Back to Journals » International Journal of General Medicine » Volume 14
Prevalence and Structure of Periodontal Disease and Oral Cavity Condition in Patients with Coronary Heart Disease (Prospective Cohort Study)
Authors Gor I, Nadeem G, Bataev H, Dorofeev A
Received 12 August 2021
Accepted for publication 6 October 2021
Published 23 November 2021 Volume 2021:14 Pages 8573—8581
DOI https://doi.org/10.2147/IJGM.S330724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ilana Gor,1 Gulrez Nadeem,2 Hizir Bataev,3 Aleksey Dorofeev4
1Department of Surgical Dentistry, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation; 2Department of Basic Medical Sciences, Ajman University, Ajman, United Arab Emirates; 3Department of Faculty Therapy, Federal State Budgetary Institution of Higher Education Kadyrov Chechen State University, Grozny, Russian Federation; 4Department of Propaedeutics of Dental Diseases of the Institute of Dentistry, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
Correspondence: Gulrez Nadeem
Ajman University, 2116, B-Block Juma-Al majid Building, Ajman, United Arab Emirates
Tel +00971501557935
Email [email protected]
Purpose: To study the incidence and structure of periodontal disease in elderly Moscow residents suffering from permanent coronary heart disease, as well as examine the oral cavity and tooth structure in patients with generalized periodontitis and coronary heart disease.
Patients and Methods: Stage 1 (studying the incidence and structure of periodontal diseases) enrolled 330 patients over 50 years old: Group 1 consisted of 180 patients (102 males and 78 females) with stable coronary heart disease; Group 2 consisted of 150 dental patients (90 males and 60 females) with periodontal pathology without associated coronary heart disease. Stage 2 enrolled 216 patients with generalized periodontitis (studying features of the generalized periodontitis course depending on the coronary heart disease presence): Group 1 consisted of 145 patients with coronary heart disease and generalized periodontitis (79 males and 66 females), Group 2 consisted of 71 patients with generalized periodontitis but without coronary heart disease (40 males and 31 females).
Results: It has been established that 172 (95.6%) patients with coronary heart disease had periodontal disease with a predominance of generalized periodontitis in its structure, present in 145 (84.3%) people with coronary heart disease. A more severe clinical course distinguishes generalized periodontitis in patients with coronary heart disease than those without comorbid coronary heart disease. Moreover, it is characterized by a higher mean number of tooth loss (6.21± 0.16 vs 4.83± 0.12 teeth, p < 0.05), more teeth defects (54.69± 2.25% vs 21.15± 1.27%, p < 0.05), higher caries intensity level (11.07± 0.32 vs 8.55± 0.41, p < 0,05), clinical attachment loss (5.76± 0.09 mm vs 4.85± 0.10 mm, p < 0.05), and greater depth of periodontal pockets (4.80± 0.17 mm vs 3.64± 0.21 mm, p < 0.05).
Conclusion: Coronary heart disease is a favorable prerequisite for the development and progression of periodontal pathology.
Keywords: cardiovascular disease, generalized periodontitis and coronary heart disease, periodontal disease and coronary heart disease, periodontal disease structure in coronary heart disease
Introduction
Among non-infectious diseases, cardiovascular disease (CVD) is characterized by the highest mortality incidence worldwide in people of working age.1 CVDs caused 17.8 million deaths in 2017 alone, with most of these deaths occurring in low- and middle-income countries.2 In the United States, according to the American Heart Association, 92.1 million adults have at least one CVD, and 43.9% of the adult population in this country may have either form of cardiovascular pathology (CVP) by 2030. In the US, almost 795.000 people experience acute cerebral circulation disorders every year, of which 185.000 are recurrent, and 61.000 are encountered for the first time.3
A significant place among all heart-related disorders is occupied by coronary heart disease (CHD), which is associated with its high prevalence and, again, mortality among persons over 35 years.4–6 Among risk factors (RF) of CHD are age, sex, heredity, which belong to the unmodified RF of CHD, as well as dyslipidemia, smoking, arterial hypertension, diabetes mellitus, metabolic syndrome, obesity, hypoestrogenemia, and irrational nutrition, which belong to the modified RF.7,8
In addition to the traditional RF of CHD, other factors can contribute to the development and progression of this nosology, particularly dental diseases. More and more studies are being conducted in the last decade to examine and establish possible relationships between periodontal disease (PD) and CHD.1,6,9 Almost 92% of people with cardiac problems are diagnosed with PD, the course of which depends on the severity of the underlying disease. The prevalence of PD is from 20% to 50%, and this disease is common in developing countries and advanced economies, among adolescents, adults, and the elders.10 Thus, in Great Britain, almost half of the adult population suffers from irreversible periodontitis (P) of different seriousness, whose severity only increases with age.4 In the USA, 47.7% (64.7 million) of people over 30 years of age suffer from PD, of which 8.9% have P.6 According to some data, PD increases the risk of heart disorders by 19%, and diabetes mellitus patients of type 2 with PD have a 3.2 times higher risk of death compared to those without the PD.11
PDs are a group of chronic inflammatory diseases that affect the teeth’ hard and soft supporting tissues and are characterized by oral dysbiosis.12 So far, the etiology and pathogenesis of PD have not been finally studied, but infectious agents (periodontopathogens) are believed to be the main etiological factors of PD.13 The main trigger for chronic gingivitis and P is the imbalance of the microflora of the oral cavity, particularly the one that forms plaque. It is most likely that the appearance of PD is associated with the interaction of a complex of factors: a change in the dynamic interaction between certain types of oral microbes, the immune response of a particular person, genetic factors, as well as adverse environmental effects.1,13,14 The role of oral microflora in the pathogenesis of development and progression of PD is indisputable. Thus, it is known that human dental plaque comprises about 800 species of bacteria. The leading role in the emergence of PD has Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Eubacterium timidum, Prevotella intermedia, Parvimonas gingivalis, and Porphyromonas micra.13,15 Mechanical contamination and infection in the oral cavity lead to blotches on the gum, which then pass on to P with subsequent involvement in the inflammatory process of the root surface of the teeth and penetration of infection into the supporting structures of the teeth. The inflammatory process typically involves many neutrophils and macrophages and is accompanied by the release of numerous inflammatory mediators like interleukins, prostaglandins (in particular, prostaglandin E2), tumor necrosis factor-α, and others. Such a reaction of the immune system aims to destroy the pathogen and utilize the resulting necrotic tissue and apoptotic neutrophils by monocytes and macrophages.1,16 Under a high bacterial load of the pathogen and a changed or inadequate immune response, the process transforms into a chronic form with the occurrence of additional mediators. It leads to the attraction of many monocytes and T-cells to the lesion area.14,16 As a result of the long inflammatory process, reabsorption of alveolar bone by osteoclasts occurs, and under the action of matrix metalloproteinases, ligament fibers degrade, and granulation tissue grows.17
In recent years, more and more research efforts have been devoted to the role of new potential inflammation biomarkers in the pathogenesis of PD, including asymmetric dimethylarginine (ADMA), galectin-3, transglutaminases (TGs), nod-like receptor family pyrin domain-containing protein-3 (NLPR3), and Il-1β.18–21 Galectin-3 is a marker of cell growth and proliferation and can both modulate and hinder the processes of oxidative stress, apoptosis and inflammation. The study revealed that the blood level of galectin-3 increases in patients with periodontitis and case of periodontitis and CHD comorbidity.18
The PD pathogenesis is highly influenced by a decreased expression of transglutaminase (TG) mRNA, particularly TG-1 and TG-3, which are essential for cell adhesion and stabilization of the cellular matrix, and, accordingly remodulation and healing of gingival tissues. TG-1 and TG-3 mRNA expression was found to decrease significantly in chronic cancer patients.19 The main inflammasome complex NLPR3 plays a significant role in PP development through activation of caspases 1 and 5, which in turn lead to intracellular processing and the formation of IL-1β and IL-18 as important inflammation mediators.20
Among the RF of PD and CVD are heredity, age, endocrine system diseases and/or other accompanying somatic pathology, and smoking.10,11,14 Nicotine has been proved to cause the development and progression of destructive and inflammatory PD by dysfunction of its tissues through vascular and immune reactions.10 Violation of periodontal tissue microcirculation, one of the main causes of dystrophic and inflammatory changes in its tissues, is most often a consequence of CVDs. Also, the development of PD is affected by the disturbances in the system hemodynamics arising in the heart pathologies, activation of the processes of free-radical lipid oxidation, etc.22
Given the high prevalence of PD in patients with CVD (including CHD), detailed epidemiologic studies are needed in each region. It may provide a clearer picture of this problem in either region, within a particular population group, and so on. Since the information about the periodontal tissues in elderly patients suffering from CHD is somewhat inconsistent, and the indicators of how PP is spreading in this category of patients vary significantly, research efforts in this area are highly relevant and of scientific and practical interest. They may be an essential resource for developing new effective measures for preventing and treating this group of diseases.
The study aims to examine the prevalence and structure of periodontal diseases in the elderly Moscow citizens who suffer from permanent CHD, as well as the state of oral cavities and teeth in patients with generalized periodontitis and CHD.
Patients and Methods
The study was conducted in 2 stages. During stage 1, 330 patients divided into 2 groups were examined: Group 1 consisted of 180 patients (102 males (56.7%) and 78 females (43.3%)) over 50 years of age (mean age 62.52 ± 4.38 years), who suffered from permanent CHD; Group 2 consisted of 150 dental patients (90 males (60.0%) and 60 females (40.0%)) over 50 years of age (mean age 59.41 ± 3.26) with PP but without associated CHD. During this stage, the incidence and structure of PDs were examined depending on the presence of CHD. On Stage 2, the study included patients from the previous stage with diagnosed generalized periodontitis (GP) in a total amount of 216 patients: Group 1 included 145 patients with CHD and GP (79 males (54.5%) and 66 females (45.5%)); Group 2 included 71 patients with GP without CHD (40 males (56.3%) and 31 females (43.7%)). At this stage, clinical course features and the state of the oral cavity in patients with GP depending on the presence of comorbid CHD were studied.
The study’s inclusion criteria were the age of 50 years or more, PP (for patients without supervised CHD), diagnosis of permanent CHD (for patients with CHD), signed informed consent to participate in the study.
Exclusion criteria were loss of teeth due to trauma; unstable course of CHD, myocardial infarction and/or severe disruption of cerebral circulation earlier than 6 months before inclusion in the study; chronic somatic pathology in the stages of acute, sub-, decompensation, acute somatic pathology, acute and chronic surgical pathology, infectious diseases, oncological pathology, mental disorders, impairment of consciousness, prolonged smoking, alcohol or drug abuse, lack of compliance.
The trial was conducted following international ethical standards and principles. Patients were included in the study only after they gave their verbal and written consent for participation. The authors declare that the work is written with due consideration of ethical standards. The study was conducted in accordance with the ethical principles approved by the Ethics Committee of I.M. Sechenov First Moscow State Medical University (Protocol № 3 of 17.06.2020). The study was conducted in accordance with the Declaration of Helsinki.
All the patients underwent a standard dental examination of the oral cavity using conventional methods with obligatory intraoral and panoramic X-ray analysis to determine the depth of the pathological process, study the state of bone tissue, detect changes in the alveolar bone tissue, and assess the state of periodontal tissue. In order to objectively assess the state of the oral cavity as a whole, teeth and periodontal tissues, the defect of dental rows, caries intensity level (CIL), the depth of periodontal pockets, the clinical attachment loss (CAL), and the gum recession were determined. Also, Russell’s periodontal index and the hygienic index of the oral cavity by Green-Vermillion were calculated. Besides, there was a thorough assessment of complaints, the study of anamnesis, and the physical status of each patient. All data were entered into the registration card.
The CIL value was calculated as the sum of decayed (D), extracted (E), and filled (F) teeth in a particular patient, ie, the DEF index value was determined. The CIL was determined based on the DEF index value, depending on the patient’s age: for patients 50 to 59 years of age, a DEF score of 1 to 7 indicated low CIL, 8 to 15 scores – moderate CIL, 16 to 30 – high CIL, and 31 and above – extremely high CIL. For people aged 60 and more, values 1 to 8 indicated low CIL, 9 to 18 – moderate CIL, 19 and above – high CIL.
The periodontal pocket depth was determined using a graded probe with a diameter of 0.5 mm and marks after every 1 mm. For this purpose, the probe’s tip was inserted into the periodontal tooth pocket, fixing the probe depth. The periodontal pocket depth was calculated as the distance from the edge of the gum to the pocket’s deepest bottom point. The depth of the periodontal pockets was determined for every tooth on all surfaces, with the deepest measurement recorded. Typically, the integrity of the tooth-axillary junction is not altered, and the periodontal pockets are not identified. Also, the CAL was determined as the distance between the enamel-cement border and the pocket bottom. Afterward, the gum recession was assessed, ie, the distance between the clinical loss of gum attachment and the depth of the pocket probe. The decline of the gingival tissues with tooth cingulum exposure for <3 mm is considered a mild form of recession, 3–5 mm is moderate form, >5 mm is severe. The Green-Vermillion Index was calculated by visual evaluation of dental plaque or dental tartar area. For this purpose, vestibular surfaces 16, 11, 26, and 31 and lingual surfaces 36 and 46 were examined. The area of a tooth covered with plaque and/or tartar was estimated from 0 to 3 points, whereas 0 - plaque/tartar is absent, 1 - plaque/tartar covers not more than one-third of a tooth, 2 – two-thirds of a tooth, 3 – almost all of a tooth). The Green Vermillion Index value of 0 to 0.6 indicates good oral hygiene, 0.7 to 1.6 – satisfactory, 1.7 to 2.5 – unsatisfactory, 2.6 or more is a sign of inadequate hygiene.
To evaluate Russell’s periodontal index, all teeth (except for the third large root teeth) were examined in detail, considering the presence of gingivitis and its severity, tooth mobility, the presence/absence of periodontal pockets, bone destruction, etc. The condition of periodontal tissues was assessed separately for each tooth. The following formula was used to calculate the index: Russell’s Index = total sum of each tooth assessment/a total number of patients’ teeth. With Russell’s Index value from 0 to 1.4, it was considered that the patient has an initial or mild periodontal lesion, the value between 1.5 and 4.0 indicated a medium periodontal lesion, and that from 4.0 to 8.0 was a sign of a severe periodontal disorder.
A cardiologist verified CHD according to the recommendations of the European Society of Cardiologists 2019,23 based on complaints and anamnesis of the disease, the patient’s physical status, data from laboratory methods of examination electrocardiography (ECG), echocardiography, treadmill test, and Holter ECG monitoring. Heart failure functional class was established according to the New York Heart Association Functional Classification.
The Student t-criterion, Fisher F-criterion, Mann–Whitney U-criterion, and odds ratio (OR) calculation were used for statistical data processing. The difference was considered statistically significant at p<0.05. The data obtained were processed and analyzed using the SPSS 13 program.
Results
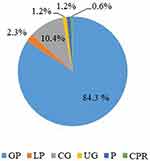
While studying the prevalence of PD in patients with CHD, periodontal tissue lesions were found in 172 (95.6%) people with CHD. It should be noted that the main periodontal pathology in the studied population is GP, which was found in 145 (84.3%) people (Figure 1). At that, GP of II severity degree prevailed, including GP of I degree of severity was present in 52 (35.9%) GP patients with CHD, GP of II degree of severity – in 75 (51.7%) GP patients with CHD, GP of III severity degree – in 18 (12.4%) GP patients with CHD. By the frequency of detection, GP was followed by catarrhal gingivitis (CG), which was present in 18 (10.4%) patients with CHD. Localized periodontitis (LP) was diagnosed in 4 (2.3%) people, ulcerative gingivitis (UG) – in 2 (1.2%) people, periodontosis (P) - in 2 (1.2%) people, and chronic pericoronaryte (CPR) – in 1 (0.6%) person. At the same time, the morbidity structure in patients with periodontal tissue lesions but without CHD and who applied for dental care was as follows (Figure 2): GP was present in 71 (47.4%) patients, LP – in 29 (19.3%) people, CG – in 44 (29.3%) people, UG - in 1 (0.7%) person, P - in 3 (2.0%) people, and CPR - in 2 (1.3%) people. A statistically significant intergroup difference in the prevalence of GP was (OR=4.61, 95% CI [2.83–7.52], p<0.05), LP (OR=10.55, 95% CI [3.62–30.77], p<0.05), and CG (OR=3.74, 95% CI [2.05–6.81], p<0.05).
It has been established that in patients with CHD, the prevalence of generalized PD increases significantly (p<0.05) with age, while the prevalence of CG decreases significantly (p<0.05). Thus, in the age group of 50–59 years, 52 (64.6%) patients with CHD suffered from GP, and among persons of 60 years old and older, 93 patients (92.1%) were diagnosed with CHD with a statistically significant difference between these age groups of (OR = 10.51, 95% CI [4.61–23.93], p<0.05). In the age group of 50–59 years old, 14 (14.1%) CHD patients suffered from GP, and at the age of 60 years and older, 4 (3.9%) people were diagnosed with this disease with a statistically significant intergroup difference of (OR=3.99, 95% CI [1.27–12.60], p<0.05).
A study of the prevalence of GP in patients with CHD by gender did not show a statistically significant difference between men and women with CHD (p> 0.05).
Besides, it has been established that the prevalence of generalized periodontal lesions raised with an increase in the functional class (FC) of heart failure (HF), namely: 10 (37.0%) out of 27 patients with the 1st FC according to NYHA had GP, 2nd FC was in 46 (76.7%) out of 60 patients, 3rd FC was revealed in 62 (95.4%) out of 65 patients, 27 (96.4%) out of 28 patients had the 4th FC with a statistically significant difference between patients with the 1st and 2nd FC (OR=5.58, 95% CI [2.09–14.94], p<0.05), 2nd and 3rd FC (OR=6.29, 95% CI [1.71–23.17], p<0.05), 1st and 3rd FC (OR=35.13, 95% CI [8.69–142.10], p<0.05), 1st and 4th FC (OR=45.90, 95% CI [5.38–391.40], p<0.05), and 2nd and 4th FC (OR=8.22, 95% CI [1.02–66.02], p<0.05).
The results of studying the features of the clinical course of GP in patients with CHD are presented in Table 1. It has been found that GP patients with CHD had 1.30 times higher tooth loss (p<0.05) than those who had GP without CHD. Accordingly, the prevalence of tooth row defects in GP patients with CHD was 2.60 times higher (p<0.05) than patients without heart disorders. Problems with teeth were also significantly higher revealed in patients with CHD - 1.30 times (p<0.05). The prevalence of pathological tooth erasure was significantly higher (p<0.05) in patients with CHD compared to those without heart disorders.
 |
Table 1 Oral and Dental Health Features of Patients with GP Depending on the Presence of CHD |
The depth of periodontal pockets in GP patients with CHD was 1.32 times (p<0.05) higher than in GP patients without CHD. Also, the CAL was 1.20 times (p<0.05) higher in patients with GP and CHD. The depth of the gum recession did not significantly differ between the comparison groups (p> 0.05). Periodontal Russell’s index was determined to be 1.35 times higher (p<0.05) in GP patients with CHD compared to those without CHD. The oral hygiene index of Green-Vermillion was also significantly higher in patients with CHD (p<0.05).
Discussion
A clinical part of the study aimed to establish the features and structure of GP in patients with stable forms of CHD. Consequently, 172 (95.6%) people with CHD were diagnosed with tooth pathology. GP significantly prevailed in the structure of teeth diseases (in particular, GP of II degree of severity), diagnosed in 145 (84.3%) people with CHD. For comparison, only 71 (47.4%) patients were diagnosed with GP among people with tooth pathology without CHD. Besides, among patients with teeth disorders and without CHD, the proportion of GP was rather high, amounting to 44 (29.3%) patients. In contrast, the number of patients with GP among patients with CHD was 18 (10.4%) (OR=3.74, 95% CI [2.05–6.81], p<0.05) (Figures 1 and 2). Therefore, CHD can be a favorable factor for the emergence and progression of teeth disorders, namely, its generalized forms. It is confirmed by the statistically significant difference in the prevalence of GP between a group of individuals with CHD and a group of individuals who suffered only from tooth pathology without CHD (OR = 4.61, 95% CI [2.83–7.52], p<0.05).
The analysis of the data obtained allows assuming that the clinical course of GP depends on the course of CHD, particularly on the FC of HF. Thus, with the increase of the FC of НF among the patients under study (p< 0.05), the frequency of HP increased, and in patients with CHD of FC III and IV according to NYHA, GP was diagnosed in 62 (95.4%) and 27 (96.4%) people, respectively, while there was a statistically significant difference in the prevalence rate of GP between patients with I and II FC (p<0.05), II and III FC (p<0.05), I and III FC (p<0.05), II and IV FC (p<0.05). There was no statistically significant difference in the prevalence of GP (p> 0.05) between patients with III and IV FC (p> 0.05), which may be because this study enrolled a small percentage of GP patients with FC IV, namely, only 28 (19.3%) people.
It has been established that the prevalence of GP in patients with CHD increases with age, which is confirmed by the presence of statistically significant difference (p<0.05) in the prevalence of GP between patients with CHD at the age of 50–59 years old and patients with CHD at the age of 60 and more years old (OR=10.51, 95% CI [4.61–23.93], p<0.05). It can be attributed to a long history of both GP and CHD in patients of the older age group, and, accordingly, more profound microcirculation disorders, which leads to the violation of periodontal tissue trophicity, and, therefore, to the development and progression of GP.
Thus, PP is widespread among patients with CHD, indicating the interrelation between the state of the oral cavity and the cardiovascular system: CVD, and stable CHD in a particular case, is favorable conditions for the development and progression of periodontal pathology. Patients with CHD should be considered those with an increased risk of PD development, especially generalized forms.
Results of oral cavity examination in patients with GP show that it has a more severe clinical course in patients with CHD than those who have GP without comorbid CHD. Thus, a significantly higher average number of tooth loss was established in the group of patients with GP with concomitant CHD compared to those with GP but without CHD (6.21±0.16 vs 4.83±0.12 teeth, p<0.05), more significant defects of teeth rows (54.69±2.25% vs 21.15±1.27%, p <0.05), the prevalence of pathological tooth abrasion (in 105 (72.5%) patients vs 20 (28.2%) patients (OR=6.69, 95% CI [3.56–12.60], p <0.05), higher CIL (11.07±0.32 vs 8, 55±0.41, p <0.05) and CAL (5.76±0.09 mm vs 4.85±0.10 mm, p <0.05), greater depth of periodontal pockets (4.80±0.17 mm vs 3.64±0.21 mm, p <0.05). Patients with GP and concomitant CHD exhibit more pronounced inflammatory-dystrophic changes of periodontal tissue than those without CHD. It is proved by quite high values of periodontal Russell Index in patients with GP and CHD (5.01±0.18 points vs 3.72±0.13 points in patients with GP but without CHD, p <0.05). Noteworthy is low level of oral hygiene in both comparison groups as per the Green–Vermillion index. The index was significantly higher in patients with GP and CHD compared to those without CHD (3.63±0.07 points vs 3.34±0.06 points, p <0.05). A low level of oral hygiene is also a significant factor in the emergence and progression of inflammatory and dystrophic changes in periodontal tissues in patients of both comparison groups. It is known that prolonged systemic persistence of pathogenic microflora in the oral cavity leads to a local and systemic chronic intoxication. As a result, numerous inflammatory mediators are formed in infected periodontal tissues, creating conditions for the development of CHD and atherosclerosis.1,24 In chronic GP, inflammation mediators, pro-inflammatory cytokines, and endotoxins are continuously fed into the bloodstream, resulting in the initiation of endothelium alteration in the vessels, infiltration of the vascular wall with lipids, hyperlipidemia, which leads to the emergence or progression of existing atherosclerosis.25
Another factor that may explain the high prevalence of teeth pathology, particularly GP, in patients with CHD is endothelial dysfunction in CHD patients and leads to the development and progression of microcirculatory disorders, which are the primary basis for inflammatory-dystrophic periodontal tissue lesions.22,26 The relationship between CHD and GP can be explained by the activation of the free-radical lipid oxidation (FRLO) processes and the imbalance in the antioxidant protection system, which underlie the pathogenesis of most diseases.22,27 In patients under study, activation of FRLO processes is probably a result of chronic hypoxia, which is a result of microcirculation disorders,22,28 activation of pro-inflammatory cytokines, and other inflammatory mediators, including ADMA,21 galectin-318, NLPR3 and IL-1β.20 In its turn, oxidative stress causes activation of the remodulation processes of target organs.22
In general, the results obtained are consistent with other studies on the relationship between PD and CHD.6,10,29 However, most of these studies are devoted to studying PD as a RF for CHD in general, the relationship between PD and atherosclerosis, and very few studies have been found on the relationship between PD and CHD. A recent meta-analysis performed by Qingwei C., which analyzed 15 studies conducted in China, including 2241 patients with CHD, shows that there is a significant relationship between periodontitis and the risk of CHD (OR=3.04, 95% CI [2.37–3.92], p<0.05).29,30
In another systematic review conducted by Batty et al,10 which analyzed a Korean study that included 975.685 people aged 35–90 years, found that 64.784 cases of CHD occurred during 21 years of observation. Also, a link between the loss of teeth and CHDs in both sexes has been established.10
In a study conducted in the US involving 6.300 people with CHD (women 56%, men 44%), who were under observation for 16.70 ± 5.50 years, it has been revealed that PPC-stage VII (Periodontal Profile Class System) of chronic periodontitis, which was characterized by severe tooth loss was significant for myocardial infarction (HR=1.59, 95% CI [1.13–2.23]), and PPC-stage V (moderate tooth loss and severe gum inflammation) was significant for fatal CHD (HR=5.27, 95% CI [1.80–15.40]).6
Conclusion
Thus, CHD is a good prerequisite for PP development and progression. Patients with CHD should be considered those who have an increased risk of PD development, especially generalized forms. GP is characterized by a more severe clinical course in patients with CHD compared to those diagnosed with GP but without comorbid CHD. Besides, there is a higher mean number of tooth loss (6.21±0.16 vs 4.83±0.12 teeth, p < 0.05), more significant defects of dental rows (54.69±2.25% vs 21.15±1.27%, p < 0.05), higher incidence of tooth abrasion (105 (72.5%) vs 20 (28.2%) patients (OR=6.69 95% CI [3.56–12.60], p < 0.05), higher CIL (11.07±0.32 vs 8.55±0.41), p < 0.05) and CAL (5.76±0.09 mm vs 4.85±0.10 mm, p < 0.05), and greater depth of periodontal pockets (4.80±0.17 mm vs 3.64±0.21 mm, p < 0.05). More pronounced inflammatory-dystrophic changes of periodontal tissue and lower level of oral cavity hygiene are more typical for patients with GP and concomitant CHD.
Prospects for Further Research
The prospect for further research is to study the general pathogenesis mechanisms of the development and progression of CHD and PDs, in particular, endothelial dysfunction, hyper-aggregation of platelets, free-radical lipid oxidation, lipid distress syndrome, cytokine homeostasis disorders, as well as the development of their integrated therapy based on the detected disorders.
Study Limitations
This study focused on the loss of teeth in patients with GP depending on the presence of CHD, but other causes of tooth losses cannot be excluded (eg, poor oral cavity care, smoking, poor diet, work in companies with exposure to toxic factors, etc.). Patients with tooth loss due to trauma or who abused alcohol or drugs for an extended period were not included in the study. However, there is no certainty that those included in the study never consumed alcohol or prohibited drugs. Experience has shown that most people hide such information.
Ethical Statement
The authors declare that the work is written with due consideration of ethical standards. The study was conducted in accordance with the ethical principles approved by the Ethics Committee of I.M. Sechenov First Moscow State Medical University (Protocol № 3 of 17.06.2020).
Informed Consent
The trial was conducted following international ethical standards and principles. Patients were included in the study only after they gave their verbal and written consent for participation.
Author Contributions
Ilana Gor – enrollment and examination of patients
Gulrez Nadeem – statistical data processing, formulation of primary statistical hypotheses, reference processing, translation of research materials into English.
Hizir Bataev – formulation and expression of primary scientific hypotheses on the relationship between coronary heart disease and the oral cavity condition in the examined patients, verification of cardiovascular pathology, enrollment of patients with coronary heart disease.
Aleksey Dorofeev – examination of patients, maintenance of primary documentation, the search of information and patents.
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27:1327–1334. doi:10.1016/j.hlc.2018.05.102
2. Zou Z, Cini K, Dong B, et al. Time trends in cardiovascular disease mortality across the BRICS: an age-period-cohort analysis of key nations with emerging economies using the global burden of disease study 2017. Circulation. 2020;141(10):790–799. doi:10.1161/CIRCULATIONAHA.119.042864
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics’2017 update: a report from the American Heart Association. Circulation. 2017;135(10):146–603.
4. Dietrich T, Webb I, Stenhouse L, et al. Evidence summary: the relationship between oral and cardiovascular disease. Br Dent J. 2017;222(5):381–385. doi:10.1038/sj.bdj.2017.224
5. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2017;15(4):230–240. doi:10.1038/nrcardio.2017.154
6. Beck JD, Philips K, Moss K, et al. Periodontal disease classifications and incident coronary heart disease in the atherosclerosis risk in communities study. J Periodontol. 2020;91(11):1409–1418. doi:10.1002/JPER.19-0723
7. Menotti A, Puddu PE, Kromhout D, Kafatos A, Tolonen H. Coronary heart disease mortality trends during 50 years as explained by risk factor changes: the European cohorts of the Seven Countries Study. Eur J Prev Cardiol. 2019;27(9):988–998. doi:10.1177/2047487318821250
8. Khukhlina OS, Kuzminska OB, Antoniv AA, Kopchuk TH, Melnychuk SP. The cytokeratin 18, adiponectin and leptin levels in patients with non-alcoholic steatohepatitis and coronary heart disease. Arch Balk Med Union. 2019;54(3):461–466. doi:10.31688/ABMU.2019.54.3.09
9. Sanz M, Marco Del Castillo A, Jepsen S, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47(3):268–288. doi:10.1111/jcpe.13189
10. Batty GD, Jung KJ, Mok Y, et al. Oral health and later coronary heart disease: cohort study of one million people. Eur J Prev Cardiol. 2018;25(6):598–605. doi:10.1177/2047487318759112
11. Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11(2):72–80.
12. Ferreira MC, Dias-Pereira AC, Branco-de-almeida LS, Martins CC, Paiva SM. Impact of periodontal disease on quality of life: a systematic review. J Periodontal Res. 2017;52(4):651–665. doi:10.1111/jre.12436
13. De Iuliis V, Ursi S, Di Tommaso LM, et al. Comparative molecular analysis of bacterial species associated with periodontal disease. J Biol Regul Homeost Agents. 2016;30(4):1209–1215.
14. Liccardo D, Cannavo A, Spagnuolo G, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 2019;20(6):1414. doi:10.3390/ijms20061414
15. Lourenço TG, Heller D, Silva-Boghossian CM, Cotton SL, Paster BJ, Colombo AP. Microbial signature profiles of periodontally healthy and diseased patients. J Clin Periodontol. 2014;41(11):1027–1036. doi:10.1111/jcpe.12302
16. Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11. doi:10.1016/j.it.2013.09.001
17. Franco C, Patricia HR, Timo S, Claudia B, Marcela H. Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci. 2017;18(2):440. doi:10.3390/ijms18020440
18. Isola G, Polizzi A, Alibrandi A, Williams RC, Lo Giudice A. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J Periodontal Res. 2021;56(3):597–605. doi:10.1111/jre.12860
19. Currò M, Matarese G, Isola G, et al. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral Dis. 2014;20(6):616–623. doi:10.1111/odi.12180
20. Isola G, Polizzi A, Santonocito S, et al. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J Periodontol. 2021. doi:10.1002/JPER.21-0049
21. Isola G, Alibrandi A, Currò M, et al. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J Periodontol. 2020;91:1076–1084. doi:10.1002/JPER.19-0446
22. Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. 2020;83:90–106. doi:10.1111/prd.12304
23. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41(1):111–188.
24. Mathews MJ, Mathews EH, Mathews GE. Oral health and coronary heart disease. BMC Oral Health. 2016;16(1):122. doi:10.1186/s12903-016-0316-7
25. Naderi S, Merchant AT. the association between periodontitis and cardiovascular disease: an update. Curr Atheroscler Rep. 2020;22(10):52. doi:10.1007/s11883-020-00878-0
26. Fujitani T, Aoyama N, Hirata F, Minabe M. Association between periodontitis and vascular endothelial function using noninvasive medical device - a pilot study. Clin Exp Dent Res. 2020;6(5):576–582. doi:10.1002/cre2.312
27. Shee F, Pralhad S, Natarajan S, et al. Cellular and biochemical changes in different categories of periodontitis: a patient-based study. J Int Soc Prev Community Dent. 2020;10(3):341–349. doi:10.4103/jispcd.JISPCD_42_20
28. Egorova SN, Bulygina IV, Vorobeva NV, Chuvashova DP, Mustafina NR. Modern Approaches to the technology of tablet dosage forms of thioctic acid (review). Drug Dev & Regist. 2021;10(2):32–41. doi:10.33380/2305-2066-2021-10-2-32-41
29. Cai QW, Ren L, Chen RX, Zou Y, Fu Q, Ma YY. Association between periodontal disease and coronary heart disease risk in Chinese population: evidence from a meta-analysis. Preprint. 2019.
30. Orlova AA, Strugar J, Shtark OY, Zhukov VA, Luzhanin VG, Povydysh MN. Use of metabolomic approaches in analysis of medicinal plants and phytopreparations (review). Drug Dev & Regist. 2021;10(1):97–105. doi:10.33380/2305-2066-2021-10-1-97-105
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.