Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Prevalence and Predisposing Factors of Intestinal Parasitic Infections Among HIV Positive Patients Visiting Nekemte Specialized Hospital, Western Ethiopia
Received 28 January 2021
Accepted for publication 24 April 2021
Published 14 May 2021 Volume 2021:13 Pages 505—512
DOI https://doi.org/10.2147/HIV.S304294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Robsen Miressa, Mebrate Dufera
Department of Biology, College of Natural and Computational Sciences, Wollega University, Nekemte, Ethiopia
Correspondence: Mebrate Dufera Email [email protected]
Background: Intestinal parasites are endemic in many regions of the world where Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome (HIV/AIDS) patients are prevalent. This study aimed to assess the extent of intestinal parasitic infection (IPI) and predisposing factors among HIV positive patients visiting Nekemte Specialized Hospital, Western Ethiopia.
Methods: A hospital-based cross-sectional study was conducted among HIV positive patients and HIV negative controls who visited Nekemte Specialized Hospital from April to August 2020. A structured questionnaire was used to collect socio-demographic and risk-factor data. Stool samples and blood were collected and tested. Data were analyzed using SPSS version 20. P< 0.05 was considered statistically significant.
Results: The occurrence of IPIs was considerably higher (73.3%) among HIV positive subjects compared to HIV negative controls (22.7%). Rate of infection with IPI was higher in individuals with CD4+ T cell count < 200 cells/μL. The species-specific distribution of parasites among HIV positive was higher for Giardia lamblia 35% followed by Entamoeba histolytica, 16% and hookworm 17.5%. Among the risk factors; age, educational status and occupation were significantly related with IPI (P< 0.05). Habit of washing hands (OR=1.146, 95% CI: 0.189– 1.936) and contact with animals (OR=2.926, 95% CI: 1.955– 4.380) were expressively associated with IPI. Furthermore, eating raw meat, lack of safe water sources and usage were meaningfully connected with IPIs with OR=1.203, 95% CI: 0.590– 2.454 and OR=0.172, 95% CI: 0.112– 0.263, respectively.
Conclusion: HIV positive individuals were highly affected by IPI than HIV negative controls. The spreading of intestinal parasites was critically affected by reduced CD4+ T cell counts. Consistent screening and treatment of IPIs and awareness creation is very vital in improving the overall quality life of HIV/AIDS patients.
Keywords: HIV positive, CD4+T cell count, intestinal parasitic infection; IPI, risk factors
Introduction
Intestinal parasites are major cause of morbidity and mortality throughout the world, particularly in resource limited tropical and subtropical regions, including sub-Saharan Africa (SSA).1,2
Intestinal parasites can be more aggressive in children and the elderly than in middle-aged people and in immunocompromised patients3 such as Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome (HIV/AIDS) patients. Parasitic helminths cause chronic immune activation,3 by tilting the immune response toward T helper-2 immune response. Although, immune modulation was shown to increase host vulnerability; consequently, promoting HIV infection and disease progression proving evidences are inadequate.4 Thus, epidemics of HIV/AIDS in Africa is adversely influenced by chronic immune activation as suggested by.5 Furthermore, the epidemiology as well as outcome of diseases caused by opportunistic parasites was significantly modified with emergence of AIDS.6,7 In general, either reinforced by HIV or independently, intestinal parasitic infections (IPIs) have continued to be main cause of illness and death in humans.8 In developing countries, the spreading of parasitic infection among HIV-infected patients is estimated to be as high as 95%.9
Ethiopia is among the developing countries with high overlapping rate of HIV and parasitic infections. In different parts of the country, targeting the prevalence of intestinal parasites some studies have been conducted about10,11 and there have been few studies to determine whether the epidemiology of intestinal parasites gives a different picture as the population with HIV/AIDS is growing.,12,13 However, epidemiological data on the occurrence of IPIs among HIV/AIDS patients are still insufficient in the study area. The present study is, therefore, intended to assess the degree of IPI and predisposing factors among people with and without HIV infection at Nekemte Specialized Hospital, Western Ethiopia.
Methods
Study Design, Period, and Area
A hospital-based cross-sectional study was conducted from April to May, 2020 at Nekemte Specialized Hospital, western Ethiopia. Nekemte is located at a distance of 328 km from the capital-Addis Ababa to the west. The city has a latitude and longitude of 9°46ʹN and 36°31ʹE with an elevation of 2,088 meters above sea level, mean annual rainfall of 2022mm and mean annual temperature of 19.85°C. Nekemte Specialized Hospital is one of the renovated hospitals providing a comprehensive package of preventive, curative and rehabilitative health services to the community. The study involved laboratory examination of stools for intestinal parasite infections and blood tests for HIV test and CD4+ T cell count and questionnaire surveys for assessing predisposing factors in HIV positive patients.
Study Population
HIV positive patients who follow the ART follow up clinic and other diarrheic HIV negative controls were taken as the study population. To be included in the study patients, irrespective of their ages and gender, were eligible to participate. Individuals who were on any anti-parasitic medication prior to data collection were excluded from the study.
Determination of Sample Size
All available participants were included and a non-probability sampling method was used for the participant selection due to the small size group of HIV/AIDS patients (230) who had been attending ART follow up clinic from April to May, 2020.
Data Collection Process
Data was collected by expertise who is working in the ART clinic with an interviewer administered questionnaire to collect the socio-demographic factors. Further information regarding clinical conditions was gathered from the medical registry of the patients. Stool and blood sample collection was performed strictly following standard operating procedures.
Stool Sample Collection and Analysis
Each study subject was provide with a labeled leak-proof container, toilet paper, applicator stick and was informed to put appropriate gram of stool using an applicator stick. Fresh stool samples were collected from consented study participants. About 2 gm of each stool sample was emulsified with 3–4 mL of 10% formal saline, mixed thoroughly and passed through gauze. About 3–4 mL of diethyl ether was added and mixed by inverting and intermittent shaking for 1 minute and centrifuged at 3,000 rpm (revolution per minute) for 5 minutes. After centrifugation, the supernatant (the layer of ether, debris and formal saline) was discarded and the sediment (containing the parasite at the bottom of the test tube) was re-suspended in formal saline. Finally, the smear was prepared from the sediment and observed under light microscope with a magnification of 100× and 400× magnifications for the presence of ova, cyst and larval stage of intestinal parasites.
Blood Collection and CD4+ T Cell Determination
Blood specimens were collected aseptically by venipuncture into evacuated tubes containing ethylene diaminetetraacetic acid (EDTA) anticoagulant, completely expanding the vacuum in the tubes. The blood specimens were mixed well to prevent clotting and were labeled. The samples were then placed on a gentle blood rocker to ensure that the samples are uniformly distributed while awaiting the analysis. After quality control had been performed and passed cluster of differentiation 4 (CD4+) T cell count analysis was performed on blood samples using a florescence-activated cell sorting (FACS) count–automated machine (Becton Dickinson, USA). Once the reagent pair of tubes had been labeled and mixed using vortex with the pair upright and upside down for about 5 seconds, the tubes were then mixed with the patient’s blood sample by inverting the tube five times after being opened using a coring station. About 50 μL blood was pipetted into each tube, and the tubes were vortexed in the upright position for 5 seconds. Then the tubes were incubated at room temperature in the dark for 60–120 minutes, 50 μL of fixative solution added to each, and mixed upright using the vortex for 5 seconds. After 30 minutes’ incubation, the FACS count was run and results printed out.
Data Quality Control
Standardized quality checks were performed throughout data collection to obtain reliable and quality research documents. Quality control procedures were strictly maintained throughout data collection. Training was given to all data collectors. Every questionnaire was coded for each patient. Structured questionnaire were prepared precisely and translated into the local language (Afan Oromo). Laboratory based assays were performed based upon standard operating procedures. The completeness of data was checked in a daily manner by the main investigator.
Data Analysis
Data were entered and analyzed using Statistical Package for the Social Sciences (SPSS) version 20 (IBM, USA). Descriptive statistics, multivariate logistic regression methods were used to interpret the data. The strength of association related to risk factors was checked using odds ratio (OR) at 95% confidence interval (CI) and its associated p-value. With 95% CI P<0.05 was considered statistically significant.
Ethical Approval and Consent/Assent
Ethical clearance was obtained from Wollega University Research Ethics Review Committee (Ref.No.WU/RD/380). Informed consent was obtained from individual study participants prior to commencing data collection. For children under the age of 18 years, assent was obtained from their parents or guardians. The study was conducted in accordance with the Declaration of Helsinki. All study subjects found positive for any intestinal parasites were treated under the permission and supervision of Nekemte Specialized Hospital ART-clinic physician according to the standard protocol.
Results
Socio-Demographic Characteristics of the Study Participants
A total of 460 (230 HIV positive and 230 HIV negative) individuals participated in this study. Among those, 121 males (26.3%) were HIV sero-positive and 104 (22.6%) were HIV negative. 109 (23.7%) and 126 (27.4%) were females with HIV sero-positive and negative respectively. The age of study participants ranges from 8–76 years old. Age of participants 32 (6.9%) were within the age range of 0–15 years, 175 (38.1%) were 16- to 30-years-old, 191 (41.5%) were 31- to 45-years-old. Regarding their educational status from a total of 460, 22.6% (104) of the participants completed secondary school while 53.9% (248) did not have a formal education. Students were more exposed (16.1%) to HIV than other study subjects (Table 1).
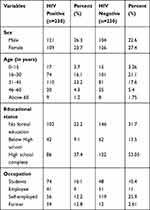 |
Table 1 Socio-Demographic Characteristics of the Study Participants (n=460) |
Species-Specific Prevalence of Intestinal Parasite
For species-specific assessment of intestinal parasites, about 185 individuals were involved during the study of which 143 of them were HIV positive and the rest 42 were HIV negative individuals. According to this study, intestinal parasites were found in 143 (77.3%) of the stool samples from HIV infected persons compared with 42 (22.7%) of those from HIV-negative individuals. The species specific prevalence of parasites among HIV positive individuals was 50 (35%) for Giardia lamblia, 23 (16%) for Entamoeba histolytica, 25 (17.5%) for hookworm, 13 (9.1%) for Ascaris lumbricoides, 15 (10.5%) for Taenia saginata, 7 (3.8%) for Strongyloides stercoralis, 4 (2.2%) for Hymenolepis nana and 3 (1.6%) for Trichuris trichiura. The predominant parasitic species among the HIV negative individuals were Giardia lamblia which accounted for 35% of the total parasites. The species-specific prevalence of parasites among HIV negative individuals were 17 (40.5%) for Giardia lamblia, 14 (33.3%) for Entamoeba histolytica, 7 (16.6%) for hookworm, 3 (7.1%) for Ascaris lumbricoides, and 1 (2.4%) for Strongyloides stercoralis. Taenia saginata, Hymenolepis nana and Trichuris trichiura infections were not observed in HIV negative patients. Regarding polyparasitism, the number of pathogens exceeds the number of patients as multiple parasites were identified in some patients (Table 2).
 |
Table 2 Association of Intestinal Parasites Among HIV Positive and Negative Study Participants (n=185) |
Correlation of Intestinal Parasites with CD4+ T Cell Count in HIV/AIDS Patients
The relationship of IPI with CD4+ T cell counts was highly significant (P=0.000) and have negative relation (−0.385) (Table 3).
 |
Table 3 Comparison of Intestinal Parasites Distribution in HIV/AIDS Patients by CD4+ T Cell Count (n=143) |
The association of socio-demographic characteristics and intestinal parasite distribution univariate logistic regression analysis of the socio demographic factor in relation to the prevalence of intestinal parasite shows that age, education and occupation of the participants’ were meaningfully associated intestinal parasite infections (P<0.05). Participants belonging to the age group of 16–30 years and living with HIV/AIDS were more exposed to intestinal parasites (Table 4).
 |
Table 4 Association of Socio Demographic Characteristics and Distribution of Intestinal Parasites Among HIV Positive and Negative Study Participants (n=185) |
Hand Washing Hygienic Conditions and Contact with Animals
Among 385 of the study subjects 128 (27%) had no habit of washing hands. However, of 75 subjects who did have good hand washing habits 55 (11.95%) were found to be positive for intestinal parasites. Presence of animals around home and close contact with them was also identified and 157 of the study subjects who had contact with animals 96 (61.1%) were found to be positive for IPIs. However, of 303 subjects who have no contact with animals 89 (29.4%) were found to be positive for IPIs (Table 5).
 |
Table 5 Association of Hand Washing Hygienic Conditions, Contact with Animals and Distribution of Intestinal Parasites Among HIV Positive and Negative Study Participants (n=185) |
Eating and Drinking Habits
The habit of eating fruit also assessed. Of 203 subjects who eat fruit directly or simply wash it with water, 102 (50.3%) were positive for infection.Of 257 subjects, those who eat fruit washed in clean water, .83 (32.3%) were found to be positive. The relationship was significantly associated with IPIs. Also multivariate logistic regression of this study shows that, eating fruit (directly or simply washing it in water (OR=1.203, 95% CI: 0.590–2.454). The habit of eating raw meat was also surveyed and out of 172 subjects who have eaten raw meat, 106 (61.6%) were positive; while of 288 subjects who have no habit of eating raw meat, 79 (27.4%) were found positive. The relation was significantly associated withIPIs (OR=0.172, 95% CI: 0.112–0.263). The risk factors for acquiring intestinal parasite were assessed in all the patients including access for safe water source and water handling practices like boiling, filtering, etc. Out of 460 participants, 99 responded that they had safe water sources and good water handling practices. Of these, only 30 (30.3%) were found to be positive for intestinal parasites. On the other hand, 351 of the participants responded that they had no safe water sources and had poor water handling practices and it is a statistically significant OR of having unsafe water source and practice (1.156, 95% CI: 0.647–2.032) (Table 6).
 |
Table 6 Association of Feeding-Drinking Habits and Distribution of Intestinal Parasites Among HIV Positive and Negative Study Participants (n=185) |
Discussion
According to this study, the overall prevalence of intestinal parasites shows a significant difference between HIV negative and HIV positive individuals. This agrees with the fact that HIV infection would increase the risk ofIPI.14 A similar finding is also evident in a research conducted in Ethiopia,13 in which, high occurrences ofIPI was found among HIV/AIDS patients and in Kenya,15 where helminthic parasites such as hookworm species and A. lumbricoides were more prevalent among HIV positive individuals.
A study conducted in Delgi, Northern Gondar, Ethiopia,16 described that higher prevalence of G. lamblia, hookworm, E. histolytica and H. nana were reported in which the present study agrees with higher prevalence of G. Lamblia and hookworm. Prevalence of G. lamblia, A. lumbricoides, hookworm and H. nana was disagree with previous studies conducted in Northern Gondar and Southern parts of Ethiopia.17
In line with the study conducted by18 in Kobo Health Center, Northeastern Ethiopia the present study shows that Giardia lamblia and hookworm were predominant parasites among HIV positive patients.CD4+ T cell count was greatly related with intestinal parasite. In this study, IPI occurrence was significantly higher in patients with CD4+ T cell count below 200 cells/μL which is in line with studies conducted elsewhere.19–21 In people living with HIV/AIDS (PLWHA) the reduction in CD4+ T cell count predisposes the patients to intestinal parasites, particularly to opportunistic IPIs. Immunodeficient patients are more vulnerable in acquiring intestinal parasites and are unable to clear the infection once it is established.22
Among HIV patients, although sex was not related with intestinal parasite infections, HIV sero-positive females were found to be greatly associated with parasitic infections. This study agrees with studies conducted in kobo health center,18 China and Kenya.23 The results may be due to the fact that most females were in a close in touch with routine household activities which facilitate the chance to be infected with intestinal parasites. Multivariate logistic regression analysis of socio demographic factor in relation to prevalence of intestinal parasite shows that age, education and occupation of the participants’ were significantly associated with intestinal parasite infection and also HIV positive individuals age group 16–30 years and CD4+ T cell range 200–499 cells/mm3 were extremely predisposed with IPI which is in agreement with study conducted by.24 Most hygienic practices, such as hand washing with water after using the toilet and hand washing with water before a meal, were significantly associated with IPI. This is in agreement with study carried out by Nekemte.25
The opposing association between hand washing practice after defecation and IPI might be due to the habit of using only water for washing in the area and inappropriate handling of ready-made foods and drinks, without washing hands using soap.26 The present study also indicated that using water from a river and unprotected springs were risk factors for IPI. This may arise from the contamination of water with animal and human waste that pollutes rivers or unprotected springs.
Contact with animals and IPI have a statistically significant relationship in this study which means that having contact with animals is 2.926 times a greater risk than not having contact with animals. This is in agreement with the fact that wild animals can also be infected with parasites that can infect people.10 The practice of eating raw vegetables and undercooked meat shows statistically significant associations with IPIs. However, sex and getting food from a restaurant did not show significant associations with IPI. The present study was in agreement with a study done in Arba Minch.27
The habit of eating raw meat was significantly associated with IPI, which means those who do not eat raw meat were less affected by intestinal parasites when compared with those who do eat raw meat. Having unsafe water sources and handling practices was statistically significant and posed a greater risk than having a safe water source and handling practices which is in agreement with the fact that intestinal parasites can transmit through unhygienic food and drinking water.26
Limitations
Due to a lack of resources such as the Kato-Katz method for parasite quantification and modified Ziehl-Neelsen staining method, detection of opportunistic intestinal parasites was not conducted. Additionally, in this study, a non-probability sampling technique was used that limits the relevance of the risk factor analysis to the entire population of PLWHA.
Conclusion
IPIs were highly prevalent in HIV positive patients, moreso than in HIV negative patients. G. lamblia trophozoite was the most commonly reported protozoan parasite in this study. Among helminths, hookworm was the most abundant in both HIV negative and positive individuals. The prevalence of IPIs was considerably higher in HIV/AIDS patients with CD4+ T cell count below 200 cells/μL. Age-wise specific occurrences of helminth and protozoan infections were highest among the 16–30 years age group. Regular screening and treatment of IPIs and awareness creation against major risk factors is vital to improve the overall quality life of HIV/AIDS patients in this study area.
Abbreviations
AOR, Adjusted Odds Ratio; ART, Antiretroviral Therapy; CDC, Center of Disease Control; COR, Crudes Odds Ratio; EDTA, Ethylene diaminetetraacetic acid; FACS, Florescence-activated cell sorting; HAART, Highly Active Antiretroviral Therapy; HIV/AIDS, Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome (HIV/AIDS); IPI, Intestinal parasitic infection; NTD, Neglected Tropical Diseases; PLWHA, People living with HIV/AIDS; SSA, Sub Saharan African; STH, Soil Transmitted Helminths; WHO, World Health Organization.
Acknowledgments
The authors would like to thank Wollega University for financial supply. Our great appreciation also goes to Nekemte Specialized Hospital ART-clinic staff members and data collectors. Likewise, we recognize the study participants for their willingness and cooperation at the time of data collection.
Funding
No external fund was attained, only institutional support from Wollega University and Nekemte Specialized Hospital.
Disclosure
The authors declare no conflicts of interests in this work.
References
1. Bangert M, Molyneux DH, Lindsay SW, et al. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect Dis Poverty. 2017;6(73). doi:10.1186/s40249-017-0288-0
2. WHO. Report on the WHO informal consultation on the use of chemotherapy for the control of morbidity due to soil-transmitted nematodes in humans. 2016.
3. Nkenfou CN, Nana CT, Payne VK. Intestinal parasitic infections in HIV infected and non-infected patients in a low HIV prevalence region, West-Cameroon. PLoS One. 2013;8(2):e57914. doi:10.1371/journal.pone.0057914
4. Kalinkovich A, Borkow G, Weisman Z, Tsimanis A, Stein M, Bentwich Z. Increased CCR5 and CXCR4 expression in Ethiopians living in Israel: environmental and constitutive factors. Clin Immunol. 2001;100(1):107–117. doi:10.1006/clim.2001.5040.
5. Fincham JE, Markus MB, Adams VJ. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Trop. 2003;86:315–333. doi:10.1016/S0001-706X(03)00063-9
6. Gupta S, Narang S, Nunavath V, Singh S. Chronic diarrhea in HIV patients: prevalence of coccidian parasites. Indian J Med Microbiol. 2008;26:172–175. doi:10.1186/1471-230X-9-7.
7. Kelly P, Todd J, Sianongo S, et al. Susceptibility to intestinal infection and diarrhoea in Zambian adults in relation to HIV status and CD4 count. BMC Gastroenterol. 2009;9:7–10. doi:10.1186/1471-230X-9-7
8. Habtamu B, Kloos H. Intestinal Parasitism. Epidemiology and Ecology of Health and Diseases in Ethiopia. Berhane Y, Hailemariam D, Kloos H, editors. Addis Ababa: Shama books; 2006:519–538.
9. Ngui R, Ishak S, Chuen CS, Mahmud R, Lim YA. Prevalence and risk factors of intestinal parasitism in rural and remote West Malaysia. PLoS Negl Trop Dis. 2011;5(3):e974. doi:10.1371/journal.pntd.0000974
10. Missaye A, Dagnew M, Alemu A. Prevalence of intestinal parasites and associated risk factors among HIV/AIDS patients with pre-ART and on-ART attending Dessie Hospital ART Clinic, Northeast Ethiopia. AIDS Res Ther. 2013;10(1):1–9. doi:10.1186/1742-6405-10-7
11. Alemu F. Prevalence of intestinal parasites and other parasites among HIV/AIDS patients with on-ART attending Dilla Referral Hospital, Ethiopia. J AIDS Clin Res. 2014;5(9):1–5. doi:10.4172/2155-6113.1000345
12. Assefa S, Erko B, Medhin G, et al. Intestinal parasitic infections in relation to HIV/ AIDS status, diarrhea and CD4 T-cell count. BMC Infect Dis. 2009;9:155. doi:10.1186/1471-2334-9-155
13. Alemayehu E, Gedefie A, Adamu A, et al. Intestinal parasitic infections among HIV-infected patients on antiretroviral therapy attending debretabor general hospital, Northern Ethiopia: a cross-sectional study. HIV/AIDS. 2020;12:647–655. doi:10.2147/HIV.S275358
14. Awole M, Gebre Selassie S, Kassa T, Kibru G. Prevalence of intestinal parasites in HIV infected adult patients in south western Ethiopia. Ethiop J Health Dev. 2003;17(1):71–78. doi:10.4314/ejhd.v17i1.9783
15. Walson JL, Stewart BT, Sangare L, et al. Prevalence and correlates of helminth co-infection in Kenyan HIV-1 infected adults. PLoS Negl Trop Dis. 2010;4(3):e644. doi:10.1371/journal.pntd.0000644
16. Gebrecherkos T, Kebede H, Addis G. Intestinal parasites among HIV/AIDS patients attending University of Gondar Hospital, northwest Ethiopia. Ethiop J Health Dev. 2019;33(2).
17. Nyantekyi LA, Legesse M, Belay M, et al. Intestinal parasitic infectionsamong under-five children and maternal awareness about the infections in SheshaKekele, Wondo Genet, Southern Ethiopia. Ethiop J Health Dev. 2010;24(3):185–190.
18. Bugassa G, Dimtsu B, Tarekegn H, Kassaw M, Tafete A. The Prevalence of Intestinal Parasites in HIV Positive and HIV Negative Individuals in Kobo Health Center, Northeastern Ethiopia. BBB Br Biomed Bull. 2014;569–576.
19. Akinbo FO, Okaka CE, Omoregie R. Prevalence of intestinal parasitic infections among HIV patients in Benin City, Nigeria. Libyan J Med. 2010;5:5506. doi:10.3402/ljm.v5i0.5506
20. Mengist HM, Taye B, Tsegaye A. Intestinal parasitosis in relation to CD4+ T cells levels and anemia among HAART initiated and HAART naive pediatric HIV patients in a model ART center in Addis Ababa, Ethiopia. PLoS One. 2015;10(2):e0117715. doi:10.1371/journal.pone.0117715.
21. Nsagha DS, Njunda AL, Assob NJ, Ayima CW, Tanue EA, Kwenti TE. Intestinal parasitic infections in relation to CD4+ T cell counts and diarrhea in HIV/AIDS patients with or without antiretroviral therapy in Cameroon. BMC Infect Dis. 2015;16(1):1–10. doi:10.1186/s12879-016-1337-1
22. Evering T, Weiss L. The immunology of parasite infections in immunocompromised hosts. Parasite Immunol. 2006;28(11):549–565. doi:10.1111/j.1365-3024.2006.00886.x
23. Tian LG, Chen JX, Wang TP, et al. Coinfection of HIV and intestinal parasites in rural area of China. Parasit Vectors. 2012;5:36. doi:10.1186/1756-3305-5-36
24. Gupta K, Bala M, Deb M, Muralidhar S, Sharma DK. Prevalence of intestinal parasitic infections in HIV-infected individuals and their relationship with immune status. Indian J Med Microbiol. 2013;31(2):161–165. doi:10.4103/0255-0857.115247.
25. Eshetu L, Dabsu R, Tadele G. Prevalence of intestinal parasites and its risk factors among food handlers in food services in Nekemte town, west Oromia, Ethiopia. Res Rep Trop Med. 2019;10:25–30. doi:10.2147/RRTM.S186723
26. Center for Disease Control. Global health, division of parasitic diseases and malaria. 2019.
27. Abyu DM, Getahun EA, Malaju MT, Bizuayehu HM. Time to increase WHO clinical stage of people living with HIV in public health facilities of Arba Minch town, south Ethiopia. Clin Med Res. 2014;3(5):119–124. doi:10.11648/j.cmr.20140305.11
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
