Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease Among Hospital Staff
Authors Zhang D , Zhang L, Chen S , Chen R, Zhang X, Bai F
Received 22 February 2023
Accepted for publication 12 April 2023
Published 27 April 2023 Volume 2023:16 Pages 1221—1234
DOI https://doi.org/10.2147/DMSO.S407657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Daya Zhang,1,* Lijun Zhang,2,* Shiju Chen,1,* Runxiang Chen,1 Xiaodong Zhang,1 Feihu Bai3,4
1Graduate School, Hainan Medical University, Haikou, People’s Republic of China; 2Medical Examination Center, The Second Affiliated Hospital of Hainan Medical University, Haikou, People’s Republic of China; 3Department of Gastroenterology, The Second Affiliated Hospital of Hainan Medical University, Haikou, People’s Republic of China; 4The Gastroenterology Clinical Medical Center of Hainan Province, Haikou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feihu Bai, Chief Physician and Professor of Department of Gastroenterology, The Second Affiliated Hospital of Hainan Medical University, Yehai Avenue, #368, Longhua District, Haikou, Hainan Province, People’s Republic of China, Tel +86-18995181963, Fax +86 898-66809168, Email [email protected]
Background: The prevalence of metabolism-related fatty liver disease (MAFLD) has been rarely reported in hospital staffs. The aim of this study was to assess the prevalence and risk factors for MAFLD in hospital staffs aged ≥ 18 years.
Methods: Based on type B ultrasonic, hospital staffs who underwent medical examinations at the second Affiliated Hospital of Hainan Medical University from January 2022 to March 2022 were classified into health control group (661 subjects) and MAFLD group (223 subjects), demographic, biochemical and blood examination information were compared between 2 groups. Independent risk factors for MAFLD were determined by logistic regression. Predictive values of risk factors of MAFLD were evaluated by receiver operating characteristic (ROC) curves.
Results: The prevalence of MAFLD was 33.7%. Older age (OR=1.08, p< 0.001), H. pylori infection (OR=0.234, p=0.02), triglyceride-glucose (TyG) (OR=7.001, p< 0.001), low-density lipoprotein cholesterol (LDL-C) (OR=2.076, p= 0.028), red blood cell (RBC) (OR=2.386, p=0.001), eating out (OR=0.048, p=0.001), regular exercise (OR=23.017, p< 0.001), and overweight (OR=3.891, p= 0.003) were independently associated factors for MAFLD. The AUC of model predicting MAFLD is 0.910 [95% CI (0.886, 0.934)], with 0.794 sensitivity, 0.908 specificity. The diagnostic value of model was higher in the female MAFLD group after stratified analysis according to gender. The model showed that TyG was the factor contributing more to MAFLD. The diagnostic value of TyG was higher in the female MAFLD group than male MAFLD group.
Conclusion: The prevalence of MAFLD among hospital staffs was 33.7%. TyG can be used to predict MAFLD especially for female hospital staffs for early intervention.
Keywords: metabolic-associated fatty liver disease, MAFLD, hospital staffs, risk factors, triglyceride-glucose index, TyG, red blood cell, RBC, H. pylori infection
Introduction
In 2020, non-alcoholic fatty liver disease (NAFLD) was changed to metabolism-associated fatty liver disease (MAFLD), and this new term was widely recognised by the academic community at home and abroad.1–3 The definition of MAFLD is recognized by multiple stakeholders globally.4 Fatty liver is a major cause of liver disease in the Asia-Pacific region and has distinct disease characteristics.5 MAFLD may be a more suitable definition for fatty liver in the Asia-Pacific region with advantages of improving clinical practice for liver disease.5,6 MAFLD includes a range of diseases such as simple steatosis of the liver, steatohepatitis and cirrhosis.7 Importantly, diagnosis of MAFLD no longer requires the exclusion of other causes of chronic liver disease, such as alcohol or viral hepatitis.8 Meta-analyses have estimated the global prevalence of MAFLD to be 37%-39%, and the incidence of MAFLD continues to increase along with the increasing prevalence of metabolic syndrome (Mets), diabetes, cardiovascular disease and other chronic metabolic diseases.9–11 The prevalence of MAFLD in China is around 26%.12,13 Guan et al found that prevalence of MAFLD among 3553 Chinese patients with type 2 diabetes could be 63.2%.14 Although MAFLD is typically associated with obesity, there is accumulating evidence that not all overweight or obesity people develop MAFLD.15 The clinical profile, natural history and pathophysiology of patients with so-called lean MAFLD are not well characterized.15 On the other hand, a considerable proportion of patients with MAFLD are of normal weight, indicating the importance of metabolic health in the pathogenesis of the disease regardless of body mass index (BMI).15
The pathogenesis of MAFLD is unclear, and liver biopsy (LB) is an effective tool to assess liver fibrosis in patients with MAFLD.16 Magnetic resonance imaging (MRI)-based proton density fat fraction (PDFF) measurements of hepatic triglyceride content have been found to be more accurate than ultrasound-controlled attenuation parameter imaging.17 Zhong et al also created a new classification of MAFLD using metabolic parameters (eg, lipids) to more accurately identify the risk of complications such as cardiovascular disease.18
It is significant to know the prevalence of MAFLD because most MAFLD patients do not have obvious clinical manifestations, while long-term intrahepatic fat accumulation can promote the progress of liver fibrosis and even cirrhosis and liver failure. There are few studies on the prevalence of MAFLD among hospital staffs, and the purpose of this study was to assess the prevalence of MAFLD and its risk factors among hospital staffs in Haikou.
Materials and Methods
Study Population and Design
Our study included hospital staffs at the Clinical and Health Screening Center of the Second Hospital of Hainan Medical University from January 2022 to March 2022. Subjects were classified as fatty liver subjects and non-fatty liver subjects based on ultrasound findings of the liver. Non-MAFLD subjects with fatty liver were excluded according to the diagnostic criteria for MAFLD, and finally 884 examinees were included. The study was approved by the Clinical Ethics Committee of the Second Hospital of Hainan Medical University, and all study subjects signed a written informed consent form (approval number: LW2022223).
The diagnosis of MAFLD is based on ultrasound evidence of hepatic steatosis and the presence of any of the three conditions of overweight/obesity, diabetes, or metabolic dysregulation.1 Experienced ultrasound physician blinded to the study further classified it as (1) mild steatosis (presence of diffuse echogenic enhancement or hepatorenal contrast) or (2) moderate or severe steatosis (both bright echogenic and hepatorenal contrast enhancement visible or ultrasound beam attenuation observed). Metabolic dysregulation were defined as the presence of two or more of the following: (1) waist circumference ≥ 102 in male and ≥ 88 cm in female; (2) blood pressure ≥ 130/85 mmHg (SBP/DBP) or specific medication; (3) TG ≥ 1.70 mmol/L or specific medication; (4) HDL-C < 1.0 mmol/L in male and HDL-C < 1.3 mmol/L in female; (5) prediabetes (ie fasting blood glucose (FPG) level is 5.6 to 6.9 mmol/L or 2-hour post-load glucose level is 7.8 to 11.0 mmol/L or HbA1c is 5.7% to 6.4%); (6) homeostatic model assessment of insulin resistance (HOMA-IR) score ≥ 2.5; (7) C-reactive protein (CRP) level >2mg/L.
Clinical and Laboratory Parameters
We collected data, including age, sex, BMI, alcohol consumption, diet styles, physical activity, diabetes, hypertension and previous medical history. Blood samples were collected for biochemical testing after overnight fasting. Our central laboratory analyzed biochemical variables including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), cholesterol (CHOL), low-density lipoprotein (LDL-C), uric acid (UA), FPG, Lymphocyte (LYM), Red blood cell distribution width (RDW), platelets (PLT) and other routine blood tests. RPR indicates the ratio of RDW to PLT and RLR indicates the ratio of RDW to LYM. TyG is calculated by the formula: TyG = ln[TG (mg-dL-1) Ⅹ FPG (mg-dL-1)/2].19
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (IQR) and compared between groups using two-sample t-test and Mann–Whitney U-test. Categorical variables were expressed as frequencies (percentages) and the two groups were compared using the chi-square test. Cutoff values, sensitivity, specificity, and area under receiver operating characteristic (ROC) were calculated to assess the diagnostic effectiveness of clinical indicators of steatosis in subjects. All statistical analyses were processed using SPSS (version 26.0, SPSS AG, Chicago, Illinois, USA) and MedCalc (version 20.1.0, Ostend, Belgium). P<0.05 was considered statistically significant.
Results
Baseline Characteristics of Enrolled Subjects
The prevalence of MAFLD was 33.7%. Their clinical characteristics are summarized in Table 1. Patients with MAFLD had a higher prevalence of H. pylori infection (53.8% vs 26.8%, p<0.001), hypertension (20.3% vs 9.7%, p<0.001), diabetes (7.2% vs 9.7%, p<0.001), and eating out (41.7% vs 24.7%, p<0.001) compared to non-MAFLD subjects. MAFLD is more prevalent in male subjects (54.7% vs 24.2%, p<0.001), older subjects [(40 (31–51) vs 32 (27–39), p<0.001)], and overweight subjects (30.0% vs 20.4%, p<0.001). MAFLD is less prevalent in subjects with regular exercise (16.1% vs 30.9%, p<0.001). In addition, MAFLD subjects had poor metabolic profiles, including lower levels of RLR and higher levels of TG, CHOL, LDL-C, FPG, TyG, RBC, Cr, UA, UA/Cr and LYM (p < 0.001).
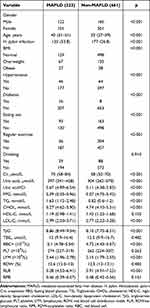 |
Table 1 Anthropometrical and Metabolic Characteristics Among MAFLD and Non-MAFLD Participants |
Independent Risk Factors Associated with MAFLD
Older age (OR=1.08, p<0.001), H. pylori infection (OR=0.234, p<0.001), eating out (OR=0.048, p<0.001), regular exercise (OR=23.017, p<0.001), and overweight (OR=3.891, p=0.003) were independently associated risk factors for MAFLD (Table 2).
 |
Table 2 Multivariate Logistic Regression Analysis of Factors Associated with MAFLD |
ROC Curve of MAFLD Predicted by Risk Factors
The results of ROC of eight indicators predicting MAFLD cases were as follows: older age had an AUC of 0.683〔95% CI (0.644, 0.721), with 0.646 sensitivity, 0.628 specificity when 34.5 was chosen as the optimal cut-off value; TyG had an AUC of 0.819〔95% CI (0.788, 0.851)〕, with 0.807 sensitivity, 0.696 specificity when 8.4037 was chosen as the optimal cut-off value; RBC had an AUC of 0.689〔95% CI (0.649, 0.728)〕, with 0.767 sensitivity, 0.545 specificity when 4.765Χ1012/L was chosen as the optimal cut-off value; H. pylori infection had an AUC of 0.635〔95% CI (0.592, 0.679)〕, with 0.538 sensitivity, 0.732 specificity; LDL-C had an AUC of 0.600〔95% CI (0.556, 0.664)〕, with 0.475 sensitivity, 0.702 specificity; eating out had an AUC of 0.585〔95% CI (0.541, 0.630)〕, with 0.417 sensitivity, 0.753 specificity; regular exercise had an AUC of 0.574〔95% CI (0.532, 0.615)〕, with 0.309 sensitivity, 0.839 specificity; overweight had an AUC of 0.593〔95% CI (0.549, 0.638)〕, with 0.422 sensitivity, 0.753 specificity; TyG combined RBC had an AUC of 0.833〔95% CI (0.803, 0.862), with 0.762 sensitivity, 0.769 specificity; TyG combined RBC as well as LDL-C had an AUC of 0.834〔95% CI (0.804, 0.864), with 0.803 sensitivity, 0.728 specificity; models constructed for all risk factors had the highest AUC of 0.910〔95% CI (0.886, 0.934), with 0.794 sensitivity, 0.908 specificity (Table 3 and Figure 1).
 |
Table 3 ROC Curve of Clinical Indices in the Diagnosis of MAFLD |
 |
Figure 1 ROC curve of clinical indices in the diagnosis of MAFLD. |
ROC Curve of MAFLD Among Different Gender
Anthropometrical and metabolic characteristics among male MAFLD participants are shown in Table 4. The results of ROC of five indicators predicting male-MAFLD cases were as follows: TyG had an AUC of 0.765〔95% CI (0.708, 0.821)〕, with 0.648 sensitivity, 0.787 specificity when 8.7873 was chosen as the optimal cut-off value; UA had an AUC of 0.666〔95% CI (0.531, 0.663)〕, with 0.828 sensitivity, 0.444 specificity when 383.5 µmol/L was chosen as the optimal cut-off value; H. pylori infection had an AUC of 0.579〔95% CI (0.512, 0.646)〕, with 0.639 sensitivity, 0.519 specificity; regular exercise had an AUC of 0.652〔95% CI (0.588, 0.716)〕, with 0.5 sensitivity, 0.803 specificity; drinking had an AUC of 0.570〔95% CI (0.504, 0.637)〕, with 0.338 sensitivity, 0.803 specificity; TyG combined UA had an AUC of 0.784〔95% CI (0.731, 0.836), with 0.639 sensitivity, 0.794 specificity; models constructed for all risk factors had the highest AUC of 0.892〔95% CI (0.855, 0.929), with 0.77 sensitivity, 0.856 specificity (Table 5 and Figure 2).
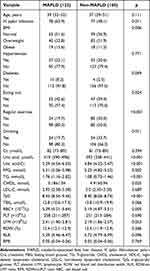 |
Table 4 Anthropometrical and Metabolic Characteristics Among Male MAFLD Participants |
 |
Table 5 ROC Curve of Clinical Indices in the Diagnosis of Male MAFLD |
 |
Figure 2 ROC curve of clinical indices in the diagnosis of male MAFLD. |
Anthropometrical and metabolic characteristics among female MAFLD participants are shown in Table 6 and Table 7. The results of ROC analysis of the performance of six indicators predicting female-MAFLD cases were as follows: older age had an AUC of 0.714〔95% CI (0.660, 0.767), with 0.535 sensitivity, 0.806 specificity when 39.5 was chosen as the optimal cut-off value; TyG had an AUC of 0.818〔95% CI (0.773, 0.863)〕, with 0.871 sensitivity, 0.631 specificity when 8.155 was chosen as the optimal cut-off value; RBC had an AUC of 0.620〔95% CI (0.559, 0.681)〕, with 0.614 sensitivity, 0.631 specificity when 4.735Χ1012/L was chosen as the optimal cut-off value; H. pylori infection had an AUC of 0.608〔95% CI (0.544, 0.672)〕, with 0.416 sensitivity, 0.8 specificity; eating out had an AUC of 0.587〔95% CI (0.524, 0.651)〕, with 0.406 sensitivity, 0.768 specificity; regular exercise had an AUC of 0.564〔95% CI (0.507, 0.622)〕, with 0.248 sensitivity, 0.881 specificity; TyG combined RBC had an AUC of 0.824〔95% CI (0.782, 0.867), with 0.703 sensitivity, 0.792 specificity; models constructed for all risk factors had the highest AUC of 0.902〔95% CI (0.867, 0.937), with 0.822 sensitivity, 0.858 specificity (Table 8 and Figure 3).
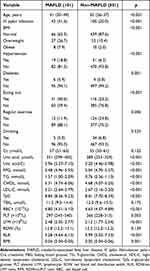 |
Table 6 Anthropometrical and Metabolic Characteristics Among Female MAFLD and Non-MAFLD Female Participants |
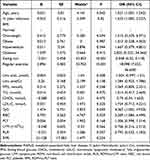 |
Table 7 Multivariate Logistic Regression Analysis of Factors Associated with Female MAFLD |
 |
Table 8 ROC Curve of Clinical Indices in the Diagnosis of Female MAFLD |
 |
Figure 3 ROC curve of clinical indices in the diagnosis of female MAFLD. |
Discussion
The transformation in name and definition from NAFLD to MAFLD represents an important milestone. Recent data robustly suggest the superior utility of MAFLD in identifying patients at high risk for metabolic dysfunction, the hepatic and extra-hepatic complications, as well as those who would benefit from genetic testing, including patients with concomitant liver diseases.20 This change from NAFLD to MAFLD also appears to have improved disease awareness among patients and physicians.20
The incidence of MAFLD has been gradually increasing with economic improvements, changes in lifestyle habits and diet, urbanisation and screening diagnostic tools. Our study found that the prevalence of MAFLD increased with age (p<0.001) and MAFLD was more prevalent in male. Among patients with NAFLD, the metabolic profiles associated with risk for severe fibrosis varied among age groups.21 The cause-specific Cox model indicated that in F0-F1 and F2 patients, age > 50 years was the only predictor of liver-related events (LRE). In F3-F4 patients, age > 55 years, obesity, PLT < 150 000/mm3 and log (GGT) were associated with LRE, while age > 55 years and previous cardiovascular events (CVE) were independent predictors of extrahepatic events (EHE).22 Males and females differ in hormonal regulation and therefore in the prevalence of MAFLD. It is hypothesised that oestrogen suppresses visceral fat accumulation and raises subcutaneous fat accumulation.13 It has been found that perimenopausal and postmenopausal women, with reduced estrogen production in the body, experience redistribution of fat and metabolic disturbances including dyslipidemia, glucose intolerance, and MAFLD.13
Previous studies have confirmed the diagnostic value of TyG in terms of risk of NAFLD and pathological changes in the liver.23,24 Insulin resistance (IR) plays a pathogenic role in MAFLD.1 The clinical application of the High Insulin Normoglycaemic Inhibition and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) is limited due to cost, time and invasiveness.25 Therefore, several IR alternative markers have appeared in the last years and TyG is recommended as a simple IR alternative marker associated with the development of MAFLD.26,27 Therefore, it is possible to assume that the TyG is sensitive in forecasting early MAFLD, which is also being supported by the ROC analysis in our research. Besides, we found that this association was more significant in the subgroup of female subjects stratified by gender. Therefore we recommend that MAFLD should be suspected when TyG levels are high, regardless of whether symptoms are present.
Erythrocytes not only reversibly transport oxygen and carbon dioxide primarily through haemoglobin (Hb), but also contain a variety of other proteins with many roles in regulating oxygen transport, vascular cell communication and inflammation.28 Studies have shown that Hb is related to insulin resistance and Mets and may be a predictor of Mets.29 Researchers have also observed an increase in peripheral blood erythrocyte aggregation in patients with obesity and insulin resistance.30 Imbalances in the MAFLD population between energy intakes and consumption may lead to insulin resistance in numerous tissues.31 Several researches have discovered that RBC is an unknown risk factor for NAFLD.32,33 There are some differences between MAFLD and NAFLD and it is significant to assess the relationship between RBC and MAFLD. In our study, elevated RBC was related to a higher risk of MAFLD, which was consistent with Dai X’s study.34
H. pylori infection can result in a variety of gastrointestinal diseases like chronic gastritis, peptic ulcer, mucosa-associated lymphoid tissue and gastric cancer, and there is a close relationship between H. pylori and a variety of extra-gastrointestinal diseases such as unexplained iron deficiency anaemia, respiratory diseases and Mets such as coronary heart disease, idiopathic thrombocytopenic purpura, type 2 diabetes and hypertension.35 Recent research has shown that H. pylori is strongly correlated with a mildly elevated risk of NAFLD,36,37 and that H. pylori eradication treatment reduces the risk of NAFLD. Our study showed that H. pylori infection was an independent risk factor for patients with MAFLD. H. pylori infection leads to MAFLD through several of these pathways. H. pylori infection may cause chronic systemic inflammation, increasing levels of inflammatory factors such as interleukin-6 and anti-tumour necrosis factor (TNF)-a, which in turn activate the IKK/NF-kB signalling pathway and lead to insulin resistance (IR); H. pylori infection may also inhibit the release of leptin from the body’s white adipose tissue, which then promotes hepatic stearoyl coenzyme A desaturase, thereby accelerating the deposition of very low density lipoprotein (VLDL)-C and fat in liver tissue; H. pylori infection may also cause dysbiosis of the gastrointestinal flora, leading to an increase in bacterial endotoxins, mainly lipopolysaccharides (LPS), which enter the liver via the portal vein, resulting in an inflammatory response, reduced lipoprotein activity and dyslipidaemia.
RDW, RLR and RPR are new non-invasive blood markers for liver fibrosis. In the past, RDW, RLR and RPR were only used clinically to diagnose anaemia or related disorders. Now, RPR, RDW and RLR have been shown to reach higher levels in the colorectal process, which is associated with lower overall survival.38 RDW has also been characterized as a marker of acute decompensated chronic heart failure and an indicator of an increased mortality in patients with myocardial infarction.39,40 RDW, RLR and RPR appear to be relatively uninvestigated parameters in patients with liver disease and fewer investigations have examined the diagnostic role of RDW, RLR and RPR in MAFLD.41,42 Michalak et al found that the AUC values and suggested cut-off values for RDW, RPR and RLR in patients with MAFLD were: 0.606 (>12.8%), 0.724 (>0.047) and 0.691 (>6.25), respectively, suggesting that RDW, RPR and RLR may be potential biomarkers of MAFLD.43 However, in our study, none of the above three indicators were independent risk factors for Hospital staffs with MAFLD.
Serum uric acid (SUA) is major product of purine metabolism produced by the liver. SUA levels increase with the progression of chronic metabolic diseases, like cardiovascular disease, diabetes and Mets.44 Studies have shown that the exposure to SUA results in hepatocellular lipid accumulation, insulin resistance and NLRP3-mediated inflammatory vesicle activation.45,46 Studies have shown that SUA levels in patients with MAFLD are positively correlated with the severity of steatosis.47,48 Disturbances in SUA metabolism may be associated with MAFLD through complex pathways involving insulin resistance, oxidative stress and inflammatory responses. Firstly, SUA induces insulin resistance through inhibition of intrahepatic IRS1 and Akt insulin signalling, thereby promoting hepatic fat accumulation.49 Secondly, SUA induces mitochondrial oxidative stress and citric acid release into the cytoplasm, increasing triglyceride synthesis.50 SUA can also cause hepatic steatosis and inflammatory damage by increasing the production of reactive oxygen species (ROS) through the activation of NADPH oxidases, particularly NOX4, which results in abnormal activation of NLRP3.50–53 However, in our study, SUA or ratio of SUA to creatinine were not independent risk factors in MAFLD.
This study also included BMI, alcohol consumption, dietary patterns, physical activity, diabetes and hypertension in the analysis of risk factors for MAFLD. Eating out, regular exercise, and overweight were independently associated risk factors for MAFLD. The level of education affects the known rate of MAFLD occurrence and prevention knowledge, which means that people with higher education may be less likely to have adverse factors such as eating imbalances and overweight. However, medical workers with heavy and stressful workload have to communicate with patients and deal with various examinations, so they will have an acute stress response producing large amounts of glucocorticoids, which can promote appetite and cause obesity. In addition, medical workers are busy working and often working night shifts, eating out for convenience, irregular lifestyle and lack of exercise have become the norm, all of which increase the incidence of MAFLD.
Whereas NAFLD is a multisystem disease, the association between MAFLD and extra-hepatic diseases is not known. MAFLD identifies patients with chronic kidney disease (CKD) better than NAFLD.54 MAFLD and MAFLD with increased liver fibrosis score are strongly and independently associated with CKD and abnormal albuminuria.54,55 Convincing evidence supports a significant association between both NAFLD and MAFLD, with increased risk of cardiovascular disease (CVD) morbidity and mortality.56 Animal studies have demonstrated a potential causal role of gut microbiota in NAFLD.57 Human studies have started to describe microbiota alterations in NAFLD and have found a few consistent microbiome signatures discriminating healthy individuals from those with NAFLD, non-alcoholic steatohepatitis or cirrhosis.57 Multiple gut microbiome sequencing tools and NAFLD diagnostic methods have been used across studies that could account for discrepant microbiome signatures.57 More advanced metagenomics and multi-omics studies using system biology approaches are needed to improve microbiome biomarkers.57
Compelling evidence suggests that MAFLD is strongly associated with the risk of multiple extrahepatic complications such as type 2 diabetes, cardiovascular disease (ie, the leading cause of death in patients with MAFLD), chronic kidney disease, and certain types of extrahepatic malignancies.58,59 There may be multiple potential mechanisms by which MAFLD increases the risk of cardiovascular disease, type 2 diabetes, and extrahepatic complications. Addressing the growing burden of MAFLD will require a multidisciplinary working group and framework to advance and embrace new collaborative approaches to provide comprehensive, person-centered care and management of patients with MAFLD.58,59
The first limitation of our study is that the causal relationship between MAFLD and influencing factors could not be determined due to the cross-sectional survey. Secondly, a lot of important parameters involved in the diagnosis of MAFLD, like blood glucose levels at 2 hours post-load, HOMA-IR scores, waist circumference, CRP, were not available, leading to an underestimation of the prevalence of MAFLD. Thirdly, ultrasound was used rather than histological assessment to diagnose MAFLD.
In conclusion, The prevalence of MAFLD among Hospital staffs was 33.7%. TyG can be used to predict MAFLD especially for female Hospital staffs for early intervention.
Abbreviations
MAFLD, metabolic-associated fatty liver disease; H. pylori, Helicobacter pylori; Cre, creatinine; FBG, fasting blood glucose; TG, Triglyceride; CHOL, cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TyG, triglyceride-glucose; PLT, platelet; LYM, lymphocyte; RDW, red blood cell distribution width; RLR, RDW-to-LYM ratio; RPR, RDW-to-PLT ratio; RBC, red blood cell; CI, Confidence interval.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The protocol was approved by the Clinical Ethics Committee of the Second Affiliated Hospital of Hainan Medical University and performed per Helsinki’s Declaration (approval number: LW2022223). All participants provided written informed consent for data collection and storage.
Author Contributions
All authors made a significant contribution to the work reported in terms of the conception, study design, execution, acquisition of data, analysis and interpretation. They took part in drafting, revising or reviewing the article; gave final approval of the final manuscript to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by grants from Hainan Province Clinical Medical Center (No. 2021818); the specific research fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202313); Hainan Provincial Health Industry Research Project (22A200078) and Hainan Provincial Postgraduate Innovation Research Project (Qhyb2022-133). The work was not funded by any industry sponsors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi:10.1053/j.gastro.2019.11.312
2. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
3. Nan Y, An J, Bao J, et al. The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol. 2021;75(2):454–461. doi:10.1016/j.jhep.2021.05.003
4. Méndez-Sánchez N, Bugianesi E, Gish RG, et al. Global multi-stakeholder consensus on the redefinition of fatty liver disease. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7(5):388–390. doi:10.1016/S2468-1253(22)00062-0
5. Kawaguchi T, Tsutsumi T, Nakano D, Eslam M, George J, Torimura T. MAFLD enhances clinical practice for liver disease in the Asia-Pacific region. Clin Mol Hepatol. 2022;28(2):150–163. doi:10.3350/cmh.2021.0310
6. Eslam M, Sarin SK, Wong VW, et al. The Asian Pacific Association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14(6):889–919. doi:10.1007/s12072-020-10094-2
7. Wang X, Wu S, Yuan X, et al. Metabolic dysfunction-associated fatty liver disease and mortality among Chinese adults: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(2):e745–e755. doi:10.1210/clinem/dgab644
8. Wang QX, Xue J, Shi MJ, et al. Association between metabolic dysfunction-associated fatty liver disease and the risk of cirrhosis in patients with chronic hepatitis B-A retrospective cohort study. Diabetes Metab Syndr Obes. 2022;15:2311–2322. doi:10.2147/DMSO.S369824
9. Lim GEH, Tang A, Ng CH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21(3):619–629.e7. doi:10.1016/j.cgh.2021.11.038
10. Ayada I, van Kleef LA, Alferink LJM, et al. Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta-analysis: focusing on the non-overlap groups. Liver Int. 2022;42(2):277–287. doi:10.1111/liv.15139
11. Chan KE, Koh TJL, Tang ASP, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10739607 individuals. J Clin Endocrinol Metab. 2022;107(9):2691–2700. doi:10.1210/clinem/dgac321
12. Wong VW, Wong GL, Woo J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol. 2021;19(10):2161–2171.e5. doi:10.1016/j.cgh.2020.10.046
13. Chen YL, Li H, Li S, et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21(1):212. doi:10.1186/s12876-021-01782-w
14. Guan C, Fu S, Zhen D, et al. Metabolic (dysfunction)-associated fatty liver disease in Chinese patients with type 2 diabetes from a subcenter of the national metabolic management center. J Diabetes Res. 2022;2022:8429847. doi:10.1155/2022/8429847
15. Eslam M, El-Serag HB, Francque S, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19(10):638–651. doi:10.1038/s41575-022-00635-5
16. Fouad Y, Esmat G, Elwakil R, et al. The Egyptian clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Saudi J Gastroenterol. 2022;28(1):3–20. doi:10.4103/sjg.sjg_357_21
17. Shao CX, Ye J, Dong Z, et al. Steatosis grading consistency between controlled attenuation parameter and MRI-PDFF in monitoring metabolic associated fatty liver disease. Ther Adv Chronic Dis. 2021;12:20406223211033119. doi:10.1177/20406223211033119
18. Ye J, Zhuang X, Li X, et al. Novel metabolic classification for extrahepatic complication of metabolic associated fatty liver disease: a data-driven cluster analysis with international validation. Metabolism. 2022;136:155294. doi:10.1016/j.metabol.2022.155294
19. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi:10.1210/jc.2010-0288
20. Alharthi J, Gastaldelli A, Cua IH, Ghazinian H, Eslam M. Metabolic dysfunction-associated fatty liver disease: a year in review. Curr Opin Gastroenterol. 2022;38(3):251–260. doi:10.1097/MOG.0000000000000823
21. Petta S, Eslam M, Valenti L, et al. Metabolic syndrome and severity of fibrosis in nonalcoholic fatty liver disease: an age-dependent risk profiling study. Liver Int. 2017;37(9):1389–1396. doi:10.1111/liv.13397
22. Pennisi G, Enea M, Romero-Gomez M, et al. Liver-related and extrahepatic events in patients with non-alcoholic fatty liver disease: a retrospective competing risks analysis. Aliment Pharmacol Ther. 2022;55(5):604–615. doi:10.1111/apt.16763
23. Wang J, Su Z, Feng Y, Xi R, Liu J, Wang P. Comparison of several blood lipid-related indexes in the screening of non-alcoholic fatty liver disease in women: a cross-sectional study in the Pearl River Delta region of southern China. BMC Gastroenterol. 2021;21(1):482. doi:10.1186/s12876-021-02072-1
24. Sheng G, Lu S, Xie Q, Peng N, Kuang M, Zou Y. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Dis. 2021;20(1):134. doi:10.1186/s12944-021-01561-2
25. Lee SB, Kim MK, Kang S, et al. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab. 2019;34(2):179–186. doi:10.3803/EnM.2019.34.2.179
26. Campos-Murguía A, Román-Calleja BM, Toledo-Coronado IV, et al. Liver fibrosis in patients with metabolic associated fatty liver disease is a risk factor for adverse outcomes in COVID-19. Dig Liver Dis. 2021;53(5):525–533. doi:10.1016/j.dld.2021.01.019
27. Wang J, Yan S, Cui Y, Chen F, Piao M, Cui W. The diagnostic and prognostic value of the triglyceride-glucose index in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): a systematic review and meta-analysis. Nutrients. 2022;14(23):4969. doi:10.3390/nu14234969
28. Crawford JH, Chacko BK, Kevil CG, Patel RP. The red blood cell and vascular function in health and disease. Antioxid Redox Signal. 2004;6(6):992–999. doi:10.1089/ars.2004.6.992
29. Wu S, Lin H, Zhang C, et al. Association between erythrocyte parameters and metabolic syndrome in urban Han Chinese: a longitudinal cohort study. BMC Public Health. 2013;13:989. doi:10.1186/1471-2458-13-989
30. Justo D, Marilus R, Mardi T, et al. The appearance of aggregated erythrocytes in the peripheral blood of individuals with insulin resistance. Diabetes Metab Res Rev. 2003;19(5):386–391. doi:10.1002/dmrr.391
31. Sakurai Y, Kubota N, Yamauchi T, et al. Role of insulin resistance in MAFLD. Int J Mol Sci. 2021;22(8):4156. doi:10.3390/ijms22084156
32. Hong F, Guan L, Lin H, et al. Red blood cell count: an unrecognized risk factor for nonalcoholic fatty liver disease. Front Endocrinol. 2021;12:760981. doi:10.3389/fendo.2021.760981
33. Wang HL, Zhang H, Wu SL, et al. Red blood cell count has an independent contribution to the prediction of ultrasonography-diagnosed fatty liver disease. PLoS One. 2017;12(2):e0172027. doi:10.1371/journal.pone.0172027
34. Dai X, Zhou G, Xu L. Associations between red blood cell count and metabolic dysfunction-associated fatty liver disease (MAFLD). PLoS One. 2022;17(12):e0279274. doi:10.1371/journal.pone.0279274
35. Ding SZ. Global whole family based-Helicobacter pylori eradication strategy to prevent its related diseases and gastric cancer. World J Gastroenterol. 2020;26(10):995–1004. doi:10.3748/wjg.v26.i10.995
36. Mantovani A, Turino T, Altomari A, et al. Association between Helicobacter pylori infection and risk of nonalcoholic fatty liver disease: an updated meta-analysis. Metabolism. 2019;96:56–65. doi:10.1016/j.metabol.2019.04.012
37. Zhao XX, Wang RL, Liu MH, Huang XJ. Is the occurrence or reversal of nonalcoholic fatty liver disease associated with long-term helicobacter pylori infection among Chinese adults? A cohort study. Gastroenterol Res Pract. 2021;2021:6696473. doi:10.1155/2021/6696473
38. Cheng KC, Lin YM, Liu CC, Wu KL, Lee KC. High red cell distribution width is associated with worse prognosis in early colorectal cancer after curative resection: a propensity-matched analysis. Cancers. 2022;14(4):945. doi:10.3390/cancers14040945
39. Xanthopoulos A, Tryposkiadis K, Dimos A, et al. Red blood cell distribution width in elderly hospitalized patients with cardiovascular disease. World J Cardiol. 2021;13(9):503–513. doi:10.4330/wjc.v13.i9.503
40. Chen M, Liao L, Yan J, Lin FQ. Predictive value of red blood cell distribution width for 1-year all-cause mortality in critically ill patients with acute myocardial infarction. Int J Gen Med. 2022;15:465–471. doi:10.2147/IJGM.S345109
41. Moreno C, Mueller S, Szabo G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol. 2019;70(2):273–283. doi:10.1016/j.jhep.2018.11.025
42. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17(4):630–637.e8. doi:10.1016/j.cgh.2018.05.059
43. Michalak A, Guz M, Kozicka J, et al. Red blood cell distribution width derivatives in alcohol-related liver cirrhosis and metabolic-associated fatty liver disease. World J Gastroenterol. 2022;28(38):5636–5647. doi:10.3748/wjg.v28.i38.5636
44. Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):210–216. doi:10.1097/BOR.0b013e32835d951e
45. Lv Q, Xu D, Ma J, et al. Uric acid drives intestinal barrier dysfunction through TSPO-mediated NLRP3 inflammasome activation. Inflamm Res. 2021;70(1):127–137. doi:10.1007/s00011-020-01409-y
46. Hu X, Rong S, Wang Q, et al. Association between plasma uric acid and insulin resistance in type 2 diabetes: a Mendelian randomization analysis. Diabetes Res Clin Pract. 2021;171:108542. doi:10.1016/j.diabres.2020.108542
47. Han AL, Lee HK. Association of the metabolic dysfunction-associated fatty liver disease with serum uric acid-to-creatinine ratio. Metab Syndr Relat Disord. 2022;20(7):370–376. doi:10.1089/met.2022.0013
48. He J, Ye J, Sun Y, Feng S, Chen Y, Zhong B. The additive values of the classification of higher serum uric acid levels as a diagnostic criteria for metabolic-associated fatty liver disease. Nutrients. 2022;14(17):3587. doi:10.3390/nu14173587
49. Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7(7–8):1040–1052. doi:10.1089/ars.2005.7.1040
50. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–96. doi:10.1152/ajpcell.00600.2006
51. Lanaspa MA, Sanchez-Lozada LG, Choi YJ, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287(48):40732–40744. doi:10.1074/jbc.M112.399899
52. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi:10.1038/nature04516
53. Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–215. doi:10.1038/nri2725
54. Sun DQ, Jin Y, Wang TY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433. doi:10.1016/j.metabol.2020.154433
55. Wang TY, Wang RF, Bu ZY, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. 2022;18(4):259–268. doi:10.1038/s41581-021-00519-y
56. Zhou XD, Cai J, Targher G, et al. CHESS-MAFLD consortium. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc Diabetol. 2022;21(1):270. doi:10.1186/s12933-022-01697-0
57. Aron-Wisnewsky J, Vigliotti C, Witjes J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279–297. doi:10.1038/s41575-020-0269-9
58. Eslam M, Ahmed A, Després JP, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. 2021;6(9):743–753. doi:10.1016/S2468-1253(21)00132-1
59. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6(7):578–588. doi:10.1016/S2468-1253(21)00020-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
