Back to Journals » Nature and Science of Sleep » Volume 12
Prevalence and Risk Factors of Restless Legs Syndrome in Hemodialysis Patients
Authors Zhang LY, Ma XY, Lin J, Liu WH, Guo W, Yin L, Wang SX, Li X, Li J, Jin LL, Tian ZL , Du YT, Tuo HZ
Received 28 October 2019
Accepted for publication 25 December 2019
Published 14 January 2020 Volume 2020:12 Pages 19—27
DOI https://doi.org/10.2147/NSS.S236393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Steven A Shea
Li-Yan Zhang, 1,* Xiao-Yang Ma, 2,* Jun Lin, 3 Wen-Hu Liu, 4 Wang Guo, 4 Le Yin, 4 Shi-Xiang Wang, 3 Xia Li, 5 Jing Li, 5 Li-Li Jin, 6 Ze-Long Tian, 7 Yi-Tong Du, 1 Hou-Zhen Tuo 1
1Department of Neurology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Neurology, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Urology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Nephrology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Blood Purification Center, Beijing No. 6 Hospital, Beijing, People’s Republic of China; 6Department of Nephrology, Beijing Zhongxing Hospital, Beijing, People’s Republic of China; 7Department of Neurology, Tianjin 4th Central Hospital, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hou-Zhen Tuo
Department of Neurology, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong’an Road, Xicheng District, Beijing 100051, People’s Republic of China
Tel/Fax +86 10-63139807
Email [email protected]
Objective: The current study aimed to investigate the prevalence and risk factors of restless legs syndrome (RLS) in patients undergoing hemodialysis, as well as the mortality and risks of cardiovascular and cerebrovascular events.
Methods: A total of 354 hemodialysis patients from four hospitals were enrolled. RLS was diagnosed using the International RLS Study Group (IRLSSG) criteria. The patients were evaluated face-to-face using the IRLSSG rating scale, Epworth Sleepiness Scale (ESS), Hamilton Anxiety Scale, Hamilton Depression Scale, and Pittsburgh Sleep Quality Index (PSQI). The patients were followed up for 9 months. Death was considered an endpoint event. The cardiovascular and cerebrovascular events were investigated.
Results: The prevalence of RLS in hemodialysis patients was 40.7% and was associated with factors such as duration of hemodialysis, hypersensitive C-reactive protein, hyperparathyroidism, glycosylated serum protein, and erythropoietin treatment. The scores of the PSQI, ESS, and Hamilton Depression Scale in the RLS group were significantly higher than those in the non-RLS group (p < 0.05). During follow-ups, the incidence rate of cardiovascular diseases was 18.8% in the RLS group and 8.6% in the non-RLS group (p < 0.005). The IRLSSG rating scores were significantly higher in RLS patients with kidney transplantation failure compared with those without transplantation (p < 0.05).
Conclusion: The prevalence of RLS was high in hemodialysis patients. The risk factors of RLS included duration of hemodialysis, hypersensitive C-reactive protein, hyperparathyroidism, glycosylated serum protein, and erythropoietin treatment. RLS affected sleep quality and emotion and increased the risk of cardiovascular diseases in hemodialysis patients. RLS was more severe in patients with kidney transplantation failure compared with those without transplantation.
Keywords: restless legs syndrome, hemodialysis, prevalence, risk factor, cardiovascular disease
Introduction
Restless legs syndrome/Willis-Ekbom disease (RLS/WED) is a common sleep-related movement disorder. It can be idiopathic or occurs in comorbidity with other medical conditions such as polyneuropathy, iron deficiency anemia, multiple sclerosis, hypertension, and cardiovascular disease (cardiovascular diseases). The RLS phenotype is an unusual composite of sensory and motor symptoms that presents with distinct circadian rhythmicity. Patients with RLS feel an overwhelming urge to move, often in conjunction with unpleasant sensations, usually in the legs. Rest and inactivity provoke the symptoms, whereas movement and other external stimuli lead to temporary relief.
RLS affects 3.5–10% of adults in the general population.1–3 A significantly higher prevalence of RLS ranging from 12% to 62% has been reported in patients with end-stage renal disease.4–7 The pathophysiology of RLS in hemodialysis patients is not well established. Researchers have proposed several risk factors, such as diabetes mellitus, use of coffee, afternoon shift of dialysis, female sex, lower hemoglobin (Hb), and homocysteine.7–9 A meta-analysis suggested that RLS in dialysis patients was strongly associated with low Hb and low iron, but not associated with sex, age, duration of dialysis, creatinine, phosphorus, calcium, parathyroid hormone, blood urea nitrogen, albumin, and body mass index.10 RLS can causes sleep disorders and affects the quality of life,11,12 leading to increased risks of depression, anxiety, psychological disorder,13 and fatigue. It has been reported that RLS was associated with an increased risk of cardiovascular diseases both in the primary2,14–16 and uremic forms.17,18 Severe RLS may increase mortality in patients with end-stage renal disease.2,17,19 However, a study with 15 years of follow-up of hemodialysis patients found that the mortality is not influenced by concomitant RLS in end-stage renal disease patients.20
RLS is also common in kidney transplant recipients with a prevalence of 51%.21 Winkelmann et al22 reported the symptoms of uremic RLS disappeared after kidney transplantation in all 11 patients but reappeared after several years in the patients with transplantation failure. This suggests that renal dysfunction is associated with the development of RLS. However, other studies have suggested that sleep disorders including RLS in kidney transplant recipients were due to the underlying illness and the surgery itself.23 A combination of renal dysfunction, surgery, and immunosuppressive agents exists in patients with kidney transplantation failure.
The current study aimed to investigate the prevalence and risk factors of RLS in hemodialysis patients in Beijing, China. The effect of RLS on sleep, emotion, and risks of cardiovascular and cerebrovascular disease was also analyzed.
Materials and Methods
Patients
The current study is a cross-sectional study including 354 hemodialysis patients recruited from four hospitals in Beijing, China (Figure 1). The four hospitals were Beijing Friendship Hospital, Beijing Chaoyang Hospital, No. 6 Beijing Hospital, and Beijing Zhongxing Hospital, each located in a distinct district of the Beijing city. The study protocol was approved by the ethics committees of all hospitals, and was conducted in accordance with the Declaration of Helsinki, and all transplant procedures had been conducted in accordance with the Declaration of Istanbul. Adult patients who had hemodialysis at least twice a week were eligible to participate. Written informed consent was acquired from all participants. The exclusion criteria were: (1) patients with impaired cognition who cannot respond to the questionnaires as assessed using the Mini-Mental State Examination; (2) patients with secondary cause of RLS such as pregnancy, epilepsy, multiple sclerosis, narcolepsy, previous diagnosis or present symptoms of sleep apnea, and involuntary movement disorders. Neurological resident doctors screened the patients and checked the medical records. The face-to-face questionnaires contained medical history and some symptoms description for excluding the secondary cause of RLS. If the resident doctors were not sure about the patient eligibility, a neurological attending doctor or consultant rechecked the patient with physical examination if necessary.
 |
Figure 1 Flow chart of patient enrollment. |
Demographic and Medical History
All questionnaires were administered by neurologist in a face-to-face manner. This part of information was derived from the interview and medical records. The patient general information included age, sex, height, weight, body mass index, smoking (Yes = ever, No = never), drinking (Yes = ever, No = never), tea or coffee (Yes = weekly, No = never or occasionally), and exercise (Yes = weekly exercise > 30 min, No = weekly exercise < 30 min). The hemodialysis information included hemodialysis schedules, etiology of the kidney disease, and kidney transplantation. Comorbidity information such as past illnesses and hyperparathyroidism was based on medical records. Use of the following medications was included in the analysis: antiplatelet agents, antihypertensive agents, antidiabetic agents, insulin, neurotrophic agents, calcium supplementation agents, iron supplementation agents, erythropoietin, antidepressant agents, sedative agents, antihistamine agents, and antiemetic agents.
RLS Questionnaire
RLS was diagnosed by neurologists using the 2014 IRLSSG criteria.24 Other information included RLS-involved limbs (1 = left upper limb, 2 = right upper limb, 3 = left lower limb, 4 = right lower limb; both lower limbs = 3 + 4, both upper limbs = 1 + 2, unilateral limb = 1, 2, 3, 4, 1 + 3, or 2 + 4), RLS symptoms on-set time (prior to or after the initiation of maintenance hemodialysis), previous diagnosis of RLS (Yes or No; if Yes, prior to or after the initiation of hemodialysis), awareness of RLS (never heard about RLS or aware of RLS symptoms), family history of RLS (Yes = at least one family member of parents, siblings, or offspring was diagnosed with RLS), RLS severity in the first and second nights after hemodialysis (subjective scores ranging from 1 to 9, higher scores mean more severe symptoms), and treatment of RLS. The IRLSSG rating scale was used to evaluate the severity of RLS,25 which was classified as mild (scores 1–10), moderate (scores 11–20), severe (scores 21–30), and critical (scores 31–40).
Sleep and Emotion Scales
The Epworth sleeping scale (ESS) was used to evaluate the excessive daytime sleepiness (scores ranging from 0 to 24, higher scores suggest worse somnolence).26 The Pittsburgh Sleep Quality Index (PSQI) was used for sleep quality evaluation with higher scores suggesting worse sleep quality.27 The Hamilton Anxiety Scale28 scale was used for anxiety evaluation, with scores ranging from 0 to 56, which grades anxiety severity as no anxiety (0–6), possible anxiety (7–13), mild anxiety (14–20), moderate anxiety (21–28), and severe anxiety (29–56). The Hamilton Depression Scale28 was used to evaluate depression, with scores ranging from 0 to 78, which grades depression severity as no depression (0–7), possible depression (8–20), mild and moderate depression (21–35), and severe depression (36–78).
Follow-Up
Patients were followed up by interview 9 months later. If the patients felt any discomfort or the patients were found to omit any hemodialysis, the doctors in nephrology or urology department would inform the neurologists for the telephone call and temporary interview. Then the neurologists would interview the patients and check the medical records. At the 9 months follow-up or temporary follow-up interview, cardiovascular and cerebrovascular events were recorded. Cardiovascular events include myocardial infarction, angina pectoris, heart failure, arrhythmia, and hypertension. Cerebrovascular events include cerebral infarction, cerebral hemorrhage, and transient ischemia attack. Death was regarded as the endpoint.
Laboratory Tests
Blood tests were conducted at once or within the last 14 days. The laboratory tests included blood routine test, hypersensitive C-reactive protein, parathyroid hormone, blood urine nitrogen, creatinine, uric acid, serum sodium, serum potassium, serum calcium, serum phosphate, serum chloride, serum glucose, serum cholesterol, triglyceride, low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), glycosylated serum protein, serum ferritin, serum iron, total iron binding capacity, unsaturated iron binding capacity, folic acid, and vitamin B12. Hyperparathyroidism was diagnosed with increased levels of parathyroid hormone.
Statistical Analysis
The estimated sample size was 92 patients per group with the α value of 0.05 and a predicted prevalence of 40%. The screening duration was 3 months and 354 patients were enrolled. The quantitative data were expressed as means ± standard deviations. The qualitative data were presented as frequencies or percentages. All analyses were carried out using the SPSS (Statistical Package version 19.0). The level of statistical significance was set at p < 0.05. All quantitative data were tested to be in normal distribution. The differences between the RLS and the non-RLS groups were analyzed using the independent samples t-test or the χ2 test. The factors with a p-value less than 0.1 were included in the multivariable logistic regression analysis to explore their association with RLS. The statistical part of the study was reviewed by a biomedical statistician.
Results
General Patient Information
A total of 354 hemodialysis patients in the 4 hospitals were enrolled in the current study during the 3 months. There were 198 (198/354, 55.9%) male patients. The mean age was 55.8 ±12.7 years and the mean hemodialysis duration was 86.0 ± 63.7 months. There were 94 (26.5%) patients with a history of kidney transplantation failure.
RLS Prevalence
Among the 354 enrolled hemodialysis patients, the prevalence of RLS was 40.7% (144/354). And 82.6% (119/144) of the RLS patients presented the symptoms after the initiation of hemodialysis within a mean time of 44.2 months. The other 17.4% (25/144) of the RLS patients presented the symptoms prior to the initiation of hemodialysis and 3 of them had a family history of RLS. The prevalence of RLS in males was significantly lower than that in females (35.9%, 71/198 vs 46.8%, 73/156; p = 0.038). The awareness rate of RLS was 27.7% (98/354) in the hemodialysis patients and 41.7% (60/144) in patients with RLS. At 9 months of follow-up, 69.7% (239/343) of the RLS patients were had been treated with medicines such as iron supplements, dopamine receptor agonists, levodopa, and sedatives.
RLS Severity
For the 144 RLS patients, the mean IRLSSG score was 15.0 ± 7.4. The severity of RLS was graded in 41 patients (28.5%) as mild, 68 patients (47.2%) as moderate, 28 patients (19.4%) as severe, and 7 patients (4.8%) as critical. RLS patients with kidney transplantation failure (n = 46) had a significantly higher IRLSSG score of 17.2 ± 7.8 than those without (n = 98, 15.4 ± 7.1, p = 0.038). Of the 144 RLS patients, 78 patients (54.2%) did not feel significant difference in RLS severity between the first night and the second night after hemodialysis. However, 38 patients (26.4%) and 26 patients had greater RLS severity on the first night and the second night, respectively. There was no significant difference in RLS severity between the patients with upper limb involvement and those with low limb involvement alone (14.7 ± 8.3 vs 16.0 ± 7.3, p = 0.552).
Clinical Features of RLS
The lower limbs were involved in 90.97% (131/144) of the RLS patients. Discomfort in the upper limbs was observed in 2.8% (4/144) of the RLS patients. Unilateral limb discomfort was observed in 6.3% (9/144) of the RLS patients.
Possible Risk Factors of RLS
Complete data were available in 307 patients (307/354, 86.7%), including 125 RLS-patients and 183 non-RLS patients. The possible risk factors and laboratory test results were compared between the RLS group and non-RLS group using the independent samples t-test or the χ2. Hemodialysis duration, female sex, erythropoietin treatment, hypertension, and drinking were significantly different between the two groups (Table 1). Significant difference was found in serum levels of chloride, potassium, and phosphorus between the RLS and non-RLS groups (Table 2).
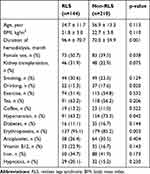 |
Table 1 Possible Risk Factors of Restless Legs Syndrome (Part 1) |
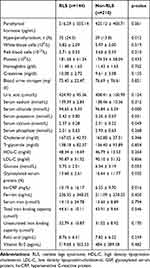 |
Table 2 Possible Risk Factors of Restless Legs Syndrome (Part 2) |
The factors with a p-value less than 0.1 in the univariate analysis were included in the logistic regression analysis. These factors were sex, duration of hemodialysis, drinking, erythropoietin treatment, hypersensitive C-reactive protein (hs-CRP), serum glucose, glycosylated serum protein, and hyperparathyroidism. Hemoglobin and serum iron were also included. The dependent variable is restless legs syndrome (present/absent) (Table 3).
 |
Table 3 Multivariable Logistic Regression Analysis of the Possible Risk Factors of Restless Legs Syndrome |
Association Between RLS and Sleep/Emotion
Significant difference was observed in the PSQI scores and the ESS scores between the RLS and non-RLS groups (independent samples t-test). In the PSQI dimensions, sleep quality, sleep latency, and daytime dysfunction were significantly different. There was no significant difference between the Hamilton Anxiety Scale scores. However, the Hamilton Depression Scale scores differed significantly between the two groups (Table 4).
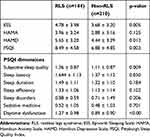 |
Table 4 Assessments of Sleep and Emotion in the RLS and Non-RLS Groups |
Association Between RLS and Risks of Mortality and Cardiovascular/Cerebrovascular Events
Our patients were followed up for 9 months. Eleven patients (3.11%, 11/354) died during the follow-up. Reasons of death included heart diseases (8/11), tumor (1/11), leukemia (1/11), and tuberculosis (1/11). Significant difference (p = 0.004, χ2 test) was observed in the percentage of newly diagnosed cardiovascular diseases, but not in newly diagnosed cerebrovascular diseases and mortality between the RLS and non-RLS groups (Table 5).
 |
Table 5 Prognosis and Follow-Up of the RLS and Non-RLS Groups |
Discussion
In the current study, the hemodialysis patients were enrolled from four hospitals located in four districts of the Beijing city, which is a representative patient group. The IRLSSG diagnostic criteria was used for RLS diagnosis. The current study included 354 hemodialysis patients and the RLS prevalence was 40.7%, which is similar to a previously reported prevalence of 40%.29 A subgroup of patients with kidney transplantation failure had an RLS prevalence of 48.9% (46/94). This might contribute to the high prevalence in the current study. Despite the high prevalence of RLS in the hemodialysis patients in the current study, the awareness rate of RLS was 27.7%, which was quite low. The low awareness rate of RLS may impede the early diagnosis of this disease.
In the current study, the on-set of RLS symptoms was after the initiation of hemodialysis in 82.6% (119/144) of the RLS patients. In addition, the current study found that the duration of hemodialysis was associated with the prevalence of RLS. It has been suggested that the internal environment disturbances and accumulation of toxins, especially the middle-molecular-weight toxins, may affect nerve conduction and lead to nerve fiber degeneration and demyelination, and finally RLS.7,30 A supporting evidence is that hemodialysis filtration and hemoperfusion can remove the middle-molecular-weight toxins and relieve the RLS symptoms.31 Another 25 RLS patients in the current study presented the RLS symptoms prior to the initiation of hemodialysis, and three of them had a family history of RLS, suggesting that genetics may play a role in the development of RLS, especially the early-onset RLS.
The most frequently affected limbs were the lower limbs in our patients. The IRLSSG rating scale was employed to evaluate the severity of RLS. Compared to the primary RLS patients with mild or moderate severity levels, most of the RLS patients with hemodialysis (66.7%) had moderate or severe RLS. Sabry et al29 reported that 65% of RLS patients had moderate or severe levels of RLS in hemodialysis patients, which is consistent with our findings.
In the current study, we focused on the subgroup of hemodialysis patients with kidney transplantation failure. Winkelmann et al22 reported that successful kidney transplantation could improve the RLS symptoms and that patients with good renal function were asymptomatic during 4–9 years of follow-ups. However, three patients with kidney transplantation failure had recurred RLS symptoms, and the severity of the RLS symptoms was reduced compared with that before transplantation. In this study, 94 patients with kidney transplantation failure were included. The current study found that kidney transplantation was not associated with RLS prevalence. However, RLS patients with kidney transplantation failure had more severe RLS symptoms in comparison with RLS patients without kidney transplantation failure. This finding suggests that the surgery or the immunosuppressive agents may affect the RLS severity.
Previous studies10,16,32 have shown that risk factors of RLS include female sex, younger age, high levels of serum β2 microglobulin and parathyroid hormone, low levels of hemoglobin and serum iron, diabetes, calcium/phosphorus imbalance, peripheral nerve damage, and renal disease. The current study found that female sex was not associated with the prevalence of RLS, which is consistent with the results of a previous meta-analysis.10 However, given that pregnancy is a cause of secondary RLS, increased estrogen levels might lead to susceptibility to RLS.
In the current study, a significantly higher proportion of RLS patients had used erythropoietin compared with the non-RLS patients, which is consistent with previous findings.16 However, the serum levels of hemoglobin and iron were not significantly different between the RLS and non-RLS groups. Renal anemia is common in hemodialysis patients and erythropoietin is frequently used to treat this condition. Long-term use of erythropoietin may relieve renal anemia and increase hemoglobin levels. We speculate that renal anemia was more serious in the RLS patients, and erythropoietin is needed to treat the anemia in these patients. There was no significant difference in hemoglobin levels between the RLS and non-RLS patients, which may be due to the use of erythropoietin in more RLS patients. In the current study, erythropoietin was found to be a protective factor for RLS. This suggests the necessity to start renal anemia treatment as soon as possible in hemodialysis patients.
It has been reported that increased levels of parathyroid hormone are associated with RLS,10,32 which is consistent with our findings. Parathyroid hormone is a middle-molecular-weight toxin and its accumulation can compromise nerve conduction and cause fiber demyelination. Elevated parathyroid hormone levels are associated with poor sleep quality in patients with uremic RLS.9 Surgical treatment of parathyroidectomy can improve the symptoms of RLS in patients with severe hyperparathyroidism.38 In the current study, nine RLS patients were treated with parathyroidectomy and the RLS symptoms were relieved. Our findings indicate a possible link between RLS severity and parathyroid function.
It has been shown that 73.5% to 88.5% of hemodialysis patients have sleep disorders and the main manifestation is difficulty falling asleep (84.5%).14,15,33 Patients with RLS are at increased risks of insomnia and excessive daytime sleepiness.7,34 RLS is also a risk factor affecting sleep quality in patients with renal diseases.35 It has been shown that patients with end-stage renal diseases and RLS had significantly higher PSQI scores compared with those without RLS.36 Similarly, the current study found that hemodialysis patients with RLS had significantly higher PSQI scores than those without RLS. In the PSQI dimensions, the scores of subjective sleep quality, sleep latency, and daytime dysfunction were significantly different between the RLS and non-RLS groups. The ESS showed that daytime somnolence was more severe in hemodialysis patients with RLS than those without RLS. These results suggest that RLS can influence sleep quality in hemodialysis patients. The Hamilton Depression Scale scores also differed significantly between the RLS and non-RLS group, showing that depression was more severe in hemodialysis patients with RLS. It is possible that sleep disorders may worsen the emotional change, especially in RLS patients with hemodialysis.
The short survival time of RLS patients with uremia might be related to the higher risk of cardiovascular diseases.37 It has been reported that end-stage renal disease patients with RLS are at increased risk of cardiovascular diseases due to poor sleep quality and deterioration of the body condition.14,38,39 In the follow-up of the current study, the mortality of RLS patients was slightly higher, though not significant. It has been suggested that RLS patients with hemodialysis are more likely to develop cardiovascular diseases due to the compromised cardiac structure and function, sarcopenia, insulin resistance, and kidney fatty infiltration.37 The current study found that the common risk factors of cardiovascular diseases such as history of diabetes, smoking, and physical inactivity were not significantly different between the RLS and non-RLS groups. The increased risks of cardiovascular diseases may be associated with renal dysfunction such as uremia, mineral metabolism disorders, inflammation, oxidative stress, and malnutrition.38
Chronic inflammation is common in hemodialysis patients and may play an important role in the development of cardiovascular diseases. In the current study, the logistic regression analysis showed that hs-CRP was associated with RLS, suggesting a possible link between inflammation and RLS. It has been shown that hs-CRP, an inflammation factor, is associated with atherosclerosis plaque and cardiovascular diseases.40 A meta-analysis suggested that hs-CRP is a risk factor for cardiovascular diseases and can increase the mortality of cardiovascular diseases.41 The increased cardiovascular diseases events in our patients may partly be associated with the high hs-CRP levels in hemodialysis patients with RLS. We speculate that sleep disturbance and depression in RLS may contribute to the elevated risks of cardiovascular diseases.
The current study has limitations. First, despite that hs-CRP was found to be associated with RLS, our cross-sectional study cannot confirm the causal relationship between hs-CRP and RLS. Second, the data of drinking, smoking, coffee, tea, and exercise were categorical, and may not provide adequate information to reveal their association with RLS. Third, the follow-up time was short and more precise categories are needed.
Conclusions
The prevalence of RLS was high in hemodialysis patients, contrasting to the low awareness rate of this disease. Risk factors of RLS included hemodialysis duration, hs-CRP, glycosylated serum protein, hyperparathyroidism, and erythropoietin treatment. RLS can affect sleep quality and emotion and increase the risk of cardiovascular diseases in hemodialysis patients. RLS was more severe in patients with kidney transplantation failure compared with those without transplantation.
Author Contributions
Li-Yan Zhang and Xiao-Yang Ma contributed to the study design, data collection, data analysis and draft of the initial manuscript. They contributed equally to the work. Jun Li, Wen-Hu Liu, Wang Guo, Xia Li, Jing Li and Li-Li Jin contributed to subject enrollment. Ze-Long Tian and Yi-Tong Du assisted in data collection and data analysis. Hou-Zhen Tuo conceptualized the study, contributed to data collection and analysis, and critically reviewed the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the submitted article is not related to any financial interest/relationship and that they have no conflicts of interest in this work.
References
1. Mallon L, Broman JE, Hetta J. Restless legs symptoms with sleepiness in relation to mortality: 20-year follow-up study of a middle-aged Swedish population. Psychiatry Clin Neurosci. 2008;62(4):457–463. doi:10.1111/j.1440-1819.2008.01831.x
2. Molnar MZ, Lu JL, Kalantar-Zadeh K, Kovesdy CP. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res. 2016;25(1):47–56. doi:10.1111/jsr.2016.25.issue-1
3. Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 2010;11(1):31–37. doi:10.1016/j.sleep.2009.03.007
4. Merlino G, Piani A, Dolso P, et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21(1):184–190. doi:10.1093/ndt/gfi144
5. Perl J, Unruh ML, Chan CT. Sleep disorders in end-stage renal disease: ‘Markers of inadequate dialysis’? Kidney Int. 2006;70(10):1687–1693.
6. Kim JM, Kwon HM, Lim CS, Kim YS, Lee SJ, Nam H. Restless legs syndrome in patients on hemodialysis: symptom severity and risk factors. J Clin Neurol. 2008;4(4):153–157. doi:10.3988/jcn.2008.4.4.153
7. Al-Jahdali HH, Al-Qadhi WA, Khogeer HA, Al-Hejaili FF, Al-Ghamdi SM, Al Sayyari AA. Restless legs syndrome in patients on dialysis. Saudi J Kidney Dis Transpl. 2009;20(3):378–385.
8. Araujo SM, de Bruin VM, Nepomuceno LA, et al. Restless legs syndrome in end-stage renal disease: clinical characteristics and associated comorbidities. Sleep Med. 2010;11(8):785–790. doi:10.1016/j.sleep.2010.02.011
9. Gade K, Blaschke S, Rodenbeck A, Becker A, Anderson-Schmidt H, Cohrs S. Uremic restless legs syndrome (RLS) and sleep quality in patients with end-stage renal disease on hemodialysis: potential role of homocysteine and parathyroid hormone. Kidney Blood Press Res. 2013;37(4–5):458–463. doi:10.1159/000355727
10. Mao S, Shen H, Huang S, Zhang A. Restless legs syndrome in dialysis patients: a meta-analysis. Sleep Med. 2014;15(12):1532–1538. doi:10.1016/j.sleep.2014.07.017
11. Gkizlis V, Giannaki CD, Karatzaferi C, et al. Uremic versus idiopathic restless legs syndrome: impact on aspects related to quality of life. ASAIO J. 2012;58(6):607–611. doi:10.1097/MAT.0b013e31826d6090
12. Dikici S, Bahadir A, Baltaci D, et al. Association of anxiety, sleepiness, and sexual dysfunction with restless legs syndrome in hemodialysis patients. Hemodial Int. 2014;18(4):809–818. doi:10.1111/hdi.2014.18.issue-4
13. DeFerio JJ, Govindarajulu U, Brar A, Cukor D, Lee KG, Salifu MO. Association of restless legs syndrome and mortality in end-stage renal disease: an analysis of the United States Renal Data System (USRDS). BMC Nephrol. 2017;18(1):258. doi:10.1186/s12882-017-0660-0
14. Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. doi:10.1212/01.wnl.0000287072.93277.c9
15. Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126(14):1689–1694. doi:10.1161/CIRCULATIONAHA.112.112698
16. Stolic RV, Trajkovic GZ, Jekic D, et al. Predictive parameters of survival in hemodialysis patients with restless leg syndrome. Saudi J Kidney Dis Transpl. 2014;25(5):974–980. doi:10.4103/1319-2442.139869
17. La Manna G, Pizza F, Persici E, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26(6):1976–1983. doi:10.1093/ndt/gfq681
18. Lin CH, Sy HN, Chang HW, et al. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol. 2015;22(1):142–149. doi:10.1111/ene.12545
19. Molnar MZ, Szentkiralyi A, Lindner A, et al. Restless legs syndrome and mortality in kidney transplant recipients. Am J Kidney Dis. 2007;50(5):813–820. doi:10.1053/j.ajkd.2007.08.003
20. Baiardi S, Mondini S, Baldi Antognini A, Santoro A, Cirignotta F. Survival of dialysis patients with restless legs syndrome: a 15-year follow-up study. Am J Nephrol. 2017;46(3):224–230. doi:10.1159/000479938
21. Naini AE, Amra B, Mahmoodnia L, Taheri S. Sleep apnea syndrome and restless legs syndrome in kidney transplant recipients. Adv Biomed Res. 2015;4:206.
22. Winkelmann J, Stautner A, Samtleben W, Trenkwalder C. Long-term course of restless legs syndrome in dialysis patients after kidney transplantation. Mov Disord. 2002;17(5):1072–1076. doi:10.1002/(ISSN)1531-8257
23. Burkhalter H, Brunner DP, Wirz-Justice A, et al. Self-reported sleep disturbances in renal transplant recipients. BMC Nephrol. 2013;14:220. doi:10.1186/1471-2369-14-220
24. Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi:10.1016/j.sleep.2014.03.025
25. Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132.
26. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
27. Buysse DJ, Reynolds CF
28. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. doi:10.3322/caac.20140
29. Sabry AA, Abo-Zenah H, Wafa E, et al. Sleep disorders in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21(2):300–305.
30. Merlino G, Lorenzut S, Romano G, et al. Restless legs syndrome in dialysis patients: a comparison between hemodialysis and continuous ambulatory peritoneal dialysis. Neurol Sci. 2012;33(6):1311–1318. doi:10.1007/s10072-012-0953-9
31. Urabe S, Hosono T, Hyodo T, et al. Restless legs syndrome effectively treated with constant-pressure predilution online hemodiafiltration. J Artif Organs. 2019;22(3):253–255. doi:10.1007/s10047-019-01100-y
32. Stefanidis I, Vainas A, Dardiotis E, et al. Restless legs syndrome in hemodialysis patients: an epidemiologic survey in Greece. Sleep Med. 2013;14(12):1381–1386. doi:10.1016/j.sleep.2013.05.022
33. Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology. 2013;81(1):52–59. doi:10.1212/WNL.0b013e318297eee0
34. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi:10.1016/S1389-9457(03)00010-8
35. Lee J, Nicholl DD, Ahmed SB, et al. The prevalence of restless legs syndrome across the full spectrum of kidney disease. J Clin Sleep Med. 2013;9(5):455–459. doi:10.5664/jcsm.2664
36. Liborio AB, Santos JP, Minete NF, de Diogenes CA, Farias Lde A, de Bruin VM. Restless legs syndrome and quality of sleep in patients with glomerulopathy. BMC Nephrol. 2013;14:113. doi:10.1186/1471-2369-14-113
37. Sakkas GK, Giannaki CD, Karatzaferi C, et al. Current trends in the management of uremic restless legs syndrome: a systematic review on aspects related to quality of life, cardiovascular mortality and survival. Sleep Med Rev. 2015;21:39–49. doi:10.1016/j.smrv.2014.07.006
38. Theofilou P. Association of insomnia symptoms with kidney disease quality of life reported by patients on maintenance dialysis. Psychol Health Med. 2013;18(1):70–78. doi:10.1080/13548506.2012.674144
39. Giannaki CD, Zigoulis P, Karatzaferi C, et al. Periodic limb movements in sleep contribute to further cardiac structure abnormalities in hemodialysis patients with restless legs syndrome. J Clin Sleep Med. 2013;9(2):147–153. doi:10.5664/jcsm.2412
40. Burke AP, Tracy RP, Kolodgie F, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105(17):2019–2023. doi:10.1161/01.CIR.0000015507.29953.38
41. Diaz CJ, Nunez AC, Flores MI, Arcaute HD, Archondo T. [Haemostatic and inflammation markers in acute coronary syndromes and its relationship with adverse cardiovascular events]. Arch Cardiol Mex. 2006;76(4):366–375.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
