Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Prevalence and Risk Factors of Comorbid Obesity in Chinese Patients with Bipolar Disorder
Authors Wu Q, Zhang X , Liu Y, Wang Y
Received 13 February 2023
Accepted for publication 10 May 2023
Published 18 May 2023 Volume 2023:16 Pages 1459—1469
DOI https://doi.org/10.2147/DMSO.S404127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Qing Wu, Xun Zhang, Yiyi Liu, Ying Wang
Department of Psychiatry, Affiliated Psychological Hospital of Anhui Medical University, Anhui Mental Health Center, Hefei Fourth People’s Hospital, Hefei, 230022, People’s Republic of China
Correspondence: Ying Wang, Department of Psychiatry, Affiliated Psychological Hospital of Anhui Medical University, 316 Huangshan Road, Hefei, 230022, People’s Republic of China, Tel +8613866136686, Email [email protected]
Purpose: Bipolar disorder (BD) predisposes patients to comorbid obesity and increases the risk of metabolic syndrome and cardiovascular disease. In this study, we investigated the prevalence of comorbid obesity and its risk factors in patients with BD in China.
Patients and Methods: We conducted a cross-sectional retrospective survey of 642 patients with BD. Demographic data were collected, physical examinations were performed, and biochemical indexes, including fasting blood glucose, alanine aminotransferase (ALT), aspartate aminotransferase, and triglycerides (TG) levels, were measured. Height and weight were measured on an electronic scale at admission, and body mass index (BMI) was in kg/m2. Pearson’s correlation analysis was used to analyze the correlation between BMI and variable indicators. Multiple linear regression analysis was used to analyze the risk factors for comorbid obesity in patients with BD.
Results: The prevalence of comorbid obesity in Chinese patients with BD was 21.3%. Obese patients had high levels of blood glucose, ALT, glutamyl transferase, cholesterol, apolipoprotein B (Apo B), TG, and uric acid in the plasma; however, the levels of high-density lipoprotein and apolipoprotein A1 were lower than those in non-obese patients. Partial correlation analysis showed that BMI was associated with ApoB, TG, uric acid, blood glucose, GGT, TC, ApoA1, HDL, and ALT levels. Multiple linear regression showed that ALT, blood glucose, uric acid, TG, and Apo B levels were important risk factors of BMI.
Conclusion: The prevalence of obesity is higher in patients with BD in China, and TG, blood glucose, liver enzymes, and uric acid are closely related to obesity. Therefore, more attention should be paid to patients with comorbid obesity. Patients should be encouraged to increase their physical activity, control sugar and fat intake, and reduce the prevalence of comorbid obesity and risk of serious complications.
Keywords: bipolar disorder, obesity, prevalence, risk factors, body mass index
Introduction
Bipolar disorder (BD) is a serious psychiatric disorder that is common in clinical practice; it manifests as recurrent, alternating, and irregular episodes of mania or hypomania and depression.1 As the condition worsens, cognitive and social functioning will continue to be impaired, affecting 1% of the global population. It has high morbidity, recurrence, disability, mortality, and comorbidity rates, resulting in significant medical, economic, and social burden.2 In the United States, the annual national economic burden of BD and its medical comorbidities is estimated at more than $195 billion.3 The studies in China have found that Chinese men and women with BD lose 6.78 and 7.35 extra life years, respectively, with comorbid respiratory diseases, cardiovascular diseases, and cancer being the causes of death in most patients.4 BD with comorbid obesity is one of the causes of the heavy burden of disease. Numerous studies have found that compared to the general population, people with BD have an increased risk of obesity.5–8 In a cross-sectional analysis of 86,028 subjects in the database of health management organizations abroad, it was found that the prevalence of obesity in BD patients (41.4%) was significantly higher than that in the general population (27.1%).7 In a study of hospitalized patients with BD, the rate of obesity increased from 25% to 36% in the first 4 weeks of acute treatment.9 Thus, after treatment, patients with BD had a higher body mass index (BMI) and a higher rate of comorbidities.10 A variety of factors contributed to the increased prevalence of obesity in patients with BD. The most explicit was the use of antipsychotics, especially atypical antipsychotics. However, weight gain has also been found in studies of untreated patients with BD.11 In addition to drug factors, BD and obesity share common neurobiological abnormality mechanisms, such as the thalamus, pituitary gland, adrenal axis (HPA), and neurotransmitter system dysfunction.12 It may also lead to an imbalance between energy intake and consumption due to reduced social function, poor living habits, and an irregular diet, resulting in obesity. In addition, the link between BD and obesity may be due to genetic factor drives.13
Obesity is a current global public health problem. It is a chronic and complex medical disease that most commonly manifests as excessive accumulation and abnormal distribution of adipose tissue in the body; as a result, the body is in a mild, chronic inflammatory state for a long time.14 If it is not controlled in a timely and effective manner it will increase the risk of cardiovascular disease, cancer, hypertension, hyperlipidemia, type 2 diabetes, and other diseases.15,16 In a meta-analysis of 239 prospective studies from four continents, obesity was independently associated with an increased risk of death after excluding confounding factors such as current smokers and pre-existing chronic conditions.17 In the United States, nearly 300,000 people die each year from obesity-related diseases.18 In China, overweight and obesity accounted for 11.1% of Non-Communicable Disease (NCD)-related deaths in 2019.19 According to the results of the 2015–2019 China Chronic Disease and Nutrition Surveillance survey, the prevalence of obesity among Chinese adults was 16.4%, which was significantly higher than the prevalence of obesity (11.9%) from 2010–2012.20 Similarly, a WHO report published in May 2022 states that 60% of adults in the European Region are overweight or obese.21
Obesity increases the risk of metabolic and cardiovascular diseases in individuals with BD, leading to premature death. Retrospective studies have found that compared with non-obese BD patients, obese BD patients have an increased frequency of depressive and manic episodes and that BMI is positively correlated with disease severity, such as a history of suicide attempts.22 Prospective studies have shown that obese patients with BD have a shorter healthy period and more frequent relapses of depression.23 In patients with BD, the harm of obesity is unique as obesity can make patients have an inferiority complex, seriously affect medication compliance, leading to drug discontinuation or drug reduction, cause relapses, thus increasing the readmission rate and consumption of medical resources, and increase medical risks. The prevalence of BD comorbid obesity investigation is conducive to risk management. However, little is known about the current status of obesity with BD comorbidities. In other countries, studies have explored the prevalence of obesity in patients who were administered medication for BD.9 In China, one study found that the prevalence of obesity in patients with BD was 21%.24 Nevertheless, few studies have explored the prevalence of obesity and its risk factors in untreated BD patients. The prevalence and risk factors for BD with obesity differ significantly between regions and ethnic populations.
In this study, we aimed to investigate the prevalence of obesity in patients with BD in the Han Chinese population. The second objective was to explore the risk factors for obesity in unmedicated patients with BD. Among them, markers of metabolic activity are the key factors in this study. Our findings will potentially help reduce the morbidity and mortality of metabolic and cardiovascular diseases in patients with BD, reduce the family and social burdens caused by the disease, and improve patients’ quality of life.
Materials and Methods
Subjects
This cross-sectional study was conducted at the Anhui Provincial Mental Health Center. This hospital is one of the largest public psychiatric hospitals in China. A total of 840 newly admitted patients with BD aged 18–60 years were enrolled. A total of 136 patients did not meet the inclusion criteria, and 62 patients were within the exclusion criteria. Finally, 642 patients with BD were included in the study. Data were extracted from electronic databases; patients’ general demographic data and biochemical test results were collected anonymously. The inclusion criteria were as follows: (1) diagnosis met the 10th revision of the International Classification of Diseases (ICD-10) diagnostic criteria for BD, confirmed by two or more attending psychiatrists; (2) age of 18–60 years; (3) Han Chinese; (4) not taking psychiatric drugs in the 3 months prior to enrollment; (5) no alcohol, tobacco, or other substance dependence; (6) convulsive electroconvulsive measures had been implemented in the past 3 months. The exclusion criteria were as follows: (1) pregnant and lactating women; (2) severe physical diseases, including severe central nervous system diseases, acute, unstable, or life-threatening diseases (eg, cancer, infection, autoimmune diseases). Finally, 642 patients with BD were included in this study. The study was approved by the Medical Ethics Committee (AMHC) of Anhui Mental Health Center. The requirement for obtaining informed consent was waived by the ethics committee because of the retrospective nature of this study and because all data, including basic personal information and detailed medical records, were encrypted. This study was performed in line with the principles of the Declaration of Helsinki.
Height and Weight Measurement
Height measurement was fixed according to the requirements, and when measuring, the shoes were removed to ensure the correct standing posture of the subject, and the horizontal plate was placed on the top of the head at a 90-degree angle to the scale. The weight scale was calibrated prior to each measurement, and all subjects removed their shoes. BMI was calculated as: body weight (kg)/height squared (m2). According to the Guidelines for Medical Nutrition Treatment of Overweight/Obesity in China, a BMI ≥ 28 kg/m2 is defined as obesity.25
Demographic Variables
The researchers used a basic patient information collection form to collect detailed demographic data of patients with BD who met the inclusion criteria. Variables included sex, age, education, age of onset, number of hospital stays, course of illness, marital status, height, and weight.
Measurement of Biochemical Indicators
All data were collected by nurses from 06:00 to 07:00 after overnight fasting (8~12 h). Blood samples were sent to the hospital laboratory department for blood sample analysis within 1h. Plasma biochemical parameters were measured with an automatic biochemistry analyzer using commercial kits. Blood glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), alanine aminotransferase (ALT), glutaminase (AST), alkaline phosphatase (ALP), glutamine transpeptidase (GGT), apolipoprotein A1 (ApoA1), apolipoprotein B (Apo B), and uric acid levels were measured.
Statistical Analysis
All analyses were performed using SPSS version 26.0. First, patients were divided into obese and non-obese groups. For descriptive analysis of general demographic data and test results, categorical variables are expressed as frequency and percentage (%), and continuous variables are expressed as mean ± standard deviation. The t-test was used for continuous variables, and the chi-square test was used for categorical variables to analyze demographic characteristics and clinical variables. Pearson’s correlation analysis was used to analyze the correlation between BMI and variable indicators. After controlling for age, sex, education level, and disease course variables as covariates, we further analyzed the partial correlation between BMI and biochemical variables. Multiple linear regression was used to detect risk factors for BMI. All statistical tests were double-tailed, and a P-value of <0.05 was considered statistically significant.
Results
Demographic Characteristics of Patients with BD and the Prevalence of Obesity
A total of 642 patients with BD were included in the study. Among them, 290 were female, and 352 were male. The mean age of the patients was 35.47 ± 11.39 years, the average number of years of schooling was 10.88 ± 4.11, the mean age of onset was 25.25 ± 9.07 years, and the mean course of illness was 10.35 ± 8.45. Among them, 137 were obese, and the prevalence of obesity was 21.3%. In addition, the obesity rate in female patients was higher than that in male patients (25.17% vs 18.18%, χ ²=4.629, P=0.031) (Table 1).
 |
Table 1 Comparison of Demographic Characteristics and Metabolic Indexes Between Patients with Bipolar Disorder with or without Obesity |
Obese Patients with BD Have Metabolic Abnormalities
As shown in Table 1, BMI was higher in the obese group than in the non-obese group (t=−30.642; P<0.001), older age (t=−1.34; P<0.05), longer BD course (t=−3.199; P=0.001), shorter years of education (t=2.304; P<0.05), higher blood glucose (t=−4.804; P<0.001), ALT level (t=−3.669; P<0.001), GGT level (t=−3.414; P=0.001), increased TC level (t=−2.582; P<0.05), increased TG level (t=−6.361; P<0.001), elevated Apo B level (t=−4.081; P<0.001), and elevated uric acid level (t=−2.613; P<0.05). However, HDL-C (t=3.207; P=0.001) and Apo A1 levels (t=2.155; P<0.05) were decreased in the obese group. There were no significant differences between the two groups in terms of marital status, presence or absence of psychotic symptoms, systolic blood pressure, diastolic blood pressure, AST, ALP, and age at BD onset (all P>0.05) (Table 1).
Analysis of Factors Related to Comorbid Obesity in Patients with BD
Correlation analysis of BMI and various indicators showed that BMI was positively correlated with blood glucose (r=0.194, P<0.001), ALT (r=0.172, P<0.001), TC (r=0.150, P=0.001), TG (r=0.311, P<0.001), Apo B (r=0.252, P<0.001), uric acid (r=0.184, P<0.001), and GGT (r=0.212, P=0.005) levels, age (r=0.143, P<0.001), and disease course (r=0.176, P<0.001). However, HDL (r=−0.209, P<0.001), education level (r=−0.143, P<0.001) were negatively correlated (P < 0.05). ApoA1 (r=−0.076, P=0.053) and sex (r=−0.030, P=0.441) were not associated with BMI. After controlling for age, sex, education level, and disease course variables as covariates, we further analyzed the partial correlation between BMI and biochemical variables. The results revealed that blood glucose (r=0.151, P<0.001), ALT (r=0.186, P<0.001), TC (r=0.134, P=0.001), TG (r=0.301, P<0.001), Apo B (r=0.240, P<0.001), uric acid (r=0.230, P<0.001), and GGT (r=0.229, P<0.001). However, HDL (r=−0.235, P<0.001) and ApoA1 (r=−0.105, P=0.008) were negatively correlated (Table 2). Scatterplots of Pearson correlation between BMI and Apo B and between BMI and uric acid are shown in Figures 1 and 2, respectively.
 |
Table 2 Matrix of Partial Correlation Coefficients Between BMI and Biochemical Indicators |
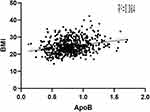 |
Figure 1 Body mass index (BMI) is positively correlated with Apo B (r =0.252, n = 642, P < 0.001). |
 |
Figure 2 Body mass index (BMI) is positively correlated with uric acid (r =0.184, n = 642, P < 0.001). |
Risk Factors for Obesity in Patients with BD
After partial correlation analysis, we found that Apo B, TG, uric acid, blood glucose, GGT, TC, ApoA1, HDL, and ALT were correlated with BMI. Hence, we used Apo B, TG, uric acid, blood glucose, GGT, TC, ApoA1, HDL, and ALT as the independent variables. BMI was the dependent variable in the regression equation analysis. Results of stepwise multiple linear regression analysis showed that Apo B (β=1.895, t=2.737, P<0.05), uric acid (β=0.005, t=3.039, P<0.05), TG (β=0.901, t=4.709, P<0.05), blood glucose (β=0.339, t=3.468, P<0.05), and ALT (β=0.014, t=2.399, P<0.05) were risk factors for high BMI. Ultimately, HDL was found to be associated with BMI (β=−2.018, t=−3.744, P<0.001) (Table 3).
 |
Table 3 Multiple Linear Regression Analysis of Factors That Influence BMI |
Discussion
Obesity and its complications are major causes of increased mortality in patients with BD. However, little is known about the current status of obesity with BD comorbidities. In China, one study found that the prevalence of obesity in patients with BD was 21%.24 In other countries, studies have explored the prevalence of obesity in patients who were administered medication for BD.9 Nevertheless, few studies have explored the prevalence of obesity and its risk factors in untreated BD patients. The prevalence and risk factors for BD with obesity differ significantly between regions and ethnic populations. This study showed that the prevalence of comorbid obesity in Chinese patients with BD was 21.3%, which was higher than the prevalence of obesity in the general population in China by 16.4%.20 We also found that several clinical variables were risk factors for obesity with BD, including level of ALT, blood glucose, TG, uric acid, and Apo B. In addition, we found that patients with obesity diagnosed with BD have low levels of HDL.
In this study, the level of Apo B in the obese group was higher than that in the non-obese group of patients with BD, and BMI increased with an increase in the Apo B level, which is an important risk factor for obesity. Plasma Apo B is the main lipid transporter in the blood, the only protein component of LDL, which transmits cholesterol to all tissues of the body through the LDL receptor pathway and induces an inflammatory response when deposited in organs, causing cell damage.26 In a study in the United States on the relationship between Apo B gene variation and obesity, Apo B gene variation was found to be associated with obesity risk.27 Obesity is a familial disease with a strong genetic component, and variants in the Apo B gene may cause obesity in a variety of various ways.28 Apo B mutations may favor liver absorption of dietary fats or VLDL secretion.29 Apo B mutations also result in altered association with capillary lipoprotein lipase, which favors triglyceride hydrolysis and fat deposition. Some previous studies have found that Apo B levels are stronger risk factors for obesity than LDL.30 In this study, BMI in patients with BD increased with elevated Apo B levels, which is an important indicator of obesity. Therefore, it is possible to provide timely clinical intervention when Apo B levels begin to be higher than normal to avoid the occurrence and development of obesity in patients with BD and reduce the incidence of metabolic diseases and cardiovascular events.
We found a positive correlation between BMI and uric acid levels, a risk factor for elevated BMI and obesity. Uric acid is the final oxidation product of purine nucleotide degradation. As an oxidant, uric acid can increase oxygen free radicals during blood circulation, promote lipid oxidation and inflammation, increase oxidative stress, and damage organ functions. Uric acid also promotes the secretion of inflammatory factors and adipocytokines, leading to the occurrence and development of metabolic diseases and cardiovascular diseases.31–33 For example, hyperuricemia has been found to be an underlying contributor to hypertension, insulin resistance, diabetes, and central obesity.34–37 Another cross-sectional study showed that in 27,009 middle-aged and elderly Chinese patients, BMI increased significantly with the increase of uric acid, which showed that uric acid is an important risk factor that promotes the occurrence and development of obesity.38 In a study that investigated the relationship between serum uric acid levels and visceral fat in Japanese men, visceral fat accumulation was found in 56.1% of hyperuricemia patients, and the visceral fat area was significantly positively correlated with serum uric acid levels.33 Recent clinical studies have shown that elevated serum uric acid levels in patients with BD compared with normal controls may be due to purine dysfunction during the course of the disease.39,40 Patients with hyperuricemia have a very high chance of developing various metabolic syndromes, including obesity. This study found that the BMI of patients with BD increased with elevated uric levels. Therefore, when elevated uric acid levels occur in patients with BD, special attention should be paid to the occurrence of obesity and cardiovascular disease. More clinical attention should be paid to patients with BD and hyperuricemia, as high uric acid not only implies a risk of gout but is also strongly associated with obesity and cardiovascular disease.
In patients with BD, TG levels were significantly higher in obese patients than in non-obese patients, and TG is a risk factor for obesity. TG is closely related to energy metabolism, and abnormal triglycerides suggest energy imbalances.41 TG is the main form of fatty acid storage and transport in cells and plasma, and excess TG leads to increased synthesis of adipose tissue, accumulation in the liver, heart, or other organs, reduced mRNA expression levels of lipoprotein lipase in adipose tissue, and decreased lipoprotein lipase activity in skeletal muscle (which hinders lipolysis), further leading to tissue accumulation of fat.42 In a cross-sectional study of 15,464 adult participants (8430 men and 7034 women, mean age 43.71 ± 8.90 years), TG was positively associated with the risk of obesity, and this association was not associated with other risk factors. Regarding psychiatric disorders, studies have found that patients with BD have a high incidence of TG.43,44 This study found that TG is an important risk factor for obesity;41 thus, more attention should be paid to TG in clinical practice to reduce the burden of physical diseases in patients with TG.
We found that elevated blood glucose levels in patients with BD were strongly associated with obesity. Elevated blood glucose levels are an important risk factor for obesity. In the past, high dietary fat was thought to be the main cause of obesity. However, a growing body of research suggests that the hyperglycemic load has a more important effect on obesity.45,46 Hyperglycemia is caused by an increase in gluconeogenesis and hepatic glucose production. The availability of substrates and the imbalance between glucagon and insulin action contribute to this process.47,48 According to the carbohydrate-insulin model, the hyperglycemic load increases insulin secretion, which allows substrates to be deposited into fat and promotes weight gain.49–51 This hypothesis is supported by several observational studies and clinical trials.52,53 Conversely, obesity is a symptom of metabolic diseases that indicates a series of metabolic disorders such as insulin resistance, which in turn leads to the development of diabetes and cardiovascular disease. For example, studies have shown that hepatic Rap1a activation can inhibit gluconeogenic gene expression and improve glucose tolerance by Akt-mediated FoxO1 inhibition, while Rap1a activation is inhibited in the liver of obese mice.54 In previous studies, the prevalence of diabetes mellitus (T2D) in patients with BD ranged from 6.7 to 26%.55–57 This study found that hyperglycemia leads to abnormal fat deposition, further increasing the risk of obesity in patients with BD; thus, patients should be advised to use a combination of low-fat and low-sugar diets to prevent and manage obesity in clinical practice.
We found that the ALT level is a risk factor for obesity. As ALT levels increase, so does the risk of obesity. ALT is the most sensitive and specific liver enzyme for the clinical assessment of liver function and is also an early sensitive indicator of liver fatty deposition and metabolic dysfunction. Elevated ALT levels are often associated with liver injury from viral hepatitis, autoimmune hepatitis, and nonalcoholic fatty liver disease, which predicts an increased risk of metabolic syndrome and cardiovascular disease in the general population.58 Elevated serum ALT levels not only increase liver and serum triglyceride levels, but are also independently associated with higher serum LDL cholesterol and lower HDL cholesterol levels.59 Elevated ALT levels are often accompanied by metabolic diseases such as hyperlipidemia, which is an important factor that promotes the development of obesity. More importantly, elevated ALT levels have been shown to correlate with higher BMI.60 The use of ALT to screen children and adolescents for overweight or obesity for NAFLD has been advocated for in the United States.61 In addition, in a retrospective analysis of natural mortality risk and protective factors for BD, ALT was found to be elevated in metabolic disorders and chronic inflammatory states, which may be risk factors for early natural mortality in patients with BD.62 Elevation of ALT is closely related to the occurrence and development of obesity. Therefore, to prevent the occurrence and progression of obesity when elevated ALT is found in patients with BD, relevant interventions should be given in a timely manner.
This study has some limitations. First, its retrospective and naturalistic design is a major limitation, which cannot prove a direct causal relationship between any variable and obesity in individuals with BD. Second, we only conducted a cross-sectional study of BD patients who had not taken psychiatric drugs 3 months before enrollment, which was an innovation in this study. However, we also failed to include the effects of psychiatric drugs. Third, we only examined the prevalence of obesity in patients with BD and did not use a control group for comparison. Fourth, this study only explored the factors related to biochemical lipid parameters and did not study the sociological factors related to obese and overweight populations, such as smoking, family history of obesity, physical activity, genetic factors, eating habits, and economic status. Not including these factors may limit our study, and these factors should be considered in future studies. Fifth, we only measured height and weight, using BMI to measure obesity. There are other ways to measure obesity, such as waist circumference and the waist-hip ratio, which may be more accurate; only obese patients were included in this study, and no grouping of normal weight, overweight, and underweight was included. However, we did not specifically explore the prevalence of obesity in patients with depression and mania in BD. In addition, owing to bias in sample size selection, our results should be confirmed by longitudinal studies with larger sample sizes.
Conclusion
In summary, the prevalence of obesity in patients with BD is significantly higher than that in the general population, and the risk factors for BD with comorbid obesity include blood glucose, ALT, ApoB, TG, and uric acid. To avoid serious medical illnesses and complications, lipids, liver enzyme levels, and BMI should be monitored regularly during treatment to actively exclude risk factors for risk management. Simultaneously, psychiatrists should pay attention to the patient’s lifestyle, encourage patients to increase their physical activity, control sugar, and fat intake and weight, and prevent the occurrence of obesity. In addition, healthcare facilities can develop specific and targeted policies to serve patients.
Acknowledgments
We would like to thank the Anhui Mental Health Center for providing data support for this study. Without the support of the hospital, this work would not have been possible.
Funding
This research was funded by the Hefei Health Applied Medicine Research Project (grant number Hwk2021yb015) and the Hospital Project of Hefei Fourth People’s Hospital (grant numbers 2019001, HFSY2022YB08 and HFSY2022ZD11). The funders had no role in the study design; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to submit the article for publication.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet. 2016;387(10027):1561–1572. doi:10.1016/S0140-6736(15)00241-X
2. Vieta E, Salagre E, Grande I, et al. Early intervention in bipolar disorder. Am J Psychiatry. 2018;175(5):411–426. doi:10.1176/appi.ajp.2017.17090972
3. Bessonova L, Ogden K, Doane MJ, et al. The economic burden of bipolar disorder in the United States: a systematic literature review. ClinicoEconomics Outcomes Res. 2020;12:481–497. doi:10.2147/CEOR.S259338
4. Chan JKN, Wong CSM, Yung NCL, et al. Excess mortality and life-years lost in people with bipolar disorder: an 11-year population-based cohort study. Epidemiol Psychiatr Sci. 2021;30:e39. doi:10.1017/S2045796021000305
5. McElroy SL. Obesity in patients with severe mental illness: overview and management. J Clin Psychiatry. 2009;70(Suppl 3):12–21. doi:10.4088/JCP.7075su1c.03
6. Keck PE, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry. 2003;64(12):1426–1435. doi:10.4088/JCP.v64n1205
7. Sicras A, Rejas J, Navarro R, et al. Metabolic syndrome in bipolar disorder: a cross-sectional assessment of a Health Management Organization database. Bipolar Disord. 2008;10(5):607–616. doi:10.1111/j.1399-5618.2008.00599.x
8. Weber NS, Fisher JA, Cowan DN, et al. Psychiatric and general medical conditions comorbid with bipolar disorder in the national hospital discharge survey. Psychiatr Serv. 2011;62(10):1152–1158. doi:10.1176/ps.62.10.pss6210_1152
9. Kim B, Kim SJ, Son JI, et al. Weight change in the acute treatment of bipolar I disorder: a naturalistic observational study of psychiatric inpatients. J Affect Disord. 2008;105(1–3):45–52. doi:10.1016/j.jad.2007.04.006
10. Menculini G, Verdolini N, Brufani F, et al. Comorbidities, depression severity, and circadian rhythms disturbances as clinical correlates of duration of untreated illness in affective disorders. Medicina. 2021;57:5.
11. Maina G, Salvi V, Vitalucci A, et al. Prevalence and correlates of overweight in drug-naïve patients with bipolar disorder. J Affect Disord. 2008;110(1–2):149–155. doi:10.1016/j.jad.2007.12.233
12. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116–121. doi:10.1176/appi.ajp.159.1.116
13. Bahrami S, Steen NE, Shadrin A, et al. Shared genetic loci between body mass index and major psychiatric disorders: a genome-wide association study. JAMA Psychiatry. 2020;77(5):503–512. doi:10.1001/jamapsychiatry.2019.4188
14. González-Muniesa P, Mártinez-González MA, Hu FB, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034. doi:10.1038/nrdp.2017.34
15. Deng T, Lyon CJ, Bergin S, et al. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421–449. doi:10.1146/annurev-pathol-012615-044359
16. Scherer PE, Hill JA. Obesity, diabetes, and cardiovascular diseases: a compendium. Circ Res. 2016;118(11):1703–1705. doi:10.1161/CIRCRESAHA.116.308999
17. Di Angelantonio E, Bhupathiraju SN, Gao P, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. doi:10.1016/S0140-6736(16)30175-1
18. Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci USA. 2018;115(5):957–961. doi:10.1073/pnas.1716802115
19. Rubin R. Profile: institute for health metrics and evaluation, WA, USA. Lancet. 2017;389(10068):493. doi:10.1016/S0140-6736(17)30263-5
20. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. doi:10.1016/S2213-8587(21)00045-0
21. Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. doi:10.1016/j.metabol.2022.155217
22. Fagiolini A, Kupfer DJ, Rucci P, et al. Suicide attempts and ideation in patients with bipolar I disorder. J Clin Psychiatry. 2004;65(4):509–514. doi:10.4088/JCP.v65n0409
23. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160(1):112–117. doi:10.1176/appi.ajp.160.1.112
24. Yi W, Wu H, Li R, et al. Prevalence and associated factors of obesity and overweight in Chinese patients with bipolar disorder. Front Psychiatry. 2022;13:984829. doi:10.3389/fpsyt.2022.984829
25. Chen W. Guidelines for medical nutrition treatment of overweight/obesity in China (2021). Asia Pac J Clin Nutr. 2022;31(3):450–482.
26. Onat A, Altay S, Karadeniz Y. Clinical significance and potential mechanism of discordance between apolipoprotein B and LDL-cholesterol. J Am Coll Cardiol. 2016;67(25):3023–3024. doi:10.1016/j.jacc.2016.02.083
27. Rajput-Williams J, Knott TJ, Wallis SC, et al. Variation of apolipoprotein-B gene is associated with obesity, high blood cholesterol levels, and increased risk of coronary heart disease. Lancet. 1988;2(8626–8627):1442–1446. doi:10.1016/S0140-6736(88)90930-0
28. Law A, Wallis SC, Powell LM, et al. Common DNA polymorphism within coding sequence of apolipoprotein B gene associated with altered lipid levels. Lancet. 1986;1(8493):1301–1303. doi:10.1016/S0140-6736(86)91222-5
29. Hassan NE, El-Masry SA, Zarouk WA, et al. Apolipoprotein B polymorphism distribution among a sample of obese Egyptian females with visceral obesity and its influence on lipid profile. J Genet Eng Biotechnol. 2015;13(2):177–183. doi:10.1016/j.jgeb.2015.09.005
30. Williams K, Sniderman AD, Sattar N, et al. Comparison of the associations of apolipoprotein B and low-density lipoprotein cholesterol with other cardiovascular risk factors in the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2003;108(19):2312–2316. doi:10.1161/01.CIR.0000097113.11419.9E
31. Kimura Y, Tsukui D, Kono H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int J Mol Sci. 2021;22:22. doi:10.3390/ijms222212394
32. Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–1293. doi:10.1161/01.HYP.0000072820.07472.3B
33. Tamba S, Nishizawa H, Funahashi T, et al. Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern Med. 2008;47(13):1175–1180. doi:10.2169/internalmedicine.47.0603
34. Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33(9):
35. Kuwabara M, Hisatome I, Niwa K, et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension. 2018;71(1):78–86. doi:10.1161/HYPERTENSIONAHA.117.10370
36. Cheang C, Law S, Ren J, et al. Prevalence of hyperuricemia in patients with severe obesity and the relationship between serum uric acid and severe obesity: a decade retrospective cross-section study in Chinese adults. Front Public Health. 2022;10:986954. doi:10.3389/fpubh.2022.986954
37. Vander Schaft N, Brahimaj A, Wen KX, et al. The association between serum uric acid and the incidence of prediabetes and type 2 diabetes mellitus: the Rotterdam study. PLoS One. 2017;12(6):e0179482. doi:10.1371/journal.pone.0179482
38. Dai X, Yuan J, Yao P, et al. Association between serum uric acid and the metabolic syndrome among a middle- and old-age Chinese population. Eur J Epidemiol. 2013;28(8):669–676. doi:10.1007/s10654-013-9829-4
39. Albert U, De Cori D, Aguglia A, et al. Increased uric acid levels in bipolar disorder subjects during different phases of illness. J Affect Disord. 2015;173:170–175. doi:10.1016/j.jad.2014.11.005
40. Chen J, Chen H, Feng J, et al. Association between hyperuricemia and metabolic syndrome in patients suffering from bipolar disorder. BMC Psychiatry. 2018;18(1):390. doi:10.1186/s12888-018-1952-z
41. Lee NY, Kim SH, Cho B, et al. Patients taking medications for bipolar disorder are more prone to metabolic syndrome than Korea’s general population. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1243–1249. doi:10.1016/j.pnpbp.2010.06.029
42. Clemente-Postigo M, Queipo-Ortuño MI, Fernandez-Garcia D, et al. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS One. 2011;6(9):e24783. doi:10.1371/journal.pone.0024783
43. Klop B, Wouter Jukema J, Rabelink TJ, et al. A physician’s guide for the management of hypertriglyceridemia: the etiology of hypertriglyceridemia determines treatment strategy. Panminerva Med. 2012;54(2):91–103.
44. Zou Y, Sheng G, Yu M, et al. The association between triglycerides and ectopic fat obesity: an inverted U-shaped curve. PLoS One. 2020;15(11):e0243068. doi:10.1371/journal.pone.0243068
45. Slabber M, Barnard HC, Kuyl JM, et al. Effects of a low-insulin-response, energy-restricted diet on weight loss and plasma insulin concentrations in hyperinsulinemic obese females. Am J Clin Nutr. 1994;60(1):48–53. doi:10.1093/ajcn/60.1.48
46. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi:10.1001/jama.287.18.2414
47. Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14(1):9–19. doi:10.1016/j.cmet.2011.06.003
48. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–587. doi:10.1038/nrendo.2017.80
49. Lucan SC, DiNicolantonio JJ. How calorie-focused thinking about obesity and related diseases may mislead and harm public health. An alternative. Public Health Nutr. 2015;18(4):571–581. doi:10.1017/S1368980014002559
50. Taubes G. The science of obesity: what do we really know about what makes us fat? An essay by Gary Taubes. BMJ. 2013;346:f1050. doi:10.1136/bmj.f1050
51. Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2167–2168. doi:10.1001/jama.2014.4133
52. Chaput JP, Tremblay A, Rimm EB, et al. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec family study. Am J Clin Nutr. 2008;87(2):303–309. doi:10.1093/ajcn/87.2.303
53. Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–2102. doi:10.1001/jama.297.19.2092
54. Wang Y, Spolitu S, Zadroga JA, et al. Hepatocyte Rap1a contributes to obesity- and statin-associated hyperglycemia. Cell Rep. 2022;40(8):111259. doi:10.1016/j.celrep.2022.111259
55. van Winkel R, De Hert M, Van Eyck D, et al. Prevalence of diabetes and the metabolic syndrome in a sample of patients with bipolar disorder. Bipolar Disord. 2008;10(2):342–348. doi:10.1111/j.1399-5618.2007.00520.x
56. Regenold WT, Thapar RK, Marano C, et al. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord. 2002;70(1):19–26. doi:10.1016/S0165-0327(01)00456-6
57. Cassidy F, Ahearn E, Carroll BJ. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry. 1999;156(9):1417–1420. doi:10.1176/ajp.156.9.1417
58. Yoo JJ, Cho EJ, Chung GE, et al. Nonalcoholic fatty liver disease is a precursor of new-onset metabolic syndrome in metabolically healthy young adults. J Clin Med. 2022;11:4. doi:10.3390/jcm11040935
59. Lorenzo C, Hanley AJ, Rewers MJ, et al. The association of alanine aminotransferase within the normal and mildly elevated range with lipoproteins and apolipoproteins: the insulin resistance atherosclerosis study. Diabetologia. 2013;56(4):746–757. doi:10.1007/s00125-012-2826-4
60. Laine S, Sjöros T, Vähä-Ypyä H, et al. Body adiposity, but not elements of objectively measured sedentary behavior or physical activity, is associated with circulating liver enzymes in adults with overweight and obesity. Front Endocrinol. 2021;12:655756. doi:10.3389/fendo.2021.655756
61. Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133(6):1814–1820. doi:10.1053/j.gastro.2007.08.077
62. Tsai SY, Lee CH, Kuo CJ, et al. A retrospective analysis of risk and protective factors for natural death in bipolar disorder. J Clin Psychiatry. 2005;66(12):1586–1591. doi:10.4088/JCP.v66n1215
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
