Back to Journals » Risk Management and Healthcare Policy » Volume 14
Prevalence and Risk Factors Associated with Hyperuricemia in the Pearl River Delta, Guangdong Province, China
Authors Liu W, Liu W, Wang S , Tong H, Yuan J, Zou Z, Liu J, Yang D, Xie Z
Received 2 December 2020
Accepted for publication 1 February 2021
Published 16 February 2021 Volume 2021:14 Pages 655—663
DOI https://doi.org/10.2147/RMHP.S293913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Marco Carotenuto
Weiqi Liu,1,* Weiling Liu,2,* Shaoling Wang,3 Huichun Tong,4 Jianmin Yuan,5 Zhenning Zou,6 Jianwen Liu,7 Donghai Yang,8 Zhongxing Xie9
1Department of Clinical Laboratory, Maternal and Children Health Care Hospital (Huzhong Hospital) of Huadu, Guangzhou, Guangdong, 510800, People’s Republic of China; 2Department of Clinical Laboratory, Chancheng Centre Hospital, Foshan, Guangdong, 528000, People’s Republic of China; 3Department of Clinical Laboratory, Taishan People’s Hospital, Jiangmen, Guangdong, 529200, People’s Republic of China; 4Department of Clinical Laboratory, Boai Hospital of Zhongshan, Zhongshan, Guangdong, 528402, People’s Republic of China; 5Department of Clinical Laboratory, Humen Hospital, Dongguan, Guangdong, 523899, People’s Republic of China; 6Department of Clinical Laboratory, Shenzhen Maternity & Child Healthcare Hospital, Shenzhen, Guangdong, 518028, People’s Republic of China; 7Department of Clinical Laboratory, Huiyang Sanhe Hospital, Huizhou, Guangdong, 516211, People’s Republic of China; 8Department of Clinical Laboratory, Sanzhao Town Health Center, Jinwan District, Zhuhai, Guangdong, 519040, People’s Republic of China; 9Department of Clinical Laboratory, The Second People’s Hospital of Zhaoqing, Zhaoqing, Guangdong, 526060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shaoling Wang Email [email protected]
Background: In China, the prevalence of HUA in the Pearl River Delta (PRD) region of Guangdong Province has not been extensively investigated. Therefore, this study investigated the prevalence of HUA and its related factors among people aged 20– 99 years in nine cities in the PRD.
Materials and Methods: We selected 6491 health check participants from 9 cities in the PRD and collected participants’ anthropometric and biochemical test results for a cross-sectional study. We included 6491 participants and assessed their blood pressure (BP), body mass index (BMI), total cholesterol (TC), triglycerides (TG), glucose (Glu) and serum uric acid (UA) to analyze the regional prevalence of HUA and its related factors. HUA was indicated when fasting serum UA level was > 420 μmol/L in men and > 360 μmol/L in women.
Results: Overall prevalence of HUA in our cohort was 34.05%; prevalence was higher in men than in women (41.53% vs 26.14%, P < 0.001). Characteristics associated with HUA were hypertension (odds ratio (OR), 5.506; 95% confidence interval (CI), 4.402– 6.889), higher body mass index (BMI; OR: 1.746; 95% CI: 1.560– 1.954), age 31– 40 years (OR: 0.829; 95% CI: 0.706– 0.973), age 61– 70 years (OR: 1.434; 95% CI: 1.194– 1.722) and age ≥ 71 years (OR: 1.742; 95% CI: 1.397– 2.173). In all subjects, serum UA was positively correlated with Glu, TG and TC. After we adjusted for age, BMI and BP, multivariate logistic regression analysis showed that HUA risk factors were high TC (OR: 1.770; 95% CI: 1.459– 2.147) and TG (OR: 1.961; 95% CI: 1.632– 2.357) in men; and high Glu (OR: 1.508; 95% CI: 1.084– 2.099), TC (OR: 1.341; 95% CI: 1.084– 1.660) and TG (OR: 1.680; 95% CI: 1.290– 2.187) in women.
Conclusion: The prevalence of HUA was relatively high in the PRD of Guangdong Province. Relevant governmental bodies should focus on early diagnosis, early treatment and early intervention.
Keywords: hyperuricemia, uric acid, prevalence, cardiovascular diseases, risk
Introduction
Gout is a crystal-associated arthropathy caused by monosodium urate (MSU) deposition, which is directly related to hyperuricemia (HUA) caused by decreased serum uric acid (UA) excretion. Serum UA is the final product of the body’s intake of purine-rich foods or the catabolism of inner core protein. The xanthine and hypoxanthine produced by purine are converted into UA by the enzymatic reaction of xanthine oxidase (XO).1 When serum UA exceeds its saturation level in blood or tissue fluid, serum urate crystals can form and be deposited in the joints, inducing local inflammation and tissue destruction, which is called gout. HUA has been recognized as an important precursor of gout.2 Studies have shown that the prevalence of HUA across different races is 2.6–36%3–5 and that of gout is 0.03–15.3%;6,7 in recent years, the prevalence of gout has significantly increased.8,9 Liu et al reported that the overall prevalence of HUA in China was 13.3%.2 More and more evidence shows that HUA and gout are independent risk factors for chronic kidney disease (CKD), hypertension, cardiovascular (CV) and cerebrovascular diseases and diabetes, as well as independent predictors of premature death.10
With continuous in-depth research on the function of serum UA, the influence thereof on clinical diagnosis of diseases has become more and more extensive. In a study on serum UA and cardiovascular disease (CVD) in 5926 subjects, Fang et al found that increased serum UA level is positively correlated with CVD mortality and that increased blood UA level is an independent risk factor for CVD.11 Magnoni et al found a relationship between serum UA and adverse CV events in 1548 patients and that serum UA concentration is closely related to hospital mortality in patients with acute coronary syndrome (ACS).12 Biscaglia et al showed that UA can penetrate the cell membrane, which has a destructive effect on the physiological activities and oxidative metabolism of cells, causing an inflammatory reaction.13 Lee et al found a relationship between blood UA levels and acute respiratory distress syndrome (ARDS). In that study, ARDS patients in the low-serum UA group experienced significant clinical improvement, and low serum UA levels were significantly associated with ARDS survival rate. However, patients with ARDS in the normal– and high-serum UA groups mostly died of sepsis. Low serum UA level could therefore be a prognostic marker in ARDS for determining risk of death during hospitalization.14 At the same time, an increase in serum UA results in vascular endothelial damage; meanwhile, cell proliferation leads to glomerular proliferation and sclerosis,15 increases oxidative stress (OS), promotes platelet activation and vascular smooth-muscle cell (VSMC) proliferation and increases the release of pro-inflammatory substances such as interleukins, ultimately leading to renal damage.16,17
The Pearl River Delta (PRD) is located in Guangdong Province, China. Its coastal location means its economy is relatively developed, with many entertainment venues; people there often consume high-fat diets that include seafood, alcohol and meat. In recent years, the number of patients with CVD has increased significantly in the PRD. Most studies in cardiology have confirmed that diseases such as dyslipidemia, diabetes, alcoholism and hypertension are risk factors for CVD,18–20 but few have focused on determining the prevalence and epidemiological characteristics of HUA in the PRD. In order to obtain more epidemiological data on chronic diseases and CV risk factors in the PRD and improve medical and health conditions there, in this study we investigated the prevalence of HUA and its related factors in people aged 20–99 years in nine cities of this region.
Materials and Methods
Study Design and Population
From June 2018 to December 2019, we randomly selected 6491 outpatients aged 20–99 years from health checkups in nine PRD cities: Dongguan, Foshan, Guangzhou, Huizhou, Jiangmen, Shenzhen, Zhaoqing, Zhongshan and Zhuhai. In all subjects, we measured blood pressure (BP), including systolic BP (SBP) and diastolic BP (DBP); height; weight; total cholesterol (TC); triglycerides (TG); glucose (Glu); and serum UA. We excluded patients with mental-health problems; malignant tumors; peritoneal dialysis as a result of severe liver or kidney failure; artificial extracorporeal liver support; hemodialysis; and pregnancy. This study was approved by the Ethics Committee of Huadu District Maternal and Child Health Hospital, Guangzhou, China and was conducted according to the principles of the Declaration of Helsinki. All participants gave informed consent after having been informed about the objectives and benefits of our study.
Data Collection
We collected blood samples from all study subjects in the morning when their stomachs were empty, taking 5 mL of venous blood using a vacuum blood collection tube at room temperature. This sample was immediately centrifuged at 3000 rpm for 10 min. No hemolysis was found in any specimen. All specimens were tested within 4 hours using an automatic biochemical analyzer. We determined UA, fasting plasma glucose (FPG), TC and TG using the enzymatic method.
Definitions
According to the Chinese Adult Dyslipidemia Prevention and Control Guidelines,21 TC ≥ 6.22 mmol/L (240 mg/dL) and TG ≥ 2.26 mmol/L (200 mg/dL) are considered elevated levels, while the American Diabetes Association’s (ADA’s) Standards of Medical Care in Diabetes guide22 considers FPG ≥ 7 mmol/L (126 mg/dL) an elevated level. Body mass index (BMI) is calculated by dividing body weight (kg) by height squared (m2). According to an earlier report,23 low BMI is <24 kg/m2, and high BMI is ≥24 kg/m2. The definition of hypertension is SBP ≥140 mmHg and/or DBP ≥ 90 mmHg, based on the 2018 Chinese Guidelines for Prevention and Treatment of Hypertension.24 Different guidelines specify different levels of serum UA for the diagnosis of HUA.25 In this study, we defined HUA as serum UA >420 μmol/L in men and >360 μmol/L in women as defined in Management of hyperuricemia and gout: Chinese experts consensus.26
Statistical Analysis
For all statistical analyses, we used SPSS version 17.0 (IBM Corp., Armonk, NY, USA) and STATA version 16.0 (StataCorp., College Station, TX, USA), and we created graphs used GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Continuous variables are represented by means and standard deviations (SDs); categorical variables are expressed as numbers and percentages. Depending on data type, we used Student’s t test, the Kruskal–Wallis test or the χ2 test for data analysis. Simple correlation and multiple linear regression were used to analyze relationships between serum UA and cardiometabolic risk factors. Multiple logistic regression analysis was used to determine the factors affecting HUA, and odds ratios (ORs) and 95% confidence intervals (95% CIs) were used to quantify the relationships. P < 0.05 was considered statistically significant.
Results
Characteristics of the Study Participants from the Pearl River Delta
Table 1 shows anthropometric and serum biochemical characteristics of subjects in the PRD. Significant differences were found between male and female in almost all the variables except TC. In terms of HUA, all the variables were significantly different between HUA and non-HUA patients (P < 0.001). Non-HUA patients were observed to have lower BMI, BP, Glu, TC, and TG. For city specified data, please refer to supplementary Table 1.
 |
Table 1 Anthropometric and Serum Biochemical Characteristics of Subjects in the Pearl River Delta, Guangdong Province, China |
Prevalence of Hyperuricemia and Serum Uric Acid Levels in Different Cities
Overall prevalence of HUA in the PRD was 34.05%. Prevalence ranged from high to low in Huizhou, Foshan, Zhaoqing, Zhongshan, Zhuhai, Dongguan, Shenzhen, Guangzhou and Jiangmen, with respective rates of 50.75%, 44.82%, 43.14%, 41.16, 36.06%, 28.18%, 20.17%, 17.91% and 7.78%. HUA prevalence among cities was found of significant difference (P < 0.001) (Figure 1). In addition, there are statistically significant (P < 0.001) differences in serum UA levels among cities (Figure 2). Shenzhen had the lowest serum UA level, while Huizhou had the highest.
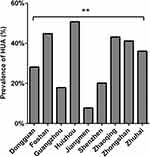 |
Figure 1 Prevalence of HUA among different cities. **P <0.001. |
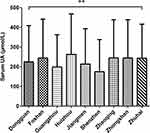 |
Figure 2 Comparison of serum UA level among different cities. **P <0.001. |
Serum UA Levels and Prevalence of Hyperuricemia Among Genders and Age Groups
Serum UA level was higher in men than in women overall (406.19 ± 104.43 vs 315.92 ± 86.64 μmol/L; P < 0.001). Across different age groups, serum UA level was significantly higher in men than in women. Overall prevalence of HUA in men was higher than in women (41.53% vs 26.14%, P < 0.001), and the disease’s prevalence was significantly higher in men than in women ≤60 years, but there was no significant difference between men and women above the age of 60 (Table 2).
 |
Table 2 Comparison of Serum UA Levels and Prevalence of Hyperuricemia Among Genders and Age Groups |
Risk Factors and Hyperuricemia
Table 3 shows the relationships between variables and HUA. Multivariable logistic regression analysis showed that hypertension (OR: 5.506; 95% CI: 4.402–6.889); high BMI (OR: 1.746; 95% CI: 1.560–1.954); and the age ranges of 31–40 years (OR: 0.829; 95% CI: 0.706–0.973), 61–70 years (OR: 1.434; 95% CI: 1.194–1.722) and ≥71 years (OR: 1.742; 95% CI: 1.397–2.173) were correlated with HUA in our subjects. Men were more likely to suffer from HUA before age 70, while women were more likely to develop it after age 50. Therefore, BMI and hypertension were risk factors for HUA.
 |
Table 3 Multiple-Regression Analyses of HUA and Associated Factors in All Subjects and Between Sexes |
Levels of Glu, TC, TG and Hyperuricemia
Correlation results showed that serum UA was positively correlated with Glu (OR: 0.061; 95% CI: 0.037–0.085), TC (OR: 0.134; 95% CI: 0.110–0.158) and TG (OR: 0.224; 95% CI: 0.201–0.247) in all subjects. Multiple linear regression analysis (Table 4) showed that after adjustments for age, sex, BMI and hypertension, serum UA of all subjects was positively correlated with TC and TG and negatively correlated with Glu. After adjustment, serum UA in men was positively correlated with TC and TG and negatively correlated with Glu, while in women it was positively correlated with Glu, TC and TG. In subjects with high levels of Glu, TC and TG, serum UA was significantly higher than in normal subjects (Figure 3), as was the prevalence of HUA (Figure 4). After we adjusted for age, BMI and BP, multivariate logistic regression analysis showed that high TC (OR: 1.770; 95% CI: 1.459–2.147) and TG (OR: 1.961; 95% CI: 1.632–2.357) were risk factors for HUA in men, while high Glu (OR: 1.508; 95% CI: 1.084–2.099), TC (OR: 1.341; 95% CI: 1.084–1.660) and TG (OR: 1.680; 95% CI: 1.290–2.187) were risk factors for HUA in women.
 |
Table 4 Glu, TC, TG Associated with Serum UA Levels in Multiple Linear-Regression Analysis |
 |
Figure 3 Serum UA level in patients with high Glu, high TC and high TG. *P <0.05. |
 |
Figure 4 Prevalence of HUA in hyperglycemia and hyperlipidemia. *P <0.05. |
Discussion
This study was based on health examination data of adults in nine cities of the PRD. We collected and used easily available anthropometric and blood biochemical indicators—including age, BMI, SBP, DBP, Glu, TC, TG and UA to analyze the risk of HUA. Before specific discussion, we would like to claim that all our results should be definitely analyzed in the context that the serum UA cut-off value is still in debate. One of the latest large-scale studies is the Uric acid Right for heArt Health (URRAH) project. The URRAH is a multicenter retrospective, observational cohort study which recruited 23,475 subjects with a minimum follow-up of 20 years. It reveals that serum UA cut-off value is varied among outcomes. For CV mortality and fatal MI, URRAH suggests a cut-off value of 5.6 mg/dL and 5.7mg/dL, respectively.27,28 This is a much lower than the classical HUA diagnostic standard 7 mg/dL (420 μmol/L) in men and 6 mg/dL (360 μmol/L) in women applied in this literature. It can be expected should the URRAH findings are applied in our research, the effect of HUA will be larger.
Overall prevalence of HUA in our sample population was 34.05%. Compared to studies using foreign country populations, our research population has a high HUA prevalence. Using the same serum UA cut-off value as ours, Maloberti A et al studied 762 Italian consecutive outpatients with essential hypertension. The HUA prevalence is 12.9% in male and 15.3% in female respectively.29 A further study on 389 health subjects has evaluated the prevalence of HUA using both the classical cut-off (>6 mg/dL in women and 7 mg/dL in men) and a latest proposed cut-off (>5.6 mg/dL, regardless of sex). In health subjects, the prevalence of HUA is 6.3% using the classical cut-off, and 28.2% using the proposed cut-off, respectively. Large differences are found between male and female.30 Redon P et al applied the classical HUA cutoff, concluded hypertensive patients tend to have high prevalence of HUA in central and east Europe. The HUA prevalence of the whole population is 25%.31 Those studies are comparable to each other.
In China, our prevalence is higher than an estimated 13.7% prevalence in Northern China.32 The high prevalence of PRD could be due to the region’s coastal location and related lifestyle. First, the PRD is the centerpiece of China’s reform and opening up; its economy is relatively developed. Studies have shown that HUA is more common in economically developed areas.32,33 Second, the PRD is located along the coast, where seafood has become a daily must. Studies have shown a positive correlation between seafood consumption and HUA.34 In addition, wine culture, meat and other dietary choices might increase the risk of HUA.35 More men had HUA than women among our subjects (41.53% vs 26.14%), and prevalence in both sexes was higher than previously reported.33 In men, the prevalence of HUA was less affected by age, while in women it increased with age. Men had a higher risk of HUA before age 70, while women had a higher risk thereof after age 50. The phenomenon might be related to the influence of female sex hormone levels on serum UA. Mumford et al36 studied the fluctuation of blood UA during the menstrual cycle in healthy young women and found that blood UA is highest in the follicular phase and lowest in the luteal phase, inversely proportional to estrogen and progesterone; it is also positively correlated with follicle-stimulating hormone (FSH). Sumino et al37 analyzed serum UA levels of postmenopausal Japanese women on an estrogen + progesterone replacement regimen and found that the average serum UA level of women in the HUA group decreased significantly after treatment, while this change was not observed in women in the normal UA group. At the same time, the third United States National Health and Nutrition Examination Survey showed average serum UA level in women increases after menopause, but postmenopausal women receiving hormone replacement therapy (HRT) had lower serum UA levels.38
We found that hypertension, high BMI, hyperglycemia and hyperlipidemia were risk factors for HUA in the PRD. In recent years, the prevalence of hypertension and HUA has shown an upward trend. Hypertension is a common and highly prevalent39 chronic disease that can increase the risk of death from CVDs. Serum UA can activate the renin–angiotensin system (RAS), damage renal blood vessels and lead to elevated BP.40 Studies have demonstrated a dose–response relationship between serum UA level and relative risk (RR) of hypertension;41 Grayson et al reported that for every 1 mg/dL increase in UA level, the pooled RR for incident hypertension after adjusting for potential confounding was 1.13.42 At the same time, long-term use of diuretics in hypertensive patients causes the body’s blood volume to decrease and UA reabsorption to increase. Maloberti A. et al43 studied UA relevant to diuretics using URRAH data, A total of 17,757 patients were included in the analysis, 17.2% of all the patients took diuretics and 58% of those people have serum UA higher than the median value (4.8 mg/dL). The literature reveals at a high SUA level (≥6.55 mg/dL), individuals with diuretics have a significant CV death hazard ratio of 2.47 (95% CI 1.05–5.80, P = 0.038). It is worth to note that although the median value is much smaller than the classical HUA cutoff, it is very close to the best cut-off for total and cardiovascular mortality in all URRAH patients (4.7 mg/dL). Compared to the classical HUA cutoff (6–7 mg/dL), the high HR in people with SUA ≥6.55 mg/dL poses a great importance that the classical HUA cutoff should be decreased to better evaluate people’s risk, especially in people who take diuretics.
Microvascular disease in patients with hypertension inhibits metabolism of UA in renal tubules; in addition, severe intrarenal arteriosclerosis in hypertensive patients leads to increased reabsorption of UA in the proximal convoluted tubules. Therefore, people with high serum UA levels are prone to hypertension. This study in PRD residents showed that high BMI was a risk factor for HUA. Other studies have shown that obesity and hypertension are important independent risk factors for HUA and gout.44 Liu45 and other studies have proven that overweight is associated with the prevalence of HUA. Among overweight or obese individuals, young people are more likely to develop HUA than elderly ones. Compared with people at normal weights, women are more likely to develop HUA with weight gain than men are. The risk of HUA in women with hyperglycemia was significantly higher than in men with hyperglycemia. Young et al46 found that gout might be independently associated with increased diabetes risk, and the degree of association is significantly greater in women than in men. Studies have shown that in high-risk middle-aged people with impaired Glu tolerance, changes in UA are associated with the risk of type 2 diabetes.47 At the same time, UA and changes thereto are closely related to changes in Glu and insulin levels. This could be due to hyperinsulinemia’s effect on renal tubular function; as insulin-mediated Glu disposal decreases, so does UA clearance. Therefore, decreased UA excretion can lead to HUA.48 We also found that PRD residents with hyperlipidemia had a significantly higher risk of HUA than those without. Men with hyperlipidemia were more likely to develop HUA than women with hyperlipidemia. Studies have shown49 that high TG levels can lead to a significant increase in the occurrence density of HUA. This could be due to TG causing disorder of free fatty acid metabolism. The increase in TG leads to an increase in free fatty acid production, accelerates the decomposition of adenosine triphosphate (ATP) and leads to an increase in UA, which is the final product of purine metabolism.50 Chen et al51 found that the joint-assessment of fasting TG and waist circumference can be used to the risk of hyperuricemia.
Limitation
As this study collected only a small amount of data from nine cities in the PRD, the sample may not be very representative in terms of the entire PRD population and could have had a certain impact on the results. A more comprehensive reflection of the prevalence of HUA in the PRD requires further data collection. The cross-sectional design of the study leads to a certain difficulty in discerning association and causality between HUA and proposed risk factors. We would like to conduct cohort study or case-control study in the future to get a more precise assessment on HUA and risk factors.
Conclusion
Age, hypertension, BMI, hyperglycemia and hyperlipidemia among study participants in the PRD region were risk factors for HUA. The prevalence of HUA was relatively high in various PRD cities.
Abbreviations
BMI, body mass index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; Glu, glucose; HUA, hyperuricemic; Non-HUA, non-hyperuricemic; OR, odds ratio; PRD, Pearl River Delta; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; UA, uric acid.
Data Sharing Statement
The datasets supporting the conclusions of this article are available from the authors on direct request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Huadu District Maternal and Child Health Hospital, Guangzhou, China. All participants gave informed consent after having been informed about the objectives and benefits of our study.
Acknowledgments
The authors would like to thank Qiaoling Liu from London School of Hygiene & Tropical Medicine for his comments and assistance in revising the manuscript.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi:10.1016/j.ijcard.2015.08.109
2. Liu R, Han C, Wu D, et al. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: a Systematic Review and Meta-Analysis. Biomed Res Int. 2015;2015:762820. doi:10.1155/2015/762820
3. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141. doi:10.1002/art.30520
4. Uaratanawong S, Suraamornkul S, Angkeaw S, Uaratanawong R. Prevalence of hyperuricemia in Bangkok population. Clin Rheumatol. 2011;30(7):887–893. doi:10.1007/s10067-011-1699-0
5. Xia Y, Wu Q, Wang H, et al. Global, regional and national burden of gout, 1990–2017: a systematic analysis of the Global Burden of Disease Study. Rheumatology. 2020;59(7):1529–1538. doi:10.1093/rheumatology/kez476
6. Mikuls TR, Saag KG. New insights into gout epidemiology. Curr Opin Rheumatol. 2006;18(2):199–203. doi:10.1097/01.bor.0000209435.89720.7c
7. Richette P, Doherty M, Pascual E, et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis. 2020;79(1):31–38. doi:10.1136/annrheumdis-2019-215315
8. Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–662. doi:10.1038/nrrheum.2015.91
9. Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–2532. doi:10.1161/CIRCULATIONAHA.106.657627
10. Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123. doi:10.1186/s12916-017-0890-9
11. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283(18):2404–2410. doi:10.1001/jama.283.18.2404
12. Magnoni M, Berteotti M, Ceriotti F, et al. Serum uric acid on admission predicts in-hospital mortality in patients with acute coronary syndrome. Int J Cardiol. 2017;240:25–29. doi:10.1016/j.ijcard.2017.04.027
13. Biscaglia S, Ceconi C, Malagu M, Pavasini R, Ferrari R. Uric acid and coronary artery disease: an elusive link deserving further attention. Int J Cardiol. 2016;213:28–32. doi:10.1016/j.ijcard.2015.08.086
14. Lee HW, Choi SM, Lee J, et al. Serum uric acid level as a prognostic marker in patients with acute respiratory distress syndrome. J Intensive Care Med. 2019;34(5):404–410. doi:10.1177/0885066617698911
15. Hsu WL, Li SY, Liu JS, et al. High uric acid ameliorates indoxyl sulfate-induced endothelial dysfunction and is associated with lower mortality among hemodialysis patients. Toxins. 2017;9(1):20. doi:10.3390/toxins9010020
16. Mancia G, Grassi G, Borghi C. Hyperuricemia, urate deposition and the association with hypertension. Curr Med Res Opin. 2015;31(Suppl 2):15–19. doi:10.1185/03007995.2015.1087981
17. Nagano S, Takahashi M, Miyai N, et al. Association of serum uric acid with subsequent arterial stiffness and renal function in normotensive subjects. Hypertens Res. 2017;40(6):620–624. doi:10.1038/hr.2017.10
18. Atherosclerosis Group GDoGPMA. [Epidemiological study on plasma lipid among patients with cardiovascular disease in 9 cities of the Pearl River Delta region]. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37(9):849–853.
19. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. doi:10.1136/bmj.i5953
20. Ding W, Li T, Su Q, Yuan M, Lin A. Integrating factors associated with hypertensive patients’ self-management using structural equation modeling: a cross-sectional study in Guangdong, China. Patient Prefer Adherence. 2018;12:2169–2178. doi:10.2147/PPA.S180314
21. Joint Committee for Developing Chinese guidelines on P. Treatment of Dyslipidemia in A. [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(5):390–419.
22. American Diabetes A. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes. 2018;36(1):14–37. doi:10.2337/cd17-0119
23. Zhou B. Cooperative Meta-Analysis Group Of China Obesity Task F. [Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population]. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23(1):5–10.
24. Joint Committee for Guideline R. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16(3):182–241. doi:10.11909/j.issn.1671-5411.2019.03.014
25. Manara M, Bortoluzzi A, Favero M, et al. Italian Society of Rheumatology recommendations for the management of gout. Reumatismo. 2013;65(1):4–21. doi:10.4081/reumatismo.2013.4
26. Endocrinology C. Management of hyperuricemia and gout: Chinese experts consensus. Chin J Endocrinol Metab. 2013;29(11):8. doi:10.3760/cma.j.issn.1000-6699.2013.11.001
27. Maloberti A, Giannattasio C, Bombelli M, et al. Hyperuricemia and Risk of Cardiovascular Outcomes: the Experience of the URRAH (Uric Acid Right for Heart Health) Project. High Blood Press Cardiovasc Prev. 2020;27(2):121–128. doi:10.1007/s40292-020-00368-z
28. Virdis A, Masi S, Casiglia E, et al. Identification of the Uric Acid Thresholds Predicting an Increased Total and Cardiovascular Mortality Over 20 Years. Hypertension. 2020;75(2):302–308. doi:10.1161/HYPERTENSIONAHA.119.13643
29. Maloberti A, Maggioni S, Occhi L, et al. Sex-related relationships between uric acid and target organ damage in hypertension. Journal of Clinical Hypertension. 2018;20(1):193–200. doi:10.1111/jch.13136
30. Maloberti A, Qualliu E, Occhi L, et al. Hyperuricemia prevalence in healthy subjects and its relationship with cardiovascular target organ damage. Nutr Metab Cardiovasc Dis. 2020. doi:10.1016/j.numecd.2020.08.015
31. Redon P, Maloberti A, Facchetti R, et al. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: findings from the Blood Pressure control rate and CArdiovascular Risk profilE study. J Hypertens. 2019;37(2):380–388. doi:10.1097/hjh.0000000000001908
32. Qiu L, Cheng XQ, Wu J, et al. Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health. 2013;13:664. doi:10.1186/1471-2458-13-664
33. Song P, Wang H, Xia W, Chang X, Wang M, An L. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci Rep. 2018;8(1):4314. doi:10.1038/s41598-018-22570-9
34. Miao Z, Li C, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol. 2008;35(9):1859–1864.
35. Hainer BL, Matheson E, Wilkes RT. Diagnosis, treatment, and prevention of gout. Am Fam Physician. 2014;90(12):831–836.
36. Mumford SL, Dasharathy SS, Pollack AZ, et al. Serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. Hum Reprod. 2013;28(7):1853–1862. doi:10.1093/humrep/det085
37. Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354(9179):650. doi:10.1016/S0140-6736(99)92381-4
38. Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women–the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2008;10(5):R116. doi:10.1186/ar2519
39. Lewington S, Lacey B, Clarke R, et al. The Burden of Hypertension and Associated Risk for Cardiovascular Mortality in China. JAMA Int Med. 2016;176(4):524–532. doi:10.1001/jamainternmed.2016.0190
40. Perlstein TS, Gumieniak O, Hopkins PN, et al. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int. 2004;66(4):1465–1470. doi:10.1111/j.1523-1755.2004.00909.x
41. Zheng R, Yang T, Chen Q, Chen C, Mao Y. Serum Uric Acid Concentrations Can Predict Hypertension: a Longitudinal Population-Based Epidemiological Study. Horm Metab Res. 2017;49(11):873–879. doi:10.1055/s-0043-119129
42. Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63(1):102–110. doi:10.1002/acr.20344
43. Maloberti A, Bombelli M, Facchetti R, et al. Relationships between diuretic-related hyperuricemia and cardiovascular events: data from the URic acid Right for heArt Health study. J Hypertens. 2021;39(2):333–340. doi:10.1097/HJH.0000000000002600
44. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Int Med. 2005;165(7):742–748. doi:10.1001/archinte.165.7.742
45. Liu DM, Jiang LD, Gan L, Su Y, Li F. Association between serum uric acid level and body mass index in sex- and age-specific groups in Southwestern China. Endocr Pract. 2019;25(5):438–445. doi:10.4158/EP-2018-0426
46. Rho YH, Lu N, Peloquin CE, et al. Independent impact of gout on the risk of diabetes mellitus among women and men: a population-based, BMI-matched cohort study. Ann Rheum Dis. 2016;75(1):91–95. doi:10.1136/annrheumdis-2014-205827
47. Niskanen L, Laaksonen DE, Lindstrom J, et al. Serum uric acid as a harbinger of metabolic outcome in subjects with impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabetes Care. 2006;29(3):709–711. doi:10.2337/diacare.29.03.06.dc05-1465
48. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–3011.
49. Hou YL, Yang XL, Wang CX, Zhi LX, Yang MJ, You CG. Hypertriglyceridemia and hyperuricemia: a retrospective study of urban residents. Lipids Health Dis. 2019;18(1):81. doi:10.1186/s12944-019-1031-6
50. Balasubramanian T. Uric acid or 1-methyl uric acid in the urinary bladder increases serum glucose, insulin, true triglyceride, and total cholesterol levels in Wistar rats. Sci World J. 2003;3:930–936. doi:10.1100/tsw.2003.90
51. Chen S, Guo X, Dong S, et al. Association between the hypertriglyceridemic waist phenotype and hyperuricemia: a cross-sectional study. Clin Rheumatol. 2017;36(5):1111–1119. doi:10.1007/s10067-017-3559-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
