Back to Journals » Journal of Inflammation Research » Volume 16
Prevalence, General and Periodontal Risk Factors of Gastroesophageal Reflux Disease in China
Authors Liu Z, Gao X, Liang L , Zhou X, Han X, Yang T, Huang K, Lin Y, Deng S, Wang Z, Wang C
Received 16 November 2022
Accepted for publication 31 December 2022
Published 17 January 2023 Volume 2023:16 Pages 235—244
DOI https://doi.org/10.2147/JIR.S395777
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Zhiqiang Liu,1,* Xiaoli Gao,1,* Lirong Liang,2 Xuan Zhou,1 Xiaozhe Han,3 Ting Yang,4– 7 Kewu Huang,8 Yingxiang Lin,8 Shu Deng,9 Zuomin Wang,1 Chen Wang4– 7,10
1Department of Stomatology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Clinical Epidemiology, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Oral Science and Translational Research, Nova Southeastern University College of Dental Medicine, Fort Lauderdale, FL, USA; 4Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 5National Clinical Research Center for Respiratory Diseases, Beijing, People’s Republic of China; 6Institute of Respiratory Medicine, Peking Union Medical College, Beijing, People’s Republic of China; 7Department of Respiratory Medicine, Capital Medical University, Beijing, People’s Republic of China; 8Beijing Key Laboratory of Respiratory and Pulmonary Circulation Disorders, Department of Pulmonary and Critical Care Medicine, Beijing Chao-Yang Hospital, Beijing, People’s Republic of China; 9Department of Immunology and Infectious Diseases, The Forsyth Institute, Harvard School of Dental Medicine Affiliate, Cambridge, MA, USA; 10WHO Collaborating Centre for Tobacco Cessation and Respiratory Diseases Prevention, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zuomin Wang, Department of Stomatology, Beijing Chao-Yang Hospital, Capital Medical University, 8 Gongti South Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86 10 85231492, Email [email protected] Chen Wang, Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, 2 Yinghuayuan Dongjie, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +86 10 65105565, Email [email protected]
Purpose: There is insufficient information about the prevalence and risk factors of gastroesophageal reflux disease (GERD) in the Chinese adult population. We aimed to assess the prevalence and identify the risk factors of GERD in China.
Methods: We collected data from a nationally representative sample (50,991 subjects) of Chinese adults from a large nation-wide cross-sectional survey. GERD was diagnosed by a standardized Chinese-language GERD questionnaire with a score of ≥ 8. The demographic characteristics, comorbidities and periodontal factors of all participants were collected.
Results: Fifty-thousands-one-hundred-eighty-three participants were finally included in this study. The overall prevalence of GERD was 5.6% (95% CI, 5.4– 5.8%) among the general Chinese population aged 20 years or older. Women, smokers, and people with older age, BMI ≥ 25.0 kg/m2, urban residence, lower education level or comorbidities were more prevalent with GERD (p < 0.001). Symptoms of severe periodontitis (OR = 1.40, 95% CI 1.28– 1.52, p < 0.001) and lower frequency of tooth brushing (OR = 2.01, 95% CI 1.76– 2.29, p < 0.001) were significantly associated with risk of GERD.
Conclusion: Symptom-based GERD is highly prevalent in the Chinese population. Overweight and smoking are major preventable risk factors for GERD. Periodontal factors are novel potential risk factors for GERD and should be given more attention in GERD prevention.
Keywords: gastroesophageal reflux disease, prevalence, risk factor, periodontitis, epidemiology
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal disorders in the world. It is defined as a condition in which the reflux of gastric contents into the esophagus results in symptoms and/or complications in the new American College of Gastroenterology (ACG) clinical guideline.1 Heartburn and regurgitation are typical symptoms of GERD, and patients can also have extraesophageal symptoms such as chest pain, chronic cough and laryngeal disorders.2 A recent meta-analysis showed that the prevalence of GERD varied from 2.5% to 51.2% in different countries and regions worldwide, and the pooled prevalence was 13.3%.3 Previously, the prevalence of GERD in China was reported to be lower than in western countries in some population-based studies.3,4 However, to our knowledge, there is no nation-wide prevalence data of China has been reported.
The pathophysiology of GERD is multifactorial.5 Many risk factors for GERD have been well established, including obesity or increased body mass index, smoking and genetic predisposition.2 Several other risk factors, including older age, low socioeconomic status and low education level, were also involved in GERD.3 The risk factors for GERD vary in different studies based on the population from different regions of China.4,6–8 To further clarify the risk factors for GERD in the national population is important for GERD prevention and the public health in China.
The oral cavity is the beginning of the digestive tract. The oral manifestations of GERD have been concerned previously.9,10 The most common oral manifestations include dental erosion and oral soft tissue disorders.10 However, whether oral health affects GERD is not clear. Periodontitis is one of the most common oral diseases. It is a chronic infection and inflammatory disease that is initially caused by periodontal pathogens and eventually leads to alveolar bone resorption, tooth mobility and tooth loss.11 Oral dysbacteriosis is a significant feature of periodontitis patients.12 Pathogens in the oral cavity of periodontitis patients easily migrate to the esophagus by swallowing food and saliva. Studies have shown that microbes in the esophagus shift in patients with GERD or reflux disorders, which may contribute to the development of GERD.13,14 Therefore, periodontitis may be an important potential risk factor for GERD.
We designed this study by collecting the data of representative Chinese adults (57,779 subjects) from a large national cross-sectional survey (China Lung Health Study, CPHS).15 In this study, we aimed to assess the prevalence and identity the general and periodontal risk factors of GERD in the general Chinese population.
Methods
Study Participants
The CPHS was a cross-sectional study conducted from 2012 to 2015 in a nationally representative sample of adults aged 20–89 in China from 2012 to 2015. The study complies with the Declaration of Helsinki and was approved by the ethics review committee of Beijing Capital Medical University (No. 11-ke-42, and approved on June 24, 2011) and other participating institutes.15 All study participants received written informed consent. Details of the study design have been published previously.15 In brief, a multistage stratified cluster sampling procedure was adopted to recruit a nationally representative sample of adults age 20 or older from the selected communities, and a total of 57,779 adults were included from 10 provinces across the country, of whom 50,183 had natural teeth in the mouth and no missing data were included in the study. The flow diagram of selecting study participants is shown in Figure 1. The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
 |
Figure 1 The flow diagram of the selection of study participants. |
Data Collection
Trained interviewers administered a standardized Chinese-language GERD questionnaire (GERDQ) that has been validated for both diagnosis and follow-up of reflux disease patients (Detailed questions are in Supplementary GERD Questionnaire).16,17 It uses a four graded scale (0–3) to score the frequency of four positive predictors of GERD, including heartburn, reflux, sleep disorders caused by reflux symptoms or the use of over-The-counter medications for reflux symptoms; and a reversed scale (3–0) for two negative predictors of GERD, including epigastric pain and nausea. Finally, the total score range of GERDQ was 0–18.16 GERD was diagnosed in participants with a score of ≥ 8 on the evaluation of GERDQ.16,18 All participants in the population with a score of < 8 on the evaluation of GERDQ were used as control participants in the analyses.
The trained interviewers conducted a standardized questionnaire, including information on demographic characteristics (sex, age, education level, geographic location, smoking status and residence) and comorbidities. Comorbidities included cancer, asthma, chronic obstructive pulmonary disease, hypertension, coronary heart disease, depression, diabetes mellitus, anemia and osteoporosis. A person who has smoked 100 cigarettes in his life and is still smoking is defined as current smoker.15 Body weight and height were determined using a calibrated scale, and body mass index (BMI) was calculated as weight/height squared.
Natural tooth loss and tooth mobility are typical symptoms of severe periodontitis.11 Trained interviewers asked all participants whether they had natural tooth loss and/or tooth mobility in the past year. The frequency of tooth brushing was also asked of all participants. Participants who had natural tooth loss and/or tooth mobility in the past year were considered to have symptoms of severe periodontitis. The tooth brushing frequency was divided into higher (≥ 1 time/day) and lower (< 1 time/day) frequency.
Statistical Analyses
The continuous variables of normal distribution were represented by the mean (standard deviation, SD). The categorical variables were presented as frequencies with percentages. SPSS statistical software (version 20.0; SPSS Inc., Chicago, IL, USA) was used for the data analyses, and statistical significance was considered at a two-sided p < 0.05.
The overall and prevalence of GERD and 95% confidence interval (CI) in total participants was calculated, as well as prevalence in participants grouped by geographic distribution, sex, age, education level, BMI, smoking status, several periodontitis and frequency of tooth brushing. P for difference or p for trend values among groups were also calculated.
Multiple logistic regression analyses were performed to calculate the odds ratios (ORs) and 95% CI for the associations between general and periodontal risk factors and GERD. The adjusted model 1 was adjusted for sex, age and BMI. The adjusted model 2 was additionally adjusted for education level, smoking status, comorbidities and residence.
In order to know whether the periodontal risk factors in sub-populations might exhibit differential risk of GERD, we investigated potential interaction between periodontal risk factors and age (20–39 years versus (vs) 40–59 years vs ≥60 years), sex, BMI (< 25 Kg/m2 vs ≥ 25Kg/m2), smoking (never smoker vs current/former smoker), education level (middle school and lower vs high school and higher), residence (urban vs rural) and comorbidities (yes vs no).
Results
The demographic characteristics of the total 50,183 participants by GERD in men and women are shown in Table 1.
 |
Table 1 Characteristics of the Study Population by GERD in Men and Women |
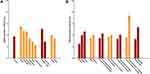
Among the general Chinese population aged 20 years or older, the overall prevalence of GERD was 5.6% (95% CI, 5.4–5.8%). The prevalence in different geographic regions of China was 8.2% (95% CI, 7.7–8.8%) in the north region, 7.1% (95% CI, 6.4–7.8%) in the northeast region, 6.9% (95% CI, 6.2–7. 5%) in the northwest region, 5.1% (95% CI, 4.6–5.5%) in the southwest region, 4.3% (95% CI, 3.9–4.8%) in the east region, and 3.4% (95% CI, 3.1–3.8%) in the central south region (p for trend < 0.001). The prevalence in the northern area (north, northeast and northwest regions) was significantly higher than in the other areas (p < 0.001). GERD was more prevalent in urban residents (6.0%, 95% CI 5.7–6.2%) than in rural residents (5.5%, 95% CI 5.3–5.8%; p < 0.001) (Figure 2).
The prevalence grew with age and was 3.7% (95% CI, 3.4–4.0%) among individuals aged 20–39 years, 6.0% (95% CI, 5.7–6.3%) among individuals aged 40–59 years, and 6.9% (95% CI, 6.5–7.4%) among those aged 60 years or older (p for trend < 0.001). Women had a higher prevalence of 6.0% (95% CI 5.7–6.3%) than men (5.2%, 95% CI 4.9–5.5%, p < 0.001). The prevalence of GERD was higher in overweight and obese participants (BMI ≥ 25.0 kg/m2), current and former smokers and participants with lower education level (middle school and lower) (all p < 0.05) (Figure 2).
Dramatically, we found that the prevalence of GERD was much higher among participants with symptoms of severe periodontitis (8.1%, 95% CI 7.5–8.6%) and lower frequency of tooth brushing (< 1 time/day) (11.1%, 95% CI 9.9–12.2%) than those without symptoms of severe periodontitis (5.6%, 95% CI 5.4–5.8%) and with higher frequency of tooth brushing (≥ 1 time/day) (5.0%, 95% CI 4.8–5.2%, both p < 0.001) (Figure 2).
In multivariable-adjusted logistic regression analyses, older age, female sex, overweight and obesity (BMI ≥ 25.0 kg/m2), lower education level (middle school and lower), smoking, urban residents and comorbidities were significantly associated with risk of GERD in adjusted model 2 (all p < 0.05) (Table 2).
 |
Table 2 Associations Between General Risk Factors and GERD by Logistic Regression Analyses |
For periodontal factors, symptoms of severe periodontitis were significantly associated with risk of GERD (OR = 1.40, 95% CI 1.28–1.52, p < 0.001) after adjusting for confounding risk factors, and lower frequency of tooth brushing (< 1 time/day) also showed higher risk of GERD (OR = 2.01, 95% CI 1.76–2.29, p < 0.001) in the multivariable-adjusted logistic regression analyses (Figure 3).
In the subgroup analyses, symptoms of severe periodontitis and lower frequency of tooth brushing (< 1 time/day) were still significantly associated with risk of GERD in all subgroup participants (all p ≤ 0.001) (Figure 4).
Discussion
As far as we know, this study is the first and largest nationwide survey on GERD for the general Chinese adult population aged 20 years and older. Our data indicated that 5.6% (95% CI, 5.4–5.8%) of the general Chinese adult population had symptom-defined GERD. Overweight and obesity, low education level and smoking were major preventable risk factors of GERD in the Chinese population. Specially, we found that symptoms of severe periodontitis and lower frequency of tooth brushing were significantly associated with risk of GERD and had higher ORs compared with other general risk factors.
No gold standard is for GERD diagnosis.1 The diagnosis of GERD is usually based on clinical symptoms, reaction to acid suppression, as well as objective testing of upper endoscopy and esophageal pH monitoring.1,2 The diagnosis of GERD only based on symptoms has some limitations, however, it may be the most applicable method for a large general population survey because objective testing with upper endoscopy and/or esophageal pH monitoring is too expensive and complicated. Symptom-based diagnostic criteria for GERD was commonly used in large epidemiology studies previously.3 In clinical practice of GERD, diagnosing and treating based on typical symptoms are pragmatic and recognized by societal guidelines.1 GERDQ was widely used in clinic and its diagnostic value has been verified in several studies.17,19–21 A recent study in China showed that the sensitivity and specificity of GERDQ were 90.30% and 92.10% for GERD diagnosis, respectively.18
A recent meta-analysis of 108 separate study populations containing 460,984 subjects showed that prevalence varied in accordance with country and criteria used to define gastro-esophageal reflux symptoms. Pooled prevalence was 13.3% (95% CI 12.0% to 14.6%) when only studies using weekly heartburn or regurgitation to define GERD were included.3 The results of our data showed that the prevalence of symptom-defined GERD in China is 5.6% (95% CI 5.4–5.8%) that is much lower than the global prevalence and the prevalence in western countries. The prevalence increased with age, and women had a slightly higher prevalence than men in our study, which was consistent with previous studies.3
In recent decades, several studies have investigated the prevalence of GERD in China. The prevalence of GERD was 23.4% in Kashgar, Xinjiang Autonomous Region from 5080 general population participants.6 19.89% in Beijing from 37,442 individuals undergoing routine physical examinations in hospital.7 10.8% in Tibet Autonomous Region from 5, 680 general population participants,22 3.8% in Hong Kong SAR from 2074 subjects,8 and 1.7% in Taizhou, Shandong province from 8831 retirees.23 The prevalence varied strikingly among these studies, which may be due to different study populations and different diagnosis criteria of GERD. In this study, we also found that the prevalence of GERD varied among different regions of China. The prevalence in the northern area of China was nearly two times that in other areas. Another population based study enrolled 16,078 subjects from five major cities in China from 2007 to 2008 found that the prevalence of symptom-defined GERD in China was 3.1%,4 which is lower than the results of our study of 50,183 subjects from a rigorous sampling design of the nation-wide population in 2012 to 2015.
Increased BMI and smoking were well-documented risk factors for GERD [2]. Our study showed overweight and obesity was significantly associated with the risk of GERD compared with normal and underweight (BMI < 25.0 kg/m2). These results were consistent with the study of Jacobson et al.24 The relationship between obesity and GERD may be the increase of intra-abdominal pressure, the prevalence of hiatal hernia, the gradient of abdominal to thoracic pressure, the level of estrogen, and the secretion of bile and pancreatic enzymes caused by obesity.2,25 Current/Former smokers had a higher risk of having GERD than never smokers, corresponding to an OR of 1.38 (95% CI, 1.23–1.56) in this study, and this is also consistent with the recent meta-analysis.3 Tobacco can prolong the clearing time of the esophageal acid and reduce the pressure in the lower esophageal sphincter, which was used to explain their association.2,25
In this study, we found that lower education level and urban residents were also significantly associated with the risk of GERD. Urban residents usually have a better socioeconomic status than rural residents, and socioeconomic status has been demonstrated to be associated with GERD.3 People with a lower education level in China usually have a worse socioeconomic status or older age. They were found to be more likely to suffer from GERD in this study, which was also consistent with previous studies.3
As far as we know, this is the first population based study to investigate periodontal risk factors for GERD. The results of our study showed that symptoms of severe periodontitis and lower frequency of tooth brushing were both significantly associated with risk of GERD, and had higher ORs (1.40, 95% CI 1.28–1.52 and 2.01, 95% CI 1.76–2.29 separately) than higher BMI and smoking after adjusting for other risk factors.
Several studies have explored the changes of microbes in the esophagus of GERD or reflux disorders. These studies showed an overall shift from gram-positive bacterial species in normal individuals to gram-negative anaerobic colonization in patients with GERD or reflux disorders.13,14,26 Gram-negative anaerobic bacteria such as Porphyromonas, Prevotella, Fusobacterium and Actinomyces were found in the distal esophagus of GERD or reflux disorders.26 The esophageal inflammation caused by microbes was supposed to be a possible link to GERD.13,14,26
Periodontitis is initially caused by periodontal pathogens infection. Periodontal pathogens are mainly gram-negative anaerobic bacteria such as Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, aggregatibacter and Fusobacterium nucleatum, which are abundant in the periodontal environment.27 These species were consistent with the bacteria that increased in the distal esophagus of GERD.26
There are more than 700 different species of bacteria in the oral cavity, most of which are attached to the tooth surface and form dental plaque biofilm.28 Most of them are anaerobic bacteria. The lower frequency of tooth brushing is associated with poor oral hygiene and more bacteria in the oral cavity. The lower frequency of tooth brushing is also an important risk factor for periodontitis.
In recent decades, there has been renewed interest in the basic principle that the oral microbiome may play an important role in overall health. Specifically, the presence of oral infections such as periodontal disease may be a pathogenic or aggravating factor for some systemic diseases.29 Three mechanisms currently support a link between periodontitis and systemic disease: metastatic infections, dissemination of bacterial toxins and immunological injury.30 Studies have found that porphyromonas gingivalis, a major cause of periodontal disease, may cause transient bacteremia during common activities such as brushing, flossing and chewing, or during dental surgery, resulting in translocation to various tissues, including brain of Alzheimer’s patients, coronary arteries, tonsil and liver.31,32 A recent study showed that 100% of patients with cardiovascular disease had porphyromonas gingivalis artery colonization.33 The oral cavity is the beginning of the digestive tract, oral microbes can easily migrate into the esophagus through the swallowing of food and saliva. Therefore, we speculated that the association between periodontal risk factors and GERD may be due to the possible microbial alteration of the esophagus caused by oral dysbacteriosis.
Our study has some limitations. First, GERD was defined based on GERDQ and no objective testing and clinical examination were performed. Second, some potential risk factors for GERD, such as genetic predisposition, were not included in this study. Third, this is a cross-sectional study, so it is impossible to establish a causal relationship between the risk factors of GERD.
Conclusion
This nationwide large-scale cross-sectional study showed that the prevalence of symptom-based GERD was 5.6% in the Chinese population, which was lower than the global average prevalence. Overweight and smoking were major preventable risk factors for GERD. Periodontal factors, including symptoms of severe periodontitis and lower frequency of tooth brushing, are novel potential risk factors for GERD and should be given more attention in GERD prevention. Long-term cohort studies are needed to confirm the associations of these periodontal risk factors in the future.
Abbreviations
GERD, gastroesophageal reflux disease; CPHS, China Pulmonary Health Study; GERDQ, GERD questionnaire; BMI, body mass index; CI, confidence interval; ORs, odds ratios; ACG, American College of Gastroenterology; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
Acknowledgments
The authors are grateful to all the participants and staff of China Pulmonary Health Study. Zhiqiang Liu and Xiaoli Gao are co-first authors for this study. Zuomin Wang and Chen Wang are co-correspondence authors for this study.
Author Contributions
All authors made significant contributions to the conception and design of the study, and reviewed the article critically. Zuomin Wang and Chen Wang supervised the study. Zhiqiang Liu, Lirong Liang, Xuan Zhou, Ting Yang, Kewu Huang and Yingxiang Lin contributed to the data collection. Zhiqiang Liu, Xiaoli Gao and Lirong Liang did the data analysis. Zhiqiang Liu, Xiaoli Gao, Lirong Liang, Xiaozhe Han, Shu Deng, Zuomin Wang and Chen Wang participated in data interpretation, drafting the manuscript. All authors have given final approval to the journal to which the article will be submitted, agreed on all versions of the article and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [grant no. 81901003] and the Special Research Foundation for Public Welfare of Health, Ministry of Health of China [grant no. 201002008].
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117(1):27–56. doi:10.14309/ajg.0000000000001538
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324(24):2536–2547. doi:10.1001/jama.2020.21360
3. Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67(3):430–440. doi:10.1136/gutjnl-2016-313589
4. He J, Ma X, Zhao Y, et al. A population-based survey of the epidemiology of symptom-defined gastroesophageal reflux disease: the systematic investigation of gastrointestinal diseases in China. BMC Gastroenterol. 2010;10:94. doi:10.1186/1471-230X-10-94
5. Zheng Z, Shang Y, Wang N, et al. Current advancement on the dynamic mechanism of gastroesophageal reflux disease. Int J Biol Sci. 2021;17(15):4154–4164. doi:10.7150/ijbs.65066
6. Tuerxun K, Balati M, Aimaiti M, et al. Epidemiological investigation, extraesophageal symptoms and risk factors of gastroesophageal reflux disease in Kashgar, Xinjiang, China. Am J Transl Res. 2021;13(12):14186–14194.
7. Gong Y, Zeng Q, Yan Y, Han C, Zheng Y. Association between lifestyle and gastroesophageal reflux disease questionnaire scores: a cross-sectional study of 37,442 Chinese adults. Gastroenterol Res Pract. 2019;2019:5753813. doi:10.1155/2019/5753813
8. Tan VP, Wong BC, Wong WM, et al. Gastroesophageal reflux disease: cross-sectional study demonstrating rising prevalence in a Chinese population. J Clin Gastroenterol. 2016;50(1):e1–e7. doi:10.1097/MCG.0000000000000304
9. Ortiz AC, Fideles SOM, Pomini KT, Buchaim RL. Updates in association of gastroesophageal reflux disease and dental erosion: systematic review. Expert Rev Gastroenterol Hepatol. 2021;15(9):1037–1046. doi:10.1080/17474124.2021.1890030
10. Durazzo M, Lupi G, Cicerchia F, et al. Extra-esophageal presentation of gastroesophageal reflux disease: 2020 update. J Clin Med. 2020;9(8):2559. doi:10.3390/jcm9082559
11. Herrera D, Sanz M, Kebschull M, et al. Treatment of stage IV periodontitis: the EFP S3 level clinical practice guideline. J Clin Periodontol. 2022;49(Suppl 24):4–71. doi:10.1111/jcpe.13639
12. Abusleme L, Hoare A, Hong BY, Diaz PI. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2021;86(1):57–78. doi:10.1111/prd.12362
13. Hasan A, Hasan LK, Schnabl B, Greytak M, Yadlapati R. Microbiome of the aerodigestive tract in health and esophageal disease. Dig Dis Sci. 2021;66(1):12–18. doi:10.1007/s10620-020-06720-6
14. Park CH, Lee SK. Exploring esophageal microbiomes in esophageal diseases: a systematic review. J Neurogastroenterol Motil. 2020;26(2):171–179. doi:10.5056/jnm19240
15. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
16. Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30(10):1030–1038. doi:10.1111/j.1365-2036.2009.04142.x
17. Jonasson C, Wernersson B, Hoff DA, Hatlebakk JG. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37(5):564–572. doi:10.1111/apt.12204
18. Wang B, Sun Q, Du Y, Mu K, Jiao J. Diagnosis and etiological analysis of gastroesophageal reflux disease by gastric filling ultrasound and GerdQ scale. J Healthc Eng. 2021;2021:5629067. doi:10.1155/2021/5629067
19. Bai Y, Du Y, Zou D, et al. Gastroesophageal Reflux Disease Questionnaire (GerdQ) in real-world practice: a national multicenter survey on 8065 patients. J Gastroenterol Hepatol. 2013;28(4):626–631. doi:10.1111/jgh.12125
20. Wang M, Zhang JZ, Kang XJ, et al. Relevance between GerdQ score and the severity of reflux esophagitis in Uygur and Han Chinese. Oncotarget. 2017;8(43):74371–74377. doi:10.18632/oncotarget.20146
21. Norder Grusell E, Mjörnheim AC, Finizia C, Ruth M, Bergquist H. The diagnostic value of GerdQ in subjects with atypical symptoms of gastro-esophageal reflux disease. Scand J Gastroenterol. 2018;53(10–11):1165–1170. doi:10.1080/00365521.2018.1503708
22. Zhang H, Gao W, Wang L, et al. A population-based study on prevalence and risk factors of gastroesophageal reflux disease in the Tibet Autonomous Region, China. PeerJ. 2019;7:e6491. doi:10.7717/peerj.6491
23. Chen T, Lu M, Wang X, et al. Prevalence and risk factors of gastroesophageal reflux symptoms in a Chinese retiree cohort. BMC Gastroenterol. 2012;12:161. doi:10.1186/1471-230X-12-161
24. Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354(22):2340–2348. doi:10.1056/NEJMoa054391
25. Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166(9):965–971. doi:10.1001/archinte.166.9.965
26. Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137(2):588–597. doi:10.1053/j.gastro.2009.04.046
27. Rylev M, Kilian M. Prevalence and distribution of principal periodontal pathogens worldwide. J Clin Periodontol. 2008;35(8 Suppl):346–361. doi:10.1111/j.1600-051X.2008.01280.x
28. Kilian M, Chapple IL, Hannig M, et al. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–666. doi:10.1038/sj.bdj.2016.865
29. Falcao A, Bullón P. A review of the influence of periodontal treatment in systemic diseases. Periodontol. 2019;79(1):117–128. doi:10.1111/prd.12249
30. Van Dyke TE, Van Winkelhoff AJ. Infection and inflammatory mechanisms. J Periodontol. 2013;84(4 Suppl):S1–S7. doi:10.1902/jop.2013.1340018
31. Nakahara T, Hyogo H, Ono A, et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J Gastroenterol. 2018;53(2):269–280. doi:10.1007/s00535-017-1368-4
32. Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333. doi:10.1126/sciadv.aau3333
33. Mougeot JC, Stevens CB, Paster BJ, Brennan MT, Lockhart PB, Mougeot FK. Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries. J Oral Microbiol. 2017;9(1):1281562. doi:10.1080/20002297.2017.1281562
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.