Back to Journals » Clinical Interventions in Aging » Volume 16
Prevalence, Incidence, and Characteristics of Adverse Drug Reactions Among Older Adults Hospitalized at Mbarara Regional Referral Hospital, Uganda: A Prospective Cohort Study
Authors Yadesa TM , Kitutu FE, Tamukong R, Alele PE
Received 2 August 2021
Accepted for publication 15 September 2021
Published 22 September 2021 Volume 2021:16 Pages 1705—1721
DOI https://doi.org/10.2147/CIA.S332251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Tadele Mekuriya Yadesa,1– 3 Freddy Eric Kitutu,4,5 Robert Tamukong,1,3 Paul E Alele6
1Department of Pharmacy, Mbarara University of Science and Technology, Mbarara, Uganda; 2Department of Pharmacy, Ambo University, Ambo, Ethiopia; 3Pharmacy Biotechnology and Traditional Medicine Center, Mbarara University of Science and Technology, Mbarara, Uganda; 4Department of Pharmacy, Makerere University, Kampala, Uganda; 5Sustainable Pharmaceutical Systems (SPS), Makerere University, Kampala, Uganda; 6Department of Pharmacology and Therapeutics, Mbarara University of Science and Technology, Mbarara, Uganda
Correspondence: Tadele Mekuriya Yadesa
Department of Pharmacy, Mbarara University of Science and Technology, P.O.Box 1410, Mbarara, Uganda
Tel +256753312571
Email [email protected]
Background: Adverse drug reactions (ADRs) are associated with significant clinical and economic effects. Among the elderly population, the risk for ADRs is even higher. Data of ADR prevalence and incidence among the elderly population in Uganda and many low- and middle-income countries are lacking.
Objective: This study determined the prevalence, incidence, and characteristics of ADRs among hospitalized elderly patients at Mbarara Regional Referral Hospital (MRRH), Uganda.
Methods and Materials: We conducted a prospective cohort of older adults admitted to medical, oncology, and surgery wards at MRRH for consecutive 6 months. The primary data were obtained by interviewing patients and caregivers and reviewing patient medication charts, taking vital signs, and physical examinations. We used Edwards and Aronson’s definition of ADR and the Naranjo ADR Causality Scale. We conducted descriptive statistics and the Kolmogorov–Smirnov test using SPSS Version 23.0.
Results: We studied a total of 523 older adults 60 to 103 years of age. During their hospital stay, 256 (48.9%) of the patients experienced at least one ADR. A total of 365 ADRs were identified during 4702 person-days of follow-up. The incidence of ADRs was 78 ADRs/1000 person-days. ADRs affecting the gastrointestinal tract were the most frequently (40.6%) identified categories. Probable and type A ADRs accounted for 260 (71.2%) and 305 (83.6%) of the total incidents, respectively. Overall, 237 (64.9%) of the ADRs were rated as mild, whereas 10 (2.8%) of them as severe. Lastly, 165 (45.2%) of the ADRs were categorized as preventable.
Conclusion: Almost half of the hospitalized patients aged 60 to 103 years experienced at least one ADR during their hospital stay, which is higher than has been previously documented. Almost three-thirds of the ADRs were probable, about 4 out of 5 were type A and almost two-thirds were mild. Nearly half of the ADRs were preventable.
Keywords: prevalence, incidence, mechanism, severity, preventability, adverse drug reaction, elderly, inpatients
Introduction
An adverse drug reaction (ADR) may be defined as an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product.1 Both the United Nations and Ugandan Ministry of Gender, Labour and Social Development define the elderly as the age group of 60 years and older.2,3 The life expectancy in Uganda has increased from 44 years in 1995 to 63 years in 2019.4 Additionally, global estimates predict that the proportion of the population 60 years or older will nearly double from the 2015 levels of 12% to 22% in 2050. The majority (80%) of these will be living in low- and middle-income countries.5
Elderly people undergo various physiological and other body changes that alter their pharmacokinetics and pharmacodynamics of drugs making them highly susceptible to experience ADRs. Frail patients of 60 years and older experience a significantly higher incidence of ADRs.6,7 Moreover, the common occurrence of multiple chronic diseases in the elderly necessitates polypharmacy, which increases the risk of ADRs by multiplying the probability of drug interactions.8–11
Systematic reviews that involved hospitalized older patients across the globe reported ADR prevalences of 11.5–24%.12–15 A study conducted in the US and another from Ethiopia as well as a systematic review that included many studies across the world showed that about 10% of hospital admissions worldwide are related to ADRs.16–18 In the US, approximately 100,000 emergency hospital admissions of older adults were attributed to ADRs every year.19 In UK, a projected annual cost of 847 million US$ and fatality of 0.15% was attributed to ADR-related hospital admissions.20
The safety data collected during the premarketing phase are inadequate due to the shorter study period, and the omission of unique groups of patients including the elderly.21 The routine ADR reporting system is poor at identifying patient safety incidents, particularly those resulting in harm.16 The hospital reporting systems significantly underestimate the incidence of ADRs in older populations, up to 100% under-reporting rate in the UK16,22 and 77.2% in Uganda.23 Thus, information about infrequent but severe ADRs as well as chronic toxicity and drug interactions often remains concealed. To this effect, pharmacovigilance studies are crucial for the identification of rare but serious ADRs among the elderly population.24
The classes of medications associated with ADRs are those that are commonly used during hospitalization.19 The most commonly implicated drug classes in ADRs among hospitalized older patients include cardiovascular agents,9,14,25–32 drugs acting on the nervous system (particularly NSAIDs, opioids, anticonvulsants, and antipsychotics),9,14,27,29,31–33 anti-infective agents25–27,30,33 and antineoplastic agents.31,33,34
ADRs are difficult to detect in older patients.35 There are numerous methods available to evaluate the likelihood that observed adverse events are due to a particular drug.36 The Naranjo algorithm, which is among the most commonly used algorithms, has a high correlation and agreement of ADR scores with the other commonly used methods like Kramer et al.36 Another recent study also showed that both the WHO causality rating criteria and the Naranjo algorithm showed similar causality ratings.37 In order to improve the identification of ADR in older adults, it is recommended for clinicians to have a high suspicion index for ADRs, as part of the differential diagnoses.38
The 2014 Ugandan National Housing and Population census estimated the elderly population (≥60 years) at over 1.2 million (3.7%).39 The Ugandan elderly population was projected to be 1.6 million (5%) in 2019 and to 5.5 million by 2050.2 Two studies from Uganda reported prevalences of 49.5%40 and 25%41 for suspected hospital-acquired ADRs. However, neither of the studies reported the prevalence of ADR among the older adult sub-group.
Previous study in Uganda reported up to half of the general adults experienced ADRs during hospitalization.40 However, evidence of ADR prevalence and incidence specifically among the elderly inpatient population in low- and middle-income countries12 including Uganda, more so from prospective cohort studies, is lacking. We, therefore, conducted a study among elderly inpatients at Medical, Oncology, and Surgical Departments at Mbarara Regional Referral Hospital to determine the prevalence and incidence of suspected ADRs. We also rated the causality of the suspected ADRs and characterized them by body system affected, causality, mechanism of occurrence, severity, preventability, and suspected causative drugs of the ADRs.
Methods and Materials
Study Setting and Period
This study was conducted at Mbarara Regional Referral Hospital (MRRH), a government-owned referral hospital, which is the largest public hospital in southwest Uganda with a 600-bed capacity. Currently, the hospital serves a population of over four million people in its catchment area including the districts of Mbarara, Bushenyi, Ntungamo, Kiruhura, Ibanda, Buhweju, Rubirizi, Mitooma, and Isingiro. The hospital also receives patients from Kabale, Masaka, Fort Portal, and neighboring countries like Rwanda and Tanzania. The hospital consists of the following wards: Emergency and Critical Care, Oncology, Medicine, Surgery, Gynecology and Obstetrics, Psychiatry, Pediatrics, and TB, in addition to several outpatient clinics.42 Most of the hospital services, in all units, are attended by postgraduate students and senior staff of Faculty of Medicine of Mbarara University of Science and Technology. The current study was conducted from 9th of November 2020 to 7th of May, 2021 for 6 consecutive months.
Study Design
We conducted a prospective observational study.
Study Population
All patients 60 years and older that were admitted to Medical, Oncology, and Surgery wards of MRRH during the study period who gave their informed consent were considered for sampling. We excluded patients suspected or confirmed of poisoning or overdose with medications before admission. We also excluded patients that died or were discharged in less than 48 hours of admission as well as those with pertinent laboratory and diagnostic test results not available within 48 hours of admission. We also excluded patients who were unable to make an effective interview: unconscious or in any level of coma, acute psychiatric condition, and hearing or speech impairment.
Sample Size
We used EPI INFOTM Version 7.2.3.1 employing Kelsey's formula at a confidence level of 95% and power of 80%; and prevalence from the previous study in a similar setting,26 the minimum sample size required was 500 for polypharmacy and between 84 and 500 for all other variables. We added 10% for possible non-response, incomplete patient files, and too early discharge of the patients, resulting in a target sample size of 556. Thus, in practice, we studied 523 patients achieving a 2-sided margin of error of less than 5% (4.3%).
Sampling Technique
Every day during the study period, in each ward, the files of newly admitted patients were assigned consecutive numbers starting from one in a sequence of times of their admission. From MRRH records of the previous year, 1st of November 2019 to 30th of April 2020, an estimate of 1150 patients 60 years and older were admitted at Medical, Oncology, and Surgery wards. Thus, to achieve a sample of 556, we used simple random sampling to select half of the daily admissions in each ward. We generated the random numbers using MS-Excel version 2016. Every patient had an equal chance of selection of 50%. We continued the same procedure until the target sample size of 556 was achieved.
Data Collection
The principal investigator trained the research team consisting of four research assistants who pharmacists pursuing master’s program in clinical pharmacy and two physicians who were final year master of medicine students in internal medicine. A pre-test study was conducted and data collection tools modified based on the experiences. Every week from Monday to Saturday between 9:00 am and 5:00 pm, we selected patients and obtained informed consent from each selected patient before enrollment.
First, on the day of enrollment, the trained research assistants collected data using the pre-tested structured questionnaire from patients and caregivers. These data included socio-demographics characteristics, social drug use, medical and medication history, drug allergies, use of over-the-counter and herbal medicines.
Second, we reviewed patients’ medical records for working diagnosis, previous allergies, and clinical and laboratory data within 48 hours of admission. The data on previous and current medications were obtained from the patient’s clinical notes, treatment sheets, drug administration charts, pill count validation, inspecting for leftover medicines, and through interviewing the patient/caregiver or ward staff.
Third, every day during their hospital stay, patients were interviewed, and their information was updated. Results of diagnostic and laboratory tests as well as vital statistics, such as body weight, height, body temperature, blood pressure, respiratory rate, pulse rate and pain scale, were documented at admission and followed up until discharge. The team’s physicians helped in conducting physical assessments, and interpreting clinical, laboratory and diagnostic data when necessary.
Then, the principal researcher (TMY) monitored each patient for adverse-events. The team’s physician (a senior physician) independently and blindly reviewed all cases with suspected adverse events and randomly selected 20% of those without suspected adverse events to replicate or even expand the principal researcher’s detection. All adverse events suspected by the principal investigator and the physician were considered for ADR causality rating and discussion by the team.
Preliminary Review and Identification and Characterization of Suspected ADRs
On admission, all the patients were screened for any community acquired ADRs that were excluded from the final analysis. In this study, we defined hepatitis as an increase of AST or ALT value of at least 2 times the upper limit normal. CNS toxicity meant any nightmares, dizziness, insomnia, nervousness, lack of concentration, depression, suicidal ideation, or psychosis. Renal failure was defined as eGFR decline to less than 60 mL/min/1.73 m2 or any increase of serum creatinine by 0.3mg/dL from baseline or reaching 1.5mg/dL. Hypotension was defined as systolic blood pressure <90mmHg or diastolic blood pressure <60 mmHg. We identified hypertension when systolic blood pressure was ≥140 mmHg or diastolic blood pressure ≥90mmHg. Extrapyramidal reaction was detected when one or more of dystonia, akathisia, parkinsonism, and tardive dyskinesia occurred. Hypoglycemia was defined as plasma glucose less than 55 mg/dL or 3 mmol/L with or without clinical symptoms. Constipation was defined as no bowel movement for at least 72 hours or less than three bowel movements per week with any two of the following features: straining, lumpy hard stools, the sensation of incomplete evacuation, use of digital maneuvers, the sensation of anorectal obstruction with 25% of bowel movements.43,44 We used different methods to identify and characterize the identified ADRs.
We employed Edwards and Aronson’s definition of ADR1 as presented above. The known adverse reaction profile of each drug was evaluated based on Ugandan Clinical Guidelines (UCG, 2016), British National Formulary (BNP),45 and Up-To-Date (2019) version 3.12.0.44 ADRs were first suspected when there is a relationship between the time of drug administration and the onset and course of the adverse reaction while excluding other potential causes.46
Second, the rating of the causal relationship between an ADR and the suspected medication was done using the Naranjo ADR assessment scale.47 We excluded all doubtful ADRs, whereas we considered those rated as possible, probable or definite for discussion and verification by the team of experts. The team of experts consisting of the principal investigator (senior clinical pharmacist), another senior pharmacist and a senior physician met daily to discuss the causality of the suspected ADRs and whenever consensus was not reached, a majority decision of the three members was applied.
Third, the principal investigator categorized the body system affected by ADRs using the International Statistical Classification of Diseases for Mortality and Morbidity Statistics (ICD-11 MMS).48 The identified ADRs were categorized as type A (dose dependent, augmented pharmacological and predictable reactions) and type B (bizarre, dose independent and non-predictable reactions) according to the Rawlins and Thompson classification method49 and the ABCDEF that added type C (dose and time dependent or chronic reactions), type D (delayed reactions), type E (withdrawal reactions), and type F (failure of therapy) to Rawlins and Thompson classification.50
Fifth, the severity of ADRs was determined by using the modified Hartwig and Siegel criteria, which has 7-items and 3 categories of severity: mild, moderate and severe ADRs.51 Sixth, ADRs were assessed for preventability using 9-item Schumock and Thornton criteria which categorizes ADRs in to “definitely preventable”, “probably preventable” or “not preventable”.52 Lastly, the medications implicated in the suspected ADRs were classified according to the WHO-Anatomical Therapeutic Chemical (ATC) classification,53 whereas Lexicomp software was used to detect potential drug–drug interactions.
Data Analysis
The data were entered and cleaned by EpiInfo version 7.2.3.1 and then transferred to and analyzed by IBM Statistical Package for the Social Sciences (SPSS version 23.0 Inc., Chicago, Illinois). The prevalence of ADR was calculated by dividing the total number of patients that incurred at least one ADR by the total number of participants. We calculated the total person-days of the study by adding the hospital stay duration (days) of all participants. We calculated incidence by dividing the total number of ADR incidents by the total person-days. Descriptive statistics were employed to analyze the frequency and percentages of causality, types, mechanism, severity, and preventability of ADRs as well as the medications implicated in the suspected ADRs. The Kolmogorov–Smirnov test was used to determine the distribution of the variables. Continuous variables without normal distribution were summarized using the median and interquartile range.
Data Management and Quality Assurance
The collected data were checked daily for completeness and consistency by the principal investigator. The principal investigator supervised the data collection process daily and gave necessary support to the research assistants who were final year master’s students in clinical pharmacy. All the decisions of the principal researcher were discussed among the team of experts consisting of the principal investigator, the second clinical pharmacist and a physician, to reach a consensus. Completed individual patient data were anonymously passed to the data manager. Data were double-entered, cross-checked, and password-protected. The data collection tools were pre-tested among 25 older adults by the trained research assistants and revised accordingly.
Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki.54 Approval to conduct the study was obtained from Mbarara University Institutional Research Ethics Committee (Reference No: MUREC 1/7) and Uganda National Council for Science and Technology (Reference No: HS992ES). We obtained site clearance to conduct the study from MRRH. We informed the participants about the study’s objectives, benefits and risks, and that their personal information would be kept confidential. We also explained their rights to autonomy, and the right to decline or to withdraw from the study. We obtained written informed consent from each participant before enrollment in this study. Data collection processes strictly adhered to Ministry of Health guidelines and Government directives for the prevention of COVID-19 transmission.
Results
Participants’ Characteristics
Out of the 1185 older patients admitted during the study period, we approached 556 and enrolled 548 while eight declined to give consent. Later, 25 of them were discharged or died in less than 48 hours and thus, 523 were included in the final analysis with a response rate of 98.6% (Figure 1).
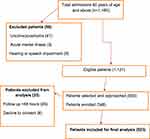 |
Figure 1 Study recruitment process of hospitalized older patients at MRRH, Uganda. |
The study patients aged 60 to 103 years; with median age of 67 (62–76) years and 269 (51.4%) of them were males. The majority (338, 64.6%) had at least one comorbid condition. Almost one-third (30.0%) stayed in the hospital for more than 10 days with a median (IQR) of 8(4–12) days (Table 1). Using ICD-11 classification of diseases, infectious diseases (ICD class-01) were the most prevalent (39%) conditions diagnosed during the current hospitalization followed by neoplasms (36.1%) (Figure 2). Anti-infective agents were used by 373 (71.3%) of the patients followed by nervous system drugs that were used by 312 (59.7%) (Figure 3).
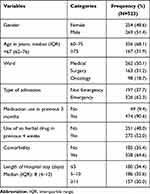 |
Table 1 Patient Characteristics of Older Adults 60 Years and Above Admitted at MRRH, Southwestern Uganda from November 2020 to May 2021 |
 |
Figure 2 Documented medical, surgical, and oncologic conditions among older adults 60 years and above admitted at MRRH, southwestern Uganda from November 2020 to May 2021. |
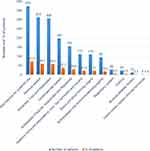 |
Figure 3 Medications used by older adults 60 years and above admitted at MRRH, Southwestern Uganda from November 2020 to May 2021. |
Prevalence and Incidence of Adverse Drug Reactions
A total of 256 patients out of 523 experienced at least one ADR incident during their hospital stay, giving a prevalence of 48.9% (95% C.I: 44.6%, 53.2%) (Figure 4). Out of the 256 patients that experienced ADRs, 171 (66.8%) experienced one ADR, while 61 (23.8%) and 24 (9.4%) of them incurred two, and three incidents of ADRs, respectively. The incidence of ADR was shown to be 78 ADRs/1000 person-days (Table 2).
 |
Table 2 The Incidence of Adverse Drug Reactions Among Older Adults 60 Years and Above Admitted at MRRH, Southwestern Uganda from November 2020 to May 2021 |
 |
Figure 4 The prevalence of adverse drug reactions among older adults 60 years and above admitted at MRRH, southwestern Uganda from November 2020 to May 2021. |
Number of Adverse Drug Reactions
Causality Rating of the ADRs
By applying the Naranjo ADR causality rating scale, out of 365 ADRs, 260 (71.2%) were rated as probable, whereas 101 (28%) and 4 (0.8%) were rated as possible and definite ADRs, respectively.
Types of Adverse Drug Reactions Detected and the Body Systems Affected
Accounting for 40.6% of the totality, ADRs affecting the gastro-intestinal tract were the most frequently identified types followed by ADRs involving the central nervous system (20.5%), endocrine and metabolic system (8.8%), and cardiovascular system (8.5%). The most frequently detected specific ADRs were constipation,42 nausea,38 nausea and vomiting,36 dizziness,29 drowsiness,16 hypotension,16 and hypoglycemia13 (Table 3).
 |
Table 3 The Categories of ADRs Detected Among Older Adults 60 Years and Above Admitted at MRRH, Southwestern Uganda from November 2020 to May 2021 |
Mechanism, Severity, and Preventability of the Suspected Adverse Drug Reactions
A total of 305 (83.6%) of the ADRs were categorized as “Type A” followed by 46 (12.6%) “Type B” and 6 (1.6%) ‘Type C ADRs. On the other hand, 237 (64.9%) of the ADRs were mild, whereas 118 (32.3%) and 10 (2.8%) were moderate and severe, respectively. Out of the 10 patients that experienced severe ADRs, 4 died, 2 recovered with a complication and 4 recovered without a complication. Lastly, 165 (45.2%) of the ADRs were preventable; 107 (29.3%) of them being definitely preventable. There was a previous ADR or allergy to the drug in 47 (43.9%), and the drug was inappropriate in 39 (36.5%) of the definitely preventable ADRs. Monitoring tests were not performed in 22 (37.9%), and drug interaction was involved in 17 (29.3%) of the probably preventable ADRs (Table 4).
 |
Table 4 The Characteristics of the Detected ADRs Among Older Adults 60 Years and Above Admitted at MRRH, Southwestern Uganda from November 2020 to May 2021 |
Potential Causative Drugs of the Adverse Drug Reactions
Out of the 365 ADRs detected, cardiovascular drugs and nervous system drugs were implicated in 76 (20.8%) and 75 (20.5%) of the ADRs, respectively. Sixty-three (84%) of the culprit nervous system drugs were analgesics. Constipation attributed to tramadol,25 electrolyte disorders by furosemide,14 and hypotension due to antihypertensives10 were the most notable ADRs by these two classes of drugs. Antipsychotics were implicated in four episodes of extrapyramidal reactions. Anti-infectives and antineoplastic and immunomodulating agents were implicated for 71 (19.4%) and 69 (18.9%) of the ADRs, respectively. Ceftriaxone alone contributing to 51 ADRs by anti-infectives and docetaxel to 12 ADRs from antineoplastic agents (Table 5).
 |
Table 5 The Drugs Implicated in the Identified ADRs and the Associated Specific ADRs Among Hospitalized Older Adults, MRRH, Uganda |
Discussion
In the current study, we achieved a very high (98.6%) response rate. This may be attributed to absence of any risk associated with the study and an extensive explanation about the procedures, risks and benefits of the study before consent was requested. Almost half (256, 48.9%) of the participants encountered at least one ADR incident during their hospital stay. The current prevalence of ADRs in hospitalized older patients is considerably higher compared to prevalences from high-income countries including 6% in Canada,55 6.5% in Italy,56 13% in the UK,27 15% in Japan,9 26% in Ireland,29 25.9% in Germany57 and 26.2% in Australia.15 The current prevalence is also higher compared to studies from middle-income countries including 10.7% in Pakistan,26 18% in Brazil,58 30% in Malaysia,28 and 32% in India.59
The current prevalence is also higher than the mean prevalence of ADR ranging from 11.5% to 24% reported by previous systematic reviews.13,14,18 All of the previous studies conducted in this setting are from middle income or high-income countries. This may be explained by absolute lack of active clinical pharmacovigilance in our setting as compared to the previous studies as none of them was conducted in low-income country or in sub-Saharan Africa. To this effect, 45.2% of the reported ADRs could have been prevented if physicians or pharmacists daily checked for and avoided the known risk factors of ADRs like a drug that had previously caused allergy or ADR and inappropriate medications, or if they regularly reported the safety monitoring tests. Studies in Uganda showed many challenges in the pharmacovigilance system including lack of training and unfamiliarity with the pharmacovigilance system, lack of necessary funding, inadequate number of trained staff, scarce training programs, indistinct roles, and poor coordination of activities as well as lack of capacity to monitor medicines and evaluate risks as well as under-reporting of ADRs.23,60 Patient factors contributing to more ADR may include lack of access to safe and effective medicines as well as inability to afford the necessary laboratory tests done for monitoring of medication safety.
The current higher prevalence may also be explained by our prospective study design and our ADR identification method that involved daily patient interviews and physical examination to detect ADRs in addition to reviewing patient records. As discussed above, previous studies showed that prospective observational studies18,61 and studies employing patient interviews in addition to reviewing patient records62 detected more ADRs compared to retrospective studies and studies that solely depended on medical records. Moreover, being conducted in a referral hospital might have resulted in a higher prevalence of ADRs as compared to some of the previous studies that were done in lower-level facilities. This may be related to a more severe and complex comorbidity as well as a wider range of available and prescribed medications including cancer chemotherapy.
On the other hand, the current prevalence is comparable with 46% from a study in Belgium32 and lower than the prevalence of 64% from India.34 The current prevalence is also comparable with the 49.5% prevalence of ADR among hospitalized general adults in Uganda.40 However, our finding was higher than an incidence of 25% among adult inpatients in Mulago hospital.41 The deviation is likely due to older adults having a higher risk of ADR because of physiologic changes resulting in pharmacokinetic variations of medicines that make them more susceptible to ADRs and often have more comorbidities and a higher number of concurrent medications.6,8,9,29,63
Similarly, the current incidence of 78 ADRs per 1000 person-days is higher than the incidence of 15.2 per 1000 person-years reported by a study in Canada55 and the incidence of 0.23 ADRs/admission in England.16 This difference is probably because Sikdar et al employed a retrospective study design and both Sikdar et al and Sari et al solely depended on the patient records, whereas our study was a prospective cohort and involved daily patient interviews and physical examination to detect ADRs in addition to reviewing patient records. Using medical record review and patient interviews detects considerably more ADRs than sole medical record review.62 Previous systematic reviews revealed that prospective observational studies have a higher ADR detection rate than retrospective studies because in prospective studies, in addition to reviewing the medical records, participants are also interrogated and observed.18,61
Out of the 365 ADRs identified, most (260, 71.2%) were rated as probable, 101 (27.7%) as possible, and 4 (1.1%) as definite ADRs using the Naranjo ADR causality scale. This result is comparable with several previous studies that revealed most of the ADRs reported among hospitalized older adults were probable including 76.5% in Australia,15 71% in Belgium,32 67% in Germany,57 66% in Ireland29 and 62–72% in India.28,59
ADRs affecting the gastro-intestinal tract were the most frequently (40.6%) identified categories followed by ADRs involving the central nervous system (20.5%), endocrine and metabolic system (8.8%), and cardiovascular system (8.5%). These results are in line with findings of previous studies that had shown ADRs affecting endocrine and metabolic system,9,25,29,30 gastrointestinal tract,9,25,30,34,64 nervous system9,29,30 and cardiovascular system29,64 to be the top three most commonly encountered types among elderly inpatients. The most frequently detected specific ADRs were constipation,42 nausea,38 nausea and vomiting,36 dizziness,29 drowsiness,16 hypotension,16 and hypoglycemia.13
The largest proportion of ADRs affecting the gastrointestinal tract was constipation (mostly associated with three nervous system agents: tramadol, morphine, and diclofenac) and nausea with or without vomiting, which was particularly associated with anti-infective/anti-parasitic and anti-neoplastic agents. Similarly, most of the ADRs affecting the nervous system were dizziness (mainly associated with anti-infective agents and cardiovascular drugs) and drowsiness (mainly associated with nervous system agents).
On the contrary, a study in India,34 as opposed to the current study and the majority of the literature, showed dermatologic system and the hematologic system as the most commonly affected by ADR next to the gastro-intestinal tract. This deviation is probably because the former solely conducted the study among cancer patients and thus, dermatological ADRs like alopecia and hematologic ADRs like bone marrow suppression were highly prevalent because of the widespread use of chemotherapeutic agents by the participants.
Most (83.6%) of the ADRs were categorized as “Type A” followed by “Type B” (12.6%) and ‘Type C (1.6%). The type C ADRs occurred during hospitalization were related to longer hospitalization of up to 34 days of the study patients. We also included ADRs like extrapyramidal reactions with antipsychotics and peripheral neuropathy with isoniazid, which occurred after hospitalization from a drug, which had been initiated before the current admission. The type F ADR that was identified in this study was treatment failure (hyperglycemia) related to dexamethasone for a patient whose fasting blood sugar was previously well controlled with insulin therapy.
The current percentage of type A ADRs is slightly lower compared to proportions of 92%59 and 94%27 type-A ADRs though both of these studies also used Rawlins and Thompson classification. This difference is likely because we employed the ABCDEF classification that added type C (dose and time dependent or chronic reactions), type D (delayed reactions), type E (withdrawal reactions), and type F (failure of therapy) types of ADRs to merely type A and B of Rawlins and Thompson classification,50 which was used by the previous studies. This resulted in the categorization of some ADRs into type C(6) E(5) and F(3) which would have been, otherwise, fallen under the type A category.
On the other hand, the proportion of type B ADRs was slightly higher compared to 6% in the UK27 and 8% in India59 probably because of our higher study population, which may have resulted in the disproportionate increase of type B ADRs as these ADRs occur rarely and are often underestimated in smaller studies. This deviation might also be explained by the involvement of patients on cancer chemotherapy that consisted of combinations of several agents associated with hypersensitivity reactions. Moreover, we might have detected type B ADRs like mild hypersensitivity reactions, fever, and skin rashes more than previous studies because used patient interview as opposed to merely reviewing medical records for ADR identification.
Almost two-thirds (64.9%) of the ADRs were mild, whereas 32.3% and 2.8% were moderate and severe, respectively. The current proportion of severe ADRs is comparable with results from two studies from India that reported 0% and 1%; however, considerably lower than proportions from high-income countries (HICs) that ranged from 9% to 72%.15,25,27,29,56,57 This difference may be related to having more complex comorbidity, advanced age, and more access to newer medications in HICs. A previous systematic review showed that the proportion of severe ADRs was higher in HICs and in patients with greater comorbidity who take more medications.61 The exclusion of patients who died or were transferred to ICU within 48 hours of admission, possibly associated with ADRs, might have reduced the proportion of severe ADRs. Likewise, delays and inconsistencies of diagnostic tools and laboratory tests in our setting might have concealed potential severe ADRs, including renal failure, hepatotoxicity, electrolyte imbalance, bleeding. Additionally, the differences between the methods used to grade the severity of the ADRs may have added to the differences.
Nearly half (45.2%) of the ADRs were preventable; 15.9% were probably preventable, whereas 29.3% were definitely preventable provided that active risk identification and intervention had been done by prescribers, pharmacists, or nurses. From the current findings, avoiding drugs that had previously caused an ADR, avoiding medications that are inappropriate to the patient, optimizing the dosage regimen, conducting the necessary monitoring tests regularly, and checking for significant drug interactions are the most important interventions to prevent ADRs. This result is comparable with 48% reported by a study in India.59 However, this proportion of preventable ADRs is lower compared to proportions ranging from 63% to 80% in studies of HICs.15,27,32,57 This is probably because the proportion of severe ADRs was higher in those studies and it had been established that severe ADRs are more preventable than mild ADRs.65
Cardiovascular drugs (20.8%) and drugs acting on the nervous system (84% of which were analgesics) (20.5%) were shown to be the most common causes of ADRs. Constipation attributed to tramadol,25 electrolyte disorders related to furosemide,14 and hypotension associated with antihypertensives10 were the most notable ADRs among others. These two classes were the fourth (used by 197 patients) and the second (used by 312 patients) most commonly used, respectively.
These findings are comparable with several previous studies that reported cardiovascular agents,9,14,25–32 drugs acting on the nervous system (particularly NSAIDs, opioids, anticonvulsants, and antipsychotics),9,14,27,29,31–33 anti-infective agents25–27,30,33 and antineoplastic agents31,33,34 as among the top three classes of drugs most frequently implicated in ADRs among hospitalized older patients.
From the descriptive statistics, a relationship was observed between how frequently each class of drug was used and how much of the ADRs were attributed to the respective class except for cardiovascular agents and antineoplastic drugs that were implicated in a disproportionally higher proportion of ADRs based on their frequency of use. This is probably because older patients are more susceptible to the ADRs commonly associated with these classes of medications like CNS side effects, renal toxicity, hypotension, electrolyte disturbances, and bone marrow suppression. With aging, physiological change and renal impairment lead to altered pharmacokinetic and pharmacodynamic changes of drugs and making the older adults highly susceptible to adverse drug reactions.6
On the other hand, the proportion of ADRs related antithrombotic agents (2.7%) is considerably lower in our study compared to the previous studies.9,14,25,28,32 This can be explained by a limitation of delays and inconsistencies in the use of coagulation tests to monitor for the ADR of these agents in our setting. This problem was observed to be widespread and was attributed to the unavailability of the tests as well as the inability of patients to afford to pay the fee out of pocket.
The strengths of the current study include being prospective design, random sampling technique, and having team of experts that comprised of senior clinical pharmacists and physicians. On the other hand, the study had some limitations. First, the unavailability of some essential laboratory or diagnostic tests that might result in missing certain types of ADRs. Secondly, being conducted at a regional referral hospital might reduce the generalizability of the results in lower-level health facilities where some classes of drugs or specific agents that have been implicated in the currently reported ADRs are not available. Thus, we recommend the future study to include different levels of facilities, be multi-centered, and be conducted for a longer period. These findings should, therefore, be carefully interpreted as the study is a single centered and conducted only for a period of 6 months.
Studies focusing on predictors of ADRs are crucial to develop preventive strategies which help to mitigate the burden of ADRs.66,67 However, such studies are scarce in low- and middle-income countries.61 These findings therefore provide a basis for future studies to develop, adapt and test context-relevant ADRs prediction models to improve patient safety and the medicine experience among the increasing elderly population in Uganda and other low- and middle-income countries.
Conclusion
Half of hospitalized older patients experienced at least one ADR during their hospital stay, whereas nearly one in ten experienced an ADR per day during hospitalization. The current ADR prevalence and incidence are considerably higher compared to results from high-income countries and slightly higher than those from middle-income countries. Almost three-thirds of the ADRs were rated as probable. ADRs affecting gastro-intestinal tract, central nervous system, endocrine and metabolic system, and cardiovascular system were the top-four most frequently identified categories. About 4 out of 5 of the ADRs were type A, whereas almost two-thirds were mild. Nearly half of the ADRs were preventable. Responsible for about one-fifth of all ADRs each, cardiovascular drugs and drugs acting on the nervous system were drug classes most frequently implicated in ADRs. Studies to develop and test context-relevant ADR prediction models among the elderly populations are thus warranted.
Abbreviations
ADR, Adverse Drug Reaction; ART, Anti-Retroviral Therapy; ATC, Anatomical Therapeutic; BNP, British National Formulary; BUN, Blood Urea Nitrogen; CCI, Charlson Comorbidity Index; CI, Confidence Interval; DDI, Drug–Drug Interaction; eGFR, Estimated Glomerular Filtration Rate; ICD, International Statistical Classification of Diseases; MRRH, Mbarara Regional Referral Hospital; MUST, Mbarara University of Science and Technology; REC, Research Ethics Committee; NSAID, Non-Steroidal Anti-Inflammatory Drugs; PIM, Potentially Inappropriate Medications; SPSS, Statistical Package for the Social Science; TB, Tuberculosis; UK, United Kingdom; UNCST, Uganda National Council for Science and Technology; US, United States of America; WHO, World Health Organization.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki (54). The study protocol was approved by the Research and Ethics Committee (REC) of MUST (Letter reference: MUREC 1/7-2020) and registered by UNCST. Then we obtained clearance from MRRH and informed consent from each participant.
Consent for Publication
All authors agreed to the submission of this manuscript for publication in addition to the consent to publish which was included in the informed consent form which attained ethical and participant approval.
Acknowledgments
The authors extend their sincere gratitude towards PHARMBIOTRAC-MUST for funding this study. We would also like to thank the research assistants and physicians: Dr. Joshua Kiptoo, Dr. Kushemererwa Oliver, Dr. John Isiiko, Dr. Bonny Luzze, Dr. Mohammed Mukhtar, Dr. Jacinta Ambaru Ojia, and the adminstrators and workers of MRRH for their assistance and collaboration throughout the study. We also thank the study participants and their caregivers for their collaboration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by PHARMBIOTRAC, one of the ACE-II projects of the World Bank at Mbarara University of Science and Technology.
Disclosure
The authors declare that they have no competing interests.
References
1. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–1259. doi:10.1016/S0140-6736(00)02799-9
2. Uganda Bureau of Statistics. Status of Older Persons in Uganda; MAKING the INVISIBLE VISIBLE. Kampala, Uganda; 2019.
3. United Nations. World Population Ageing. ST/ESA/SER.A/348 ; 2013.
4. World Bank. World population prospects: 2019 Revision, life expectancy at birth 2019. Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=UG.
5. World Health Organisation. Ageing and health 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text. Accessed February 11, 2021.
6. Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi:10.1046/j.1365-2125.2003.02007.x
7. Wang Y, Zhang R, Shen Y, Su L, Dong B, Hao Q. Prediction of chemotherapy adverse reactions and mortality in older patients with primary lung cancer through frailty index based on routine laboratory data. Clin Interv Aging. 2019;14:1187–1197. doi:10.2147/CIA.S201873
8. Khandeparkar A, Rataboli P. A study of harmful drug–drug interactions due to polypharmacy in hospitalized patients in Goa Medical College. Perspect Clin Res. 2017;8:180. doi:10.4103/picr.PICR_132_16
9. Kojima T, Matsui T, Suzuki Y, et al. Risk factors for adverse drug reactions in older inpatients of geriatric wards at admission: multicenter study. Geriatr Gerontol Int. 2019;20:144.
10. Aguiar JP, Heitor Costa L, Alves da Costa F, Leufkens HG, Martins AP. Identification of potentially inappropriate medications with risk of major adverse cardiac and cerebrovascular events among elderly patients in ambulatory setting and long-term care facilities. Clin Interv Aging. 2019;14:535–547. doi:10.2147/CIA.S192252
11. Lin HQ, Wu JY, Chen ML, et al. Prevalence of dyslipidemia and prediction of 10-year CVD risk among older adults living in southeast coastal regions in China: a cross-sectional study. Clin Interv Aging. 2019;14:1119–1129. doi:10.2147/CIA.S207665
12. Yadesa TM, Kitutu FE, Deyno S, Ogwang PE, Tamukong R, Alele PE. Prevalence, characteristics and predicting risk factors of adverse drug reactions among hospitalized older adults: a systematic review and meta-analysis. SAGE Open Med. 2021;9:20503121211039099. doi:10.1177/20503121211039099
13. Jennings EL, Murphy KD, Gallagher P, O’Mahony D. In-hospital adverse drug reactions in older adults; prevalence, presentation and associated drugs—a systematic review and meta-analysis. Age Ageing. 2020;49(6):948–958. doi:10.1093/ageing/afaa188
14. Jennings E, Murphy K, Gallagher P, O’Mahony D. Adverse drug reactions in hospitalised older adults - a systematic review. Age Ageing. 2018;47(suppl_5):v13–v60.
15. Alhawassi TM, Krass I, Pont LG. Prevalence and risk factors for adverse drug reactions in older adults in the acute care setting. Pharmacoepidemiol Drug Saf. 2015;24(Suppl. 1):91.
16. Sari -AB-A, Sheldon TA, Cracknell A, Turnbull A. Sensitivity of routine system for reporting patient safety incidents in an NHS hospital: retrospective patient case note review. BMJ. 2007;334(7584):79. doi:10.1136/bmj.39031.507153.AE
17. Angamo M, Curtain C, Chalmers YD, Bereznicki L. Predictors of adverse drug reaction-related hospitalisation in Southwest Ethiopia: a prospective cross-sectional study. PLoS One. 2017;12:e0186631. doi:10.1371/journal.pone.0186631
18. Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–2086.
19. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi:10.1056/NEJMsa1103053
20. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi:10.1136/bmj.329.7456.15
21. UMC-WHO. Safety Monitoring of Medicinal Products: Guidelines for Setting Up and Running a Pharmacovigilance Centre. WHO Collaborating Centre for International Drug Monitoring; 2000:28.
22. Barrow P, Waller P, Wise L. Comparison of hospital episodes with ‘drug-induced’ disorders and spontaneously reported adverse drug reactions. Br J Clin Pharmacol. 2006;61(2):233–237. doi:10.1111/j.1365-2125.2005.02554.x
23. Katusiime B, Semakula D, Lubinga SJ. Adverse drug reaction reporting among health care workers at Mulago National Referral and Teaching hospital in Uganda. Afr Health Sci. 2015;15(4):1308–1317. doi:10.4314/ahs.v15i4.34
24. JFDA. Guidelines for detecting & reporting adverse drug reactions: individual case safety reports for healthcare professionals, rational drug use and pharmacovigilance department. Version 1; 2014.
25. Conforti A, Costantini D, Zanetti F, Moretti U, Grezzana M, Leone R. Adverse drug reactions in older patients: an Italian observational prospective hospital study. Drug Healthc Patient Saf. 2012;4:75–80. doi:10.2147/DHPS.S29287
26. Ahmed B, Nanji K, Mujeeb R, Patel MJ. Effects of polypharmacy on adverse drug reactions among geriatric outpatients at a tertiary care hospital in Karachi: a prospective cohort study. PLoS One. 2014;9(11):e112133. doi:10.1371/journal.pone.0112133
27. Tangiisuran B, Davies J, Wright J, Rajkumar C. Adverse drug reactions in a population of hospitalized very elderly patients. Drugs Aging. 2012;29:669–679.
28. Abd NM, Aziz N, Hassan Y, Abdulrazzaq Al-Ani H, Ghazali R, Ali Z. Predictors of polypharmacy and adverse drug reactions among geriatric in patients at Malaysian hospital. Healthmed. 2010;4:273–283.
29. O’Mahony D, Gallagher P, O’Connor MN, Byrne S. Adverse drug reactions in older patients during hospitalisation: are they predictable? Age Ageing. 2012;41(6):771–776. doi:10.1093/ageing/afs046
30. Harugeri APG, Ramesh M, Guido S, Basavanagowdappa H. Frequency and nature of adverse drug reactions in elderly in-patients of two Indian medical college hospitals. J Postgrad Med. 2011;57(3):189–195.
31. Sikdar K, Dowden J, Alaghehbandan R, Macdonald D, Wang P, Gadag V. Adverse drug reactions in elderly hospitalized patients: a 12-year population-based retrospective cohort study. Ann Pharmacother. 2012;46:960–971.
32. De Paepe P, Petrovic M, Outtier L, Van Maele G, Buylaert W. Drug interactions and adverse drug reactions in the older patients admitted to the emergency department. Acta Clin Belg. 2013;68(1):15–21. doi:10.2143/ACB.68.1.2062714
33. Liao P-J, Mao C-T, Chen T-L, Deng S-T, Hsu K-H. Factors associated with adverse drug reaction occurrence and prognosis, and their economic impacts in older inpatients in Taiwan: a nested case–control study. BMJ Open. 2019;9(5):e026771. doi:10.1136/bmjopen-2018-026771
34. Sneha SG, Simhadri K, Subeesh VK, Sneha SV. Predictors of adverse drug reactions in geriatric patients: an exploratory study among cancer patients. South Asian J Cancer. 2019;8(2):130. doi:10.4103/sajc.sajc_218_18
35. Davies EA, O’Mahony MS. Adverse drug reactions in special populations – the elderly. Br J Clin Pharmacol. 2015;80(4):796–807. doi:10.1111/bcp.12596
36. Murayama H, Sakuma M, Takahashi Y, Morimoto T. Improving the assessment of adverse drug reactions using the Naranjo Algorithm in daily practice: the Japan Adverse Drug Events Study. Pharmacol Res Perspectives. 2018;6(1):e00373. doi:10.1002/prp2.373
37. Fasipe O, Akhideno P, Isah A, Owhin O. A prospective study on causality assessment rating, mortality rate, and case fatality rate for adverse drug reactions among medical inpatients at the University of Benin Teaching Hospital, Nigeria. Med J Dr DY Patil Vidyapeeth. 2019;12(5):398–407. doi:10.4103/mjdrdypu.mjdrdypu_224_18
38. Tangiisuran B, Wright J, Van der Cammen T, Rajkumar C. Adverse drug reactions in elderly: challenges in identification and improving preventative strategies. Age Ageing. 2009;38(4):358–359. doi:10.1093/ageing/afp050
39. Uganda Bureau of Statistics. The National Population and Housing Census 2014; 2016.
40. Tumwikirize WA, Ogwal-Okeng JW, Vernby A, Anokbonggo WW, Gustafsson LL, Lundborg SC. Adverse drug reactions in patients admitted on internal medicine wards in a district and regional hospital in Uganda. Afr Health Sci. 2011;11(1):72–78.
41. Kiguba R, Karamagi C, Bird SM. Incidence, risk factors and risk prediction of hospital-acquired suspected adverse drug reactions: a prospective cohort of Ugandan inpatients. BMJ Open. 2017;7(1):e010568. doi:10.1136/bmjopen-2015-010568
42. MRRH Brief History of Mbarara Regional Referral Hospital 2021. Available from: https://mbararahospital.go.ug/.
43. Martin E. Concise Colour Medical Dictionary. Oxford University Press; 2015.
44. UpToDate Contributors. ©UpToDate; 2021.
45. Committee Joint Formulary. British National Formulary; 2019.
46. Handler SM, Hanlon JT, Perera S, Roumani YF, Nace DA. Consensus list of signals to detect potential adverse drug reactions in nursing homes. J Am Geriatr Soc. 2008;56(5):808–815. doi:10.1111/j.1532-5415.2008.01665.x
47. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi:10.1038/clpt.1981.154
48. World Health Organisation. ICD-11 for mortality and morbidity statistics; 2020. Available from: https://icd.who.int/browse11/l-m/en.
49. Rawlins MD. Clinical pharmacology adverse reactions to drugs. Br Med J. 1981;282(6268):974–976. doi:10.1136/bmj.282.6268.974
50. Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003;327(7425):1222–1225. doi:10.1136/bmj.327.7425.1222
51. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232.
52. Schumock G, Thornton P. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538.
53. World Health Organisation. WHO Collaborating Centre for Drug Statistics Methodology; 2020. Available from: https://www.whocc.no/atc_ddd_index/.
54. Association WM. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Bull World Health Organ. 2001;79(4):373.
55. Sikdar KC, Dowden J, Alaghehbandan R, MacDonald D, Peter P, Gadag V. Adverse drug reactions in elderly hospitalized patients: a 12-year population-based retrospective cohort study. Ann Pharmacother. 2012;46(7–8):960–971.
56. Onder G, Petrovic M, Tangiisuran B, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. JAMA Intern Med. 2010;170(13):1142–1148.
57. Dormann H, Krebs S, Muth-Selbach U, et al. Adverse drug reactions in patients with gastroenterological diseases: does age increase the risk? Aliment Pharmacol Ther. 2001;15(2):171–180. doi:10.1046/j.1365-2036.2001.00922.x
58. Galli T, Reis W, Andrzejevski V. Potentially inappropriate prescribing and the risk of adverse drug reactions in critically ill older adults. Pharm Pract (Granada). 2016;14:818. doi:10.18549/PharmPract.2016.04.818
59. Harugeri A, Parthasarathi G, Madhan R, Guido S, Basavanagowdappa H. Frequency and nature of adverse drug reactions in elderly in-patients of two Indian medical college hospitals. J Postgrad Med. 2011;57:189–195. doi:10.4103/0022-3859.85201
60. Maigetter K, Pollock AM, Kadam A, Ward K, Weiss MG. Pharmacovigilance in India, Uganda and South Africa with reference to WHO’s minimum requirements. Int J Health Policy Manage. 2015;4(5):295–305. doi:10.15171/ijhpm.2015.55
61. Angamo MT, Chalmers L, Curtain CM, Bereznicki LRE. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Safety. 2016;39(9):847–857. doi:10.1007/s40264-016-0444-7
62. Kongkaew C, Noyce P, Ashcroft D. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42:1017–1025. doi:10.1345/aph.1L037
63. Gray SL, Sager M, Lestico MR, Jalaluddin M. Adverse drug events in hospitalized elderly. J Gerontol. 1998;53A(1):M59–M64. doi:10.1093/gerona/53A.1.M59
64. Rawat RS. Evaluation of potentially inappropriate medication use and risk of adverse drug reactions in hospitalized older adults: an observational study in a tertiary care hospital. Int J Pharm Res. 2018;11(2):79–85.
65. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi:10.1001/jama.289.9.1107
66. Ogoina D, Obiako RO, Muktar HM, et al. Morbidity and mortality patterns of hospitalised adult HIV/AIDS patients in the era of highly active antiretroviral therapy: a 4-year retrospective review from Zaria, Northern Nigeria. AIDS Res Treat. 2012;2012:940580. doi:10.1155/2012/940580
67. Namme Luma H, Doualla M-S, Choukem S-P, et al. Adverse drug reactions of Highly Active Antiretroviral Therapy (HAART) in HIV infected patients at the General Hospital, Douala, Cameroon: a cross sectional study. Pan Afr Med J. 2012;12:87.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
