.1186/s13104-018-3679-9
19. Nute AW, Endeshaw T, Stewart AEP, et al. Prevalence of soil-transmitted helminths and schistosoma mansoni among a population-based sample of school-age children in Amhara region, Ethiopia. Parasit Vectors. 2018;11(1):431. doi:10.1186/s13071-018-3008-0
20. Mengist HM, Zewdie O, Belew A. Intestinal helminthic infection and anemia among pregnant women attending antenatal care (ANC) in East Wollega, Oromia, Ethiopia. BMC Res Notes. 2017;10(1):440. doi:10.1186/s13104-017-2770-y
21. Hajian-Tilaki K. Sample size estimation in epidemiologic studies. Caspian J Intern Med. 2011;2(4):289–298.
22. Worku L, Damte D, Endris M, Tesfa H, Aemero M. Schistosoma mansoni infection and associated determinant factors among school children in Sanja Town, Northwest Ethiopia. J Parasitol Res. 2014;2014:1–7. doi:10.1155/2014/792536
23. World health organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee; 2002. Available from: https://apps.who.int/iris/handle/10665/42588.
24. Tefera E, Belay T, Mekonnen SK, Zeynudin A, Belachew T. Therapeutic efficacy of different brands of albendazole against soil transmitted helminths among students of Mendera Elementary School, Jimma, Southwest Ethiopia. Pan Afr Med J. 2015;22. doi:10.11604/pamj.2015.22.252.6501.
25. Abossie A, Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health. 2014;14(1):166. doi:10.1186/1471-2458-14-166
26. Abera A, Nibret E. Prevalence of gastrointestinal helminthic infections and associated risk factors among schoolchildren in Tilili town, northwest Ethiopia. Asian Pac J Trop Med. 2014;7(7):525–530. doi:10.1016/S1995-7645(14)60088-2
27. Mekonnen Z, Suleman S, Biruksew A, Tefera T, Chelkeba L. Intestinal polyparasitism with special emphasis to soil-transmitted helminths among residents around Gilgel Gibe Dam, Southwest Ethiopia: a community based survey. BMC Public Health. 2016;16(1):1185. doi:10.1186/s12889-016-3859-2
28. World Health Organization. Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiases. Geneva World Health Organ; 2013. Available from: https://apps.who.int/iris/handle/10665/79019.
29. Samuel F, Demsew A, Alem Y, Hailesilassie Y. Soil transmitted helminthiasis and associated risk factors among elementary school children in ambo town, western Ethiopia. BMC Public Health. 2017;17(1):791. doi:10.1186/s12889-017-4809-3
30. Tefera E, Mohammed J, Mitiku H. Intestinal helminthic infections among elementary students of Babile town, eastern Ethiopia. Pan Afr Med J. 2015;20. doi:10.11604/pamj.2015.20.50.5251.
31. Shiferaw MB, Mengistu AD. Helminthiasis: hookworm infection remains a public health problem in Dera District, South Gondar, Ethiopia. Aroian RV, ed. PLoS One. 2015;10(12):e0144588. doi:10.1371/journal.pone.0144588
32. Sturrock SL, Yiannakoulias N, Sanchez AL. The geography and scale of soil-transmitted helminth infections. Curr Trop Med Rep. 2017;4(4):245–255. doi:10.1007/s40475-017-0126-2
33. Mathewos B, Alemu A, Woldeyohannes D, et al. Current status of soil transmitted helminths and schistosoma mansoni infection among children in two primary schools in North Gondar, Northwest Ethiopia: a cross sectional study. BMC Res Notes. 2014;7(1):88. doi:10.1186/1756-0500-7-88
34. Ridley JW. Parasitology for Medical and Clinical Laboratory Professionals. Delmar Cengage Learning; 2012.
35. Alemayehu B, Tomass Z, Wadilo F, Leja D, Liang S, Erko B. Epidemiology of intestinal helminthiasis among school children with emphasis on schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health. 2017;17(1):587. doi:10.1186/s12889-017-4499-x
36. Brooker S, Clements ACA, Bundy DAP. Global epidemiology, ecology and control of soil-transmitted helminth infections. In: Advances in Parasitology. Vol. 62. Elsevier;2006:221–261. doi:10.1016/S0065-308X(05)62007-6
37. Blum AJ, Hotez PJ, Liang S. Global “worming”: climate change and its projected general impact on human helminth infections. Liang S, ed. PLoS Negl Trop Dis. 2018;12(7):e0006370. doi:10.1371/journal.pntd.0006370
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
