Back to Journals » International Journal of General Medicine » Volume 15
Prevalence, Clinical Profile and Risk Factors of Nosocomial Infection in Ayder Pediatric Intensive Care Unit, Tigray, Ethiopia
Authors Mohamed AA , Haftu H , Hadgu A, Seyoum D , Gebrekidan G, Ebrahim MM , Yusuf AA, Mustefa M
Received 4 August 2022
Accepted for publication 2 September 2022
Published 9 September 2022 Volume 2022:15 Pages 7145—7153
DOI https://doi.org/10.2147/IJGM.S384233
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Abdikarin Ahmed Mohamed,1 Hansa Haftu,1 Amanuel Hadgu,1 Dawit Seyoum,1 Goitom Gebrekidan,1 Mohamedawel Mohamedniguss Ebrahim,2 Abdisalam Abdullahi Yusuf,3 Mohammed Mustefa1
1Department of Pediatrics and Child Health, College of Health Science, Mekelle University, Tigray, Ethiopia; 2Department of Public Health, College of Health Science, Mekelle University, Tigray, Ethiopia; 3Department of Pediatrics, and Child Health, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia
Correspondence: Abdikarin Ahmed Mohamed, Email [email protected]
Background: Hospital-acquired infection (HAI) is a significant cause of increased morbidity and mortality amongst hospitalized patients and represents a considerable health and economic burden worldwide. However, evidence about HAI in pediatric ICU is limited.
Objective: To identify the prevalence of hospital-acquired infection (HAI), clinical profile, and its risk factors for nosocomial infection in patients admitted to the pediatric intensive care unit (PICU).
Methodology: From a two-year retrospective chart review admitted from 2019 to 2020 to the PICU, 223 patients were selected by systematic random sampling. Data were analyzed in SPSS version 23.0. P-values < 0.05 were considered significant for all tests.
Results: Forty-five (20.2%) patients developed nosocomial infection (NI). The median age was 4 years with 25– 50th IQR of (0.6– 9). About invasive procedures done, the most common was nasogastric tube (57%), followed by mechanical ventilation (17.9%) and urinary catheter (13.9%). The main focus of the infection was chest (53.3%), followed by bloodstream infection (22%) and gastrointestinal infection (9%). The odds of HAI were 3.3 times higher among under-five compared to those aged between 5 and 18 years (AOR: 3.3, 95% CI = 1.4– 8.0, p = 0.008). The odds of HAI were also 4.1 times higher in those who stayed for more than two weeks compared to those who stayed in the pediatric ICU 2 to 14 days (AOR: 4.1, 95% CI = 2.0– 8.6, p < 0.001). The mean duration of mechanical ventilation in those patients with and without NI was 1.65 days and 13.96 days, respectively (AOR = 3.46, 95% CI = 1.44– 9.81, p = 0.02). Patients who started antibiotics at admission and patients who were on nasogastric tube feeding were also statistically significant risk factors for developing NI (AOR = 2.67, 95% CI = 1.37– 9.64, p = 0.02; AOR = 2.45, 95% CI = 1.64– 6.53, p = 0.03).
Conclusion: The rate of infection in this study was higher compared to some developing countries. Younger age and prolonged length of hospital stay were found to be significant risk factors for HAI.
Keywords: prevalence, PICU, NI, risk factor
Introduction
Hospital-acquired infection (HAI) is a substantial public health problem that occurs during the patient care process.1,2 The occurrence of HAI in intensive care units (ICU) is much higher in developing than developed countries. The recently reviewed and published data in World Health Organization (WHO) showed that in low and middle-income countries, the incidence of HAI in ICU is 2–3 times higher than in high-income countries.3 In another review on HAI done in Africa by the WHO, there were significant differences in the prevalence and incidence, though it ranges from 2.5% to 14.8%.4,5 The average prevalence is significantly higher in high than low-quality studies (15.5% vs 8.5%, respectively).4 The European Centre for Disease Prevention and Control reports an average prevalence of 7.1% in European countries.5
Different studies report the incidence of HAI in Pediatric ICU (PICU) ranges between 6.1% and 15.1%. Even though there are limited studies in PICU about NI, especially from developing countries, children hospitalized in PICU are a unique population concerning specific risk factors. Young children have increased susceptibility to infections because of the immaturity of their immune system.6 In a study done in Peru, PICU revealed an incidence of HAI of 19.5%, and bloodstream infection (BSI), nosocomial pneumonia (NP), and urinary tract infection (UTI) were the most common types of NI.7 As aforementioned, in Pakistan, the rate of HAI in PICUs was 4.7%,8 in Rwanda (15.1%), and in two PICUs, Egypt was 15.6%.9,10
Significant identified risk factors for acquiring NI were young age, MV, re-intubation, sedation, NGT feeding, prolonged hospital stay, underlying illness, impaired consciousness, neuromuscular disease, and aspiration of gastric content.1,4,9,11,12 The current worldwide focus on improving the cost-effectiveness of health care, along with reports of successful nosocomial infection-control programs, stimulates hospital administrators, infection-control teams, and researchers to try to understand their own local situations in order to improve health-care programs and an essential prerequisite for developing effective infection control measures.2,13–15 Little is known about the prevalence of nosocomial infections and associated risk factors among children in Ethiopia. Additionally, the magnitude of HAI in pediatric ICU is not available in open access publications. This study aims to assess the prevalence, clinical profile, and risk factors for acquiring HAI in our setting for further planning.
Methodology
Ayder is one of the biggest institutions in the Tigray, established in 2008. The Department of Pediatrics and Child Health is one of the major specialties, with a total of 162 beds. Pediatric ICU is one of the main wards for critically ill patients and is staffed with 25 nurses, 3 residents, and two senior pediatricians. Moreover, it is equipped with mechanical ventilators, monitors, and perfusers.
A two-year retrospective chart review was done from 2019 to 2020. All pediatric patients admitted to the Pediatric Intensive Care Unit who stayed for more than 48 hours with complete data were included in the study. Incomplete chart documentation and patients who stayed in PICU for less than 48 hours were excluded from the study.
The sample size was calculated with a single proportional formula, and the minimum sample size required after adding 10% was 223. The systematic random sampling technique was used for a total of 630 patients who were admitted to pediatric ICU between 2018 and 2020. The total patients admitted during that period were divided into our sample size to assess equal patients in each month of the study period. For this, every third chart was recruited by excluding incomplete charts and those who stayed less than 48 hours in the pediatric intensive care unit.
Clinically investigated parameters included age, sex, underlying disease, patient feeding, duration of maintenance fluid, invasive procedures, hospital stays, mechanical ventilation, duration of mechanical ventilation, NGT feeding, and urinary catheterization.
A locally prepared structured questionnaire was used to collect data from patient’s charts. The questionnaire was developed in English. Trained residents collected the data from the charts, and the principal investigator checked for completeness of the questionnaire daily.
Ethical clearance was obtained from the Institutional Review Board (IRB) of the College of Health Sciences, Mekelle University, with an IRB number of 1853/2021. The permission letter was also obtained from the ACSH Chief Clinical director’s office before the commencement of the study. Consent was taken from the care givers after they were told about the objective and purpose of the study. They were also told about their right to refuse to participate in the study at any time.
The collected data were checked for completeness. Descriptive and analytical statistics, including univariate, frequency distribution, percentage, ratios, and the measure of dispersion, were used for univariate analysis. Visual (histogram) and analytical methods (Kolmogorov–Smirnov test) were used to determine whether variables exhibited normal distribution. Variables with normal distribution were expressed as mean plus/minus standard deviation, while not normally distributed data were expressed as median (minimum-maximum) values. Categorical data were compared using Pearson’s Chi-square test or Fisher’s exact test. Correlations were determined using Pearson’s or Spearman correlation analyses. Binary logistic regression analysis was performed to assess the predictors of hospital-acquired infection (HAI). The odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. P-values <0.05 were considered significant for all tests. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS-IBM) for Windows version 26.
Results
Socio-Demographic Profile
From a total of 680 admitted patients to PICU over two years, 223 patients were selected by systematic random sampling after exclusion was done for incomplete data records. Among 223 enrolled patients, 45 (20.2%) acquired Nosocomial infection. The median age of the children who participated in this study was 4 years, with a 25–50th IQR of (0.6–9). Male patients accounted for 138 (61.9%) of study participants making an M:F ratio of 1.6:1. The main source of admission was emergency department 151 (67.7%), followed by pediatric ward 49 (22%). The most common organ/system affected was neurology (25.6%), followed by infectious (19.3%) and respiratory (13.5%). Severe and moderate malnutrition was reported in 25.6% and 19.3% of them, respectively. Fifty-seven percent (57.0%) of children had an additional diagnosis and the main reason for their ICU admission (Table 1).
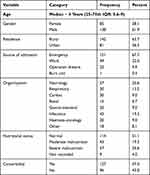 |
Table 1 Socio-Demographic and Admission-Related Characteristics of Pediatric ICU Patients of ACSH, Mekelle, Tigray (N = 223) |
ICU Care and Invasive Procedures Done
Regarding patient feeding, 98 (43.9%) patients were on maintenance fluid, with the majority (50%) of them put for 24–72 hours. About invasive procedures done, the most common was nasogastric tube (57%), followed by mechanical ventilation (17.9%) and urinary catheter (13.9%). The culture was done for a total of 110 (49.3%) patients, of which 39.1% (43) were patients who developed HAI. The majority (62.8%) of ICU patients were improved and transferred to the ward, but 20.6% of them died (Table 2)
 |
Table 2 Pediatric ICU Care and Different Procedures, Ayder, Mekelle, Tigray (N = 223) |
Hospital-acquired infection (HAI) was diagnosed in 45. The main clinical indicators of nosocomial infection were heterogeneous, with the most dominant feature of Fever (39%) followed by urinary compliant (5%). The main focus of infection for NI was chest (53.3%), followed by BSI (22%) and GI (9%) (Figure 1).
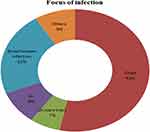 |
Figure 1 Focus of infection for nosocomial infection in pediatric ICU, Ayder, Mekelle (n=45). |
A total of 127 samples were taken for culture, of which 38.6% (49/127) were from patients with NI. The culture was positive, and etiologic diagnosis was made in 27.3% (30/110 pts), with the majority (56.7%, 17/30 pts) of them with hospital-acquired infection.
In the culture growth, a total of 37 bacteria were isolated. The most common bacteria isolated in PICU were Klebsiella pneumonia (27%, 10/37), followed by Staphylococcus aureus and E. coli, accounting for 13.5% (5/37) for each. Concerning the dominant etiology for those with and without HAI, there was no difference (in both Klebsiella pneumonia commonest bacteria), but Staphylococcus is more common in those ICU patients without HAI than with HAI (8.2% vs 5.4%). Acinetobacter and E. Coli were isolated more in those patients with NI than those without (8.2% vs 5.4%) (Figure 2).
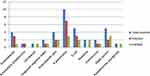 |
Figure 2 Identified bacterial etiologies from different samples in patients admitted to PICU (n=37). |
Predictors of Hospital-Acquired Infection (HAI)
In the binary logistic regression model, different factors have been identified that increase the risk of hospital-acquired infection. The mean length of PICU stays in those patients with NI and without NI were 24.33 days and 2.36 days, respectively (AOR: 4.1, 95% CI: 2.0, 8.6, p < 0.001). The mean duration of mechanical ventilation in those patients with and without NI was 1.65 days and 13.96 days, respectively (AOR = 3.46, 95% CI = 1.44–9.81, p = 0.02). The longer the mechanical ventilation and hospital stay duration, the higher the risk of developing HAI, which was statistically significant. The younger age (<5 years), those patients who started antibiotics at admission, and patients who were on nasogastric tube feeding were also statistically significant risk factors for developing NI (AOR: 3.3, 95% CI: 1.4, 8.0, p < 0.008; AOR = 2.67, 95% CI = 1.37–9.64, p = 0.02; AOR = 2.45, 95% CI = 1.64–6.53, p = 0.03). Other risk factors which increase the risk of developing HAI were sedated than conscious patients (33.3% Vs 21.1%), on maintenance than oral feeding (25.5% vs 8.3%), those who were on maintenance Fluid for greater than 72 hours than less than 24 hours (35.3% vs 13.3%), but they were not statistically significant (p > 0.05) (Table 3).
 |
Table 3 Predictors of HAI Among Pediatric ICU Patients, Ayder, Tigray (N = 223) |
Discussion
Developing and developed countries are suffering from a high burden of hospital-acquired infection. According to a WHO study in 55 hospitals in 14 countries, about 8.7% of admitted patients had NI.11 As NI is more rampant and its prevention is the critical step in improving healthcare quality, surveillance is crucial for further interventional plans.6,7 In this study, 20.2% of admitted patients develop HAI, with the main focus of infection being the chest. Our study differs as we only assessed the focus, and it does not differentiate the type of infection, including whether it was ventilator-associated or not, and causative etiology. Even though the prevalence of HAI in our study (20.2%) was higher than in studies done in Pakistan (4.7%), Lithuania (13.6%), Egypt (15.6%), American study (11.9%), Rwanda (12.1%) and Peru (20%),6–8,16,17 but it was lower than studies done in India (27.3–30.5%), Egypt (27.7%), Serbia (32.7%), Nigeria (45%).1,3,10,11,18 The differences in the prevalence of HAI in developing countries may be due to the disparities in quality of care, strong infection prevention mechanism implementation, the study design we used, and the study area (we used only PICU, but in others, adult ICU and General ICU were included) and the type of patients where NI was assessed. According to our findings, the main focus of infection was the chest which was consistent with a study done in Lithuania (58.8%)6 and Egypt, but in other studies, BSI was more prevalent than respiratory infection like in Peru7 and Nigeria.3
Despite the differences between studies with regard to causative organisms, Gram-Negatives showed the most common pathogens identified in this study, which is consistent with other studies.1,3,18,19 Among body fluid analysis, 36 isolates of organisms were found, of which Gram-Negatives accounted 75% and the rest were Gram-Positives.
Klebsiella pneumonia was the leading bacterial etiology of HAI, followed by Escherichia coli and Acinetobacter baumannii. This is parallel to the study reported in the literature.6,7,12 This may be due to differences in the site of infection (chest vs BSI), sampling site, demographic differences, and the degree of infection prevention implementations.
According to our findings, statistically significant predictors which increase the risk of nosocomial infection were younger age (under five years), prolonged duration of mechanical ventilation (more than 7 days), presence of nasogastric tube, patients started antimicrobial at admission and long duration of hospital stay (more than two weeks). Some of these findings were compatible with that of previous studies like duration of hospital stay,6 mechanical ventilation, presence of NGT,11 preceding use of antibiotics,3 and using the medical device.20 The new finding of our study is the younger the age, the more they are at risk of developing HAI. This could be attributed to young age groups' immature immune systems3,11,19,20.
Despite the fact that we failed to evaluate reintubation rate, other studies have consistently demonstrated as a strong predictor of NI. And others we did not assess as risk factors were prophylactic use for stress ulcers, beta-blocker medication, neuromuscular block, and the severity of illness at admission. As in our study, knowing risk factors is crucial, and efforts can be made to prevent subsequent infections. All necessary precautions, including timely removal of medical devices like NGT and urinary catheters, must be taken to minimize HAI.
This study has certain limitations, including, first, it is a retrospective study challenging to examine other lab-related investigations to correlate the clinical profile. Second, the study did not provide data regarding re-intubation. Third, the sample was also not taken from the trachea and/or respiratory samples. Besides these limitations, our study is one of the few studies done in PICU on the prevalence and risk factors for HAI, which may further help us to strategize our infection prevention plans.
Conclusion
Our study revealed that the prevalence of NI in PICU was 20.2% which was higher compared to some developing countries, and K. pneumonia was the leading etiology. Young age (<5 years), hospital stay for more than 14 days, mechanical ventilation >7 days, NGT, and initiation of antibiotics at admission were significantly associated with HAI. Further studies are needed to implement and evaluate adequate infection prevention plans. Therefore, priority should be given to implementing an effective infection control team. Hospital management and the Ministry of Health should give precedence to this high rate of nosocomial infection and implement infection prevention control programs.
Abbreviations
NI, Nosocomial Infection; WHO, World Health Organization; HAI, Hospital Acquired Infection; PICU, Pediatric Intensive Care Unit; ACSH, Ayder Comprehensive Specialized hospital; NGT, Naso Gastric tube; MV, Mechanical Ventilation; BSI, Blood Stream Infection.
Data Sharing Statement
The data included in the manuscript.
Ethical Consideration
Ethical clearance was obtained from the Institutional Review Board (IRB) of the College of Health Sciences, Mekelle University, with an IRB number of 1853/2021. The permission letter was also obtained from the ACSH Chief Clinical director’s office before the commencement of the study. Moreover, it confirms that this study compiles all the requirements of the latest 2013 version of the Declaration of Helsinki.
Informed Consent
Consent was taken from the care givers after they were told about the objective and purpose of the study. They were also told about their right to refuse to participate in the study at any time.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Deep A, Ghildiyal R, Kandian S, et al. Clinical and microbiological profile of nosocomial infections in the Pediatric Intensive Care Unit (PICU). Indian Pediatr. 2004;41:1238–1246.
2. Maluf Lopes M, Goulart M, Starling CEF, et al. Pediatric mortality due to nosocomial infection: a critical approach. Braz J Infect Dis. 2007;11(5):515–519. doi:10.1590/s1413-86702007000500013
3. Iwuafor A, Ogunsola T, Oladele RO. Incidence, clinical outcome and risk factors of intensive care unit infections in the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria. PLoS One. 2016;11(10):e0165242. doi:10.1371/journal.pone.0165242
4. European Centre for Disease Prevention and Control. Annual Epidemiological Report on Communicable Diseases in Europe 2008. Stockholm: European Centre for Disease Prevention and Control; 2008.
5. European Centre for Disease Prevention and Control. Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC; 2013.
6. Ašembergienė J, Gurskis V, Kėvalas R, Valintėlienė R. Nosocomial infections in the pediatric intensive care units in Lithuania. Medicina. 2009;45(1):29–35. doi:10.3390/medicina45010005
7. Becerra R, Tantaleán A, Suárez VJ, et al. Epidemiologic surveillance of nosocomial infections in a Pediatric Intensive Care Unit of a developing country. BMC Pediatr. 2010;10(1):1–9. doi:10.1186/1471-2431-10-66
8. Haque A, Bano S. Clinical profile and outcome in a pediatric intensive care unit in Pakistan. BMJ. 2009;19(8):534–535.
9. Gomaa HE, Helmy NA, El-Sahrigy SA, et al. Prevalence and anti-microbial susceptibility of hospital acquired infections in two pediatric intensive care units in Egypt. Open Access Maced J Med Sci. 2019;7:1744–1749.
10. Lukas et al, Establishment of a HAI on surveillance system,internatioal Journal of Infection Control.
11. Mansour E, Bendary S. Hospital acquired pneumonia in critically ill children: incidence, risk factors, outcome and diagnosis with insight on the novel diagnostic technique of multiplex polymerase chain reaction. Egypt J Med Human Genet. 2012;13:99–105. doi:10.1016/j.ejmhg.2012.01.002
12. Patra K, Jayashree M, Singhi S, et al. Nosocomial pneumonia in a pediatric intensive care unit. Indian Pediatr. 2007;44:511–518.
13. Atif ML, Bezzaoucha A, Mesbah S, et al. Evolution of nosocomial infection prevalence in an Algeria university hospital. Med Mal Infect. 2006;36:423–428. doi:10.1016/j.medmal.2006.05.002
14. Pittet D, Allegranzi B, Storr J, et al. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4–10. doi:10.1016/j.jiph.2008.08.006
15. Grisaru-Soen G, Sweed Y, Lerner-Geva L, et al.Nosocomial bloodstream infections in a pediatric intensive care unit: 3-year survey. Med Sci Monit. 2007;13(6):251–257.
16. Sahrigy E, Shouman M, Ibrahim H, et al. Prevalence and anti-microbial susceptibility of hospital acquired infections in two pediatric intensive care units in Egypt. Maced J Med Sci. 2019;7(11):1744–1749. doi:10.3889/oamjms.2019.485
17. Jaballah NB, Bouziri A, Mnif K, Hamdi A, Khaldi A, Kchaou W. Epidemiology of hospital-acquired bloodstream infections in a Tunisian pediatric intensive care unit: a 2-year prospective study. Am J Infect Control. 2007;35:613–618. doi:10.1016/j.ajic.2006.09.007
18. Despotovic A, Miloservic B, Milosevic I, et al. Hospital-acquired infections in the adult intensive care unit—Epidemiology, antimicrobial resistance patterns, and risk factors for acquisition and mortality. Am J Infect Control. 2020;48(10):1211–1215. doi:10.1016/j.ajic.2020.01.009
19. Putra I, Irwanto I, Dharmawati I, et al. Microbial pattern and antibiotic susceptibility in pediatric intensive care unit Dr. Soetomo hospital, Surabaya. Indones J Trop Infect Dis. 2019;7(5):122. doi:10.20473/ijtid.v7i5.5737
20. Chang J, Yeh L, Li Y-C, et al. Predicting hospital-acquired infections by scoring system with simple parameters. PLoS One. 2011;6(8):e23137. doi:10.1371/journal.pone.0023137
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
