Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Prevalence and Predictors of Virological Failure Among Adults Living with HIV in South Wollo Zone, Northeast Ethiopia: A Retrospective Cohort Study
Authors Fentie Wendie T , Workneh BD
Received 6 June 2020
Accepted for publication 14 August 2020
Published 7 September 2020 Volume 2020:12 Pages 393—402
DOI https://doi.org/10.2147/HIV.S266460
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Teklehaimanot Fentie Wendie, Birhanu Demeke Workneh
Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Correspondence: Teklehaimanot Fentie Wendie Email [email protected]
Background: Highly active antiretroviral therapy has dramatically altered progression of HIV infection and significantly improved patients’ quality of life. However, drug resistance with consequent treatment failure raises the need for much more expensive and toxic second-line regimens. Thus, this study aimed at investigating the predictors of virologic failure among adults in Northeast Ethiopia.
Methods: A retrospective cohort study was carried out among adults who started first-line antiretroviral treatment from September 2005 to January 2018. Data were collected from patients’ medical records, entered and validated using EpiData version 3.1 and then exported to SPSS version 20 for analysis. Binary logistic regression was carried out; odds ratio with 95% CI was used to identify covariates associated with virologic failure. Statistical significance was considered at p-value < 0.05.
Results: A total of 384 patients with mean age of 35.73± 9.44 years were consecutively enrolled; of which, 213 (55.5%) were females, 255 (66.4%) had WHO clinical stage III/IV, and 130 (33.9%) had baseline CD4 count < 100 cells/mm3. Mean baseline CD4 count was 179 cells/mm3 (range: 2– 853 cells), and 158 (41.1%) participants were on AZT/3TC/NVP. Virological failure was diagnosed among 61 (15.9%) patients. The mean time to virologic failure after initiation of ART was 63.80 months (range: 17– 150 months). After adjusting for other confounders, risk of experiencing virologic failure was significantly associated with being divorced (AOR 3.40, 95% CI 1.20– 9.59), being naïve to ART (AOR 2.55, 95% CI 1.23– 5.28), low (< 100) baseline CD4 count (AOR 2.39, 95% CI 1.03– 5.54) and nonadherence (AOR 6.73, 95% CI 3.29– 13.76).
Conclusion: In this study, the prevalence of antiretroviral treatment failure was 15.9%. Being divorced, being naïve to antiretroviral therapy, low (< 100 cells/mm3) baseline CD4 count and nonadherence were found to be significant predictors of virologic failure. ART programs should focus on early HIV diagnosis and ART initiation as well as enhanced adherence support.
Keywords: adult, virological failure, risk factors, Northeast Ethiopia
Introduction
Combatting the epidemics of human immunodeficiency virus (HIV) continues to be a major challenge to global health remaining in the top 10 leading causes of death worldwide. By the end of 2018, approximately 37.9 million people were living with HIV worldwide. Of which, 23.3 million people were accessing antiretroviral therapy and global coverage of antiretroviral therapy among adults aged 15 years and older reached 62%. Antiretroviral therapy (ART) coverage in Sub-Saharan Africa increased from 24% in 2010 to 54% in 2015. Annual AIDS-related deaths have decreased by 43% and 36% worldwide and in Sub-Saharan Africa, respectively.1–3
Highly active antiretroviral therapy (HAART) has dramatically altered the natural progression of HIV infection, and significantly improved patients’ quality of life.4 Virologic response to HAART results in a substantial rise in CD4 cell counts which is central to the restoration of integrity of the immune system.5,6 However, studies around the world revealed that antiretroviral treatment failure and the need for much more expensive and much more toxic second-line regimens are a major concern.7–13 WHO’s Report on HIV drug resistance 2017 demonstrates a steady increase in the prevalence of HIV drug resistance in individuals initiating first-line ART since 2001, most notably in Southern and Eastern Africa.14 Several studies have also investigated risk factors for poor responses to HAART including old age, poor adherence, CD4 cell count <200 cells/mm3, advanced clinical stage, nucleoside-only regimen, viral load, pretreatment, prior virologic failure and financial insecurity.5,7-9,15–21
Monitoring of HIV RNA and CD4 count every 3 to 6 months to identify treatment failure is essential to ensure that ART remains potent and to enable timely switches from first- to second-line therapy.22 However, global and local studies revealed that the probability of detecting treatment failure and switching to second-line therapy was limited. Delayed switching increases levels of resistance to commonly used antiretroviral drugs and further jeopardizes the success of antiretroviral therapy; on the other hand, early, unnecessary switching may reduce treatment options and increase costs.6,23,24 Viral load is recommended as the preferred monitoring approach, though WHO guidelines suggest using immunologic and clinical criteria for making decisions about treatment failure in resource-limited settings.25,26
Knowledge of treatment failure trends and predictors among HIV-positive patients in the era of HAART in developing countries like Ethiopia is virtually limited.6,7,27–30 The emergence of resistant viruses resulting in treatment failure is inevitable and should be anticipated proactively so that patients will be timely switched to second-line regimen in order to have a sustained viral suppression. Understanding the magnitude of the problem and factors that are associated with treatment failure could be a key step in offering stringent care for those at risk. To date, the prevalence of virological failure and its predictors among adults on highly active antiretroviral therapy have not been investigated at hospitals of South Wollo Zone. Thus, this study was aimed at filling this gap so that, strategies can perhaps be developed to address these risk factors, or subjects at risk can become candidates for close monitoring.
Methods and Participants
Study Design, Area and Period
A retrospective cohort study was conducted from September 2018 to January 2019 among HIV-positive adults on a standard first-line antiretroviral treatment at two governmental hospitals in Dessie town. These were Boru Meda and Dessie Referral Hospitals. The study was conducted in compliance with the Declaration of Helsinki after approval by institutional review committee of Wollo University and a subsequent permission from each hospital.
Study Population and Eligibility Criteria
All HIV-infected patients aged 15 years and above who started HAART from September 2005 to January 2018 and had been treated for at least 12 months with two consecutive documented viral load test results as well as pre- and post-ART CD4 cell counts were eligible for enrolment in the study. On the other hand, transfer in/out cases, lost to follow-up and patients with incomplete medical records were excluded.
Sample Size Determination and Sampling Procedure
The required sample size was calculated using single population proportion formula with the following assumptions: true population proportion or estimated prevalence (P) = 0.5, absolute precision (d) = 0.05, and 95% confidence level to be 384. Since there was no prior similar study in the study area, the true population proportion was taken to be 50%. Participants were consecutively enrolled based on their refill appointment; thus, simple random sampling technique was used.
Study Variables
Virological failure, defined as a persistently detectable viral load exceeding 1000 copies/mL based on two consecutive measurements within a three-month interval after at least six months of antiretroviral treatment, was the primary outcome variable of the study. Socio-demographic characteristics (age, gender, educational level, marital status, social drug use), nutritional status, tuberculosis coinfection, WHO clinical stage at initiation, baseline CD4 cell count previous exposure to ART, first-line regimen given and level of adherence were the predictor variables.
Data Collection Procedure
Socio-demographic characteristics, baseline and follow-up clinical and laboratory data, and treatment outcomes were collected from patients’ medical records using a structured and pretested data abstraction format. Unique ART numbers were used to identify individual participants. The data abstraction format was adopted from ART intake and follow-up forms of ART clinics. It was pretested on transferred-in patients found in one of the study facilities. Patient intake form, follow-up card and ART registers as well as the electronic information database were used as data sources. Other clinical charts including laboratory test results were also used to collect retroviral load and CD4 cell counts. Patients were retrospectively followed from the date of enrolment to HAART initiation.
Data Quality Assurance
The data collection tool was pretested for applicability. Data collectors and supervisors were trained. Data were checked for completeness within 24 hours. Data coding, cleaning and verification were done before entry. Ambiguities were cleared by discussion among the research team.
Data Processing and Analysis
Data were entered into computer using EpiData version 3.1 and exported to the Statistical package for Social Science (SPSS) version 20.0 for analysis. Descriptive statistics were applied for the analysis of patient characteristics. Survival analysis was performed to determine the mean time to treatment failure after initiation of the first-line regimen. Binary logistic regression models were used to identify significant predictors of treatment failure. The following variables were considered as potential predictors: gender, age, residence, educational status, marital status, social drug use, BMI, baseline WHO clinical stage, TB, ART regimen, baseline CD4 cells, and adherence. Odds ratios (OR) with the 95% confidence intervals were presented. A 2-sided p-value of <0.05 was considered to be statistically significant.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki ethical principles for medical research involving human subjects after approval by institutional review committee of Wollo University and a subsequent permission from each hospital. Informed consent was waived by Institutional Review Committee of Wollo University and respective hospitals due to the retrospective nature of the study, as all the data were extracted from patients’ medical records. The confidentiality of information obtained from each study participant was guaranteed by omitting names or any personal identifiers. Moreover, the collected data were kept safe throughout the whole process of the research work to limit data accessibility to a third party.
Definition of Terms and Explanations
- Baseline CD4 count is the latest value measured before initiation of therapy.
- HAART is the combination of antiretroviral treatment with at least three drugs, including at least one non-nucleoside reverse-transcriptase inhibitor (NNRTI) or a protease inhibitor (PI), and/or abacavir, or a treatment regimen with a combination of a NNRTI and a boosted PI.
- Virological failure is defined by a persistently detectable viral load exceeding 1000 copies/mL (based on two consecutive measurements within a three-month interval) after at least six months of using antiretroviral drugs; defined per World Health Organization criteria.25,26
- Adherence was assessed by patient self-report of missed doses and calculated using the following formula:
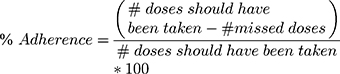
Adherence is considered optimal when its rate is greater than or equal to 95%.
Results
Sociodemographic and Baseline Clinical Characteristics of Study Participants
Three hundred eighty-four eligible participants were enrolled in the study. Of which, 213 (55.5%) of them were females. The mean age of patients at the time of HAART initiation was 35.73 ± 9.44 (minimum 15, maximum 80) years. More than half of the patients, 195 (50.8%) were married; 147 (38.3%) attended primary school; and 214 (55.7%) were urban dwellers. Of the total 384 study participants, 125 (32.6%) were underweight with body mass index of <18.5 kg/m2; 60 (15.6%) use social drugs like Khat, alcohol, or cigarette; 57 (14.8%) were diagnosed with active tuberculosis; and 226 (58.9%) of them were ART naïve.
Regarding the severity of disease, 255 (66.4%) of the participants were categorized under WHO clinical stage III/IV and 130 (33.9%) of them had a baseline CD4 cell count of <100 cells/mm3. The mean baseline CD4 cell count was 179 cells/mm3 (minimum 2, maximum 853). Concerning primary prophylaxis against common opportunistic infections, 349 (90.9%) and 270 (70.3%) of the participants were appropriately prescribed with cotrimoxazole and isoniazid preventive therapies, respectively. The predominant HAART regimen was a combination of Zidovudine, Lamivudine and Nevirapine (AZT/3TC/NVP) accounting for 158 (41.1%) and 199 (51.8%) of the study participants were initiated on NVP-based regimen (Table 1).
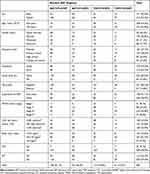 |
Table 1 Sociodemographic and Baseline Clinical Characteristics of Patients on HAART in Northeast Ethiopia: 2005–2019 (N: 384) |
Treatment Outcome After Initiation of HAART
Of the total 384 study participants, 65 (16.9%) were not adherent to their recommended ART regimen; 162 (42.2%) had documented history of at least one adverse drug reaction; and 61 (15.9%) experienced antiretroviral treatment failure confirmed by retroviral load test. The mean time to develop treatment failure after initiation of first-line regimen was 63.80 months which ranges from 17 to 150 months. The mean duration of follow-up on HAART was 76.91±31.9 months with minimum 15 months and maximum 150 months of follow-up (Table 2).
 |
Table 2 Treatment Outcome After Initiation of HAART Among Adults in Northeast Ethiopia: 2005–2019 (N: 384) |
Predictors of Antiretroviral Treatment Failure
Bivariate analysis of the factors revealed that being naïve to antiretroviral therapy (crude odds ratio (COR) 1.83, 95% confidence interval (CI) 1.01–3.31) and nonadherence (COR 5.96, 95% CI 3.24–10.94) were associated with the risk of antiretroviral treatment failure. On the other hand, the antiretroviral therapy regimen TDF/3TC/NVP was found to be protective of treatment failure (COR 0.11, 95% CI 0.02–0.84). But in multivariate logistic regression being divorced, being naïve to antiretroviral therapy, low (<100 cells/mm3) baseline CD4 cell count and nonadherence were found to be significant predictors of antiretroviral treatment failure (Table 3).
 |
Table 3 Sociodemographic and Clinical Variables as Predictors of Antiretroviral Treatment Failure Among Adults in Northeast Ethiopia: 2005–2019 (N: 384) |
Nonadherence was the strongest predictor of treatment failure as patients with poor adherence were found to be greater than six times at risk of facing treatment failure compared to their counterparts (adjusted odds ratio (AOR) 6.73, 95% CI 3.29–13.76). The risk of experiencing treatment failure was more than three times (AOR 3.40, 95% CI 1.20–9.59) among divorced individuals than those who never get married. Patients who were naïve to antiretroviral therapy were more than twice at risk of having treatment failure than patients who were on any other antiretroviral regimen (AOR 2.55, 95% CI 1.23–5.28).
Similarly, patients with low (<100 cells/mm3) baseline CD4 cell count were greater than twice more likely to have treatment failure compared to patients with baseline CD4 cell count of >200 cells/mm3 (AOR 2.39, 95% CI 1.03–5.54). However, no statistically significant association was found by multivariate analysis between treatment failure and the following covariates: sex, age, residency, body mass index, educational level, social drug use, tuberculosis coinfection, baseline WHO clinical stage and first-line ART regimen.
Discussion
Prevention, monitoring and timely response to population levels of HIV drug resistance are critical to achieving the WHO/UNAIDS 90–90–90 targets for 2020 that 90% of people living with HIV know their HIV status, 90% of those who know their HIV-positive status are accessing treatment and 90% of the people receiving treatment having suppressed viral loads. However, assessment of programmatic quality of care indicators (ie, retention on ART at 12 months after initiation, viral load testing coverage, viral load suppression at 12 months, drug stock out and proportion of people on second-line ART) associated with the prevention of HIV drug resistance shows that Ethiopia attained none of these key indicators as reported during the years 2015–2017.31
Timely identification and switching of antiretroviral treatment failure are key components for HIV programs. Staying on a failing regimen is associated with an increased risk of mortality. In addition to this, it promotes the transmission of resistant viruses in the community and also it limits the development of potent and tolerable regimens in the future. This study was aimed to determine the prevalence and associated factors of treatment failure among HIV/AIDS patients on HAART.
According to this study, the prevalence of treatment failure, defined as retroviral load >1000 copies/mL, was 15.9%. This finding was in agreement with studies done in Southern Ethiopia 11.1%,24 Northwest Ethiopia 14.7%,10 Northern Ethiopia 11.5%,32 Addis Ababa, Ethiopia 19.8%,33 Uganda 11%,34 Tanzania 19%,27 India 16.5%35 and China 11.8%.11 However; our finding was lower than findings of prior researches conducted in Southwest Ethiopia 25.1%,36 Eastern Ethiopia 22.7%,37 Mozambique 24.4%,8 Europe 23.4%,38 Canada 37.1%,12 Vietnam 23%,13 Saudi Arabia 23.8%,39 and Peru 24%.40 On the other hand, this result was significantly higher than what were reported by studies conducted in different parts of the world like Northern Ethiopia 4.1%,9 South Eastern Ethiopia 2.4%,41 a multicenter cohort in Ethiopia 7.4%,42 Kenya 6%,43 India 3.9%,44 and Vietnam 6.6%.45 The observed discrepancy might be due to the differences in health literacy, study design, sample size, interventions for adherence support, inclusion criteria, first-line ART regimen and definition of virological failure. Some authors used ≥400 copies/mL or ≥5000 copies/mL as a cut-off point for virological failure.13,35,43
The mean time to develop treatment failure after initiation of first-line regimen was 63.80 months which ranges from 17 to 150 months. This finding is less than the finding reported in Southwest Ethiopia and Tanzania where the mean times to treatment failure were 121.9 (120.3–123.5) months and 72 months, respectively.27,36 However, it was much greater than reports from Northwest Ethiopia 17.5 (8–36) months,9 Southern Ethiopia 21.6 (6.1–95.0) months,24 Northern Ethiopia 36 months,32 Addis Ababa 41.2 (39.7–42.6) months,33 Kenya 40 months,43 Mozambique22.2 (12.1–46.7) months,8 Canada 29 months,12 Vietnam 14 months,13 and Peru 35 (29–41) months.40 The possible explanation for this might be the delay in the diagnosis of treatment failure in the study setting though it needs further study.
Multivariate logistic regression analysis shows that nonadherence, being divorced, low baseline CD4 cell count and being naïve to antiretroviral therapy were independent predictors of antiretroviral treatment failure. Nonadherence to ART regimens was the strongest predictor of treatment failure as patients with poor adherence were found to be greater than six times at risk of facing treatment failure compared to their counterparts (AOR 6.73, 95% CI 3.29–13.76). Similarly, studies conducted in Northern Ethiopia,9,32,46 Northeast Ethiopia,47 Southeast Ethiopia,41 Eastern Ethiopia,37 Uganda,34 Mozambique,8 India,35,48 China,11 Canada12 and Peru40 showed that nonadherence was one of the main factors significantly associated with antiretroviral treatment failure.
The risk of experiencing treatment failure was more than three times (AOR 3.40, 95% CI 1.20–9.59) among divorced individuals than those who never get married. Possible reasons might be social drug use, sedentary way of life and other high-risk behaviors which could adversely affect adherence to antiretroviral therapy and health–seeking behavior. Likewise, patients who were naïve to antiretroviral therapy were more than twice at risk of having treatment failure than patients who were on any other antiretroviral regimen (AOR 2.55, 95% CI 1.23–5.28). This was inconsistent with the finding of a study done in Peru where history of antiretroviral use before starting HAART was associated with virologic failure.40 The discrepancy might be due to the acquisition of drug-resistant viral strains. However, it needs further investigation.
This research also revealed that patients with low (<100 cells/mm3) baseline CD4 cell count were greater than twice more likely to have treatment failure compared to patients with baseline CD4 cell count of >200 cells/mm3 (AOR 2.39, 95% CI 1.03–5.54). Similar findings were reported by several studies conducted in Ethiopia,9,18,37,41,42 Europe,38 Vietnam,45 and Peru.40 However, no statistically significant association was found by multivariate analysis between treatment failure and the following covariates: sex, age, residency, body mass index, educational level, social drug use, tuberculosis coinfection, baseline WHO clinical stage and first-line ART regimen. These were inconsistent with the findings of various studies done around the globe.10,11,13,32–35,40–42
Our study was not without limitation. The retrospective nature of this study might be a limitation as the accuracy of analysis depends on the completeness of the records, and hence, information bias might have occurred because of underreporting/missing data elements. The other limitations might be the assessment of adherence based on records without using standardized set of questions and the exclusion of transferred out patients.
Conclusions
In this study, the prevalence of antiretroviral treatment failure was 15.9%. Being divorced, being naïve to antiretroviral therapy, low (<100 cells/mm3) baseline CD4 cell count and nonadherence were found to be independent predictors of treatment failure. ART programs should focus on early HIV diagnosis and ART initiation as well as enhanced adherence support. Further studies are needed to evaluate whether the use of these factors can help to identify prospectively patients at high risk of failure, and to design interventions aimed to reduce this risk.
Data Sharing Statement
The datasets are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to express our sincere gratitude to our colleagues in department of pharmacy, Wollo University for their dedicated and constructive comments. Our special thanks go to the management and workers of the selected hospitals for their cooperation and data collectors for their commitment.
Authors’ Contribution
Both authors made a significant contribution to the work reported in the conception, study design, acquisition of data, analysis and interpretation, drafting, revising or critically reviewing the article; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Finally, both the authors proofread and approved the final version of this manuscript to be published.
Disclosure
The authors declare that they have no potential competing interests for this work.
References
1. Global AIDS update; 2018. https://www.unaids.org/en/resources/fact-sheet.
2. Global AIDS Response Progress Reporting (GARPR) 2016. UNAIDS 2016 estimates.
3. Ethiopia HIV/AIDS Progress in 2014 WHO update March 2015.
4. Marconi VC, Grandits GA, Weintrob AC, et al. Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the U.S. Military HIV natural history study. AIDS Res Ther. 2010;7(1):14. doi:10.1186/1742-6405-7-14
5. Vaamonde CM, Hoover DR, Anastos K, et al. Factors associated with poor immunologic response to virologic suppression by highly active antiretroviral therapy in HIV-infected women. AIDS Res Hum Retroviruses. 2006;22(3):222–231. doi:10.1089/aid.2006.22.222
6. Palombi L, Marazzi MC, Guidotti G, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral treated patients in Sub-Saharan African sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48:115–122. doi:10.1086/593312
7. Robbins GK, Daniels B, Zheng H, Chueh H, Meigs JB, Freedberg KA. Predictors of antiretroviral treatment failure in an urban HIV clinic. J Acquir Immune Defic Syndr. 2007;44(1):30–37. doi:10.1097/01.qai.0000248351.10383.b7
8. Palladino C, Briz V, Bellon JM, et al. Predictors of attrition and immunological failure in HIV-1 patients on highly active antiretroviral therapy from different healthcare settings in Mozambique. PLoS One. 2013;8(12):e82718. doi:10.1371/journal.pone.0082718
9. Ayalew MB, Kumilachew D, Belay A, et al. First-line antiretroviral treatment failure and associated factors in HIV patients at the University of Gondar Teaching Hospital, Gondar, Northwest Ethiopia. HIV/AIDS (Auckland, Nz). 2016;8:141–146.
10. Ayele G, Tessema B, Amsalu A, Ferede G, Yismaw G. Prevalence and associated factors of treatment failure among HIV/AIDS patients on HAART attending University of Gondar Referral Hospital Northwest Ethiopia. BMC Immunol. 2018;19(1):37. doi:10.1186/s12865-018-0278-4
11. Kan W, Teng T, Liang S, et al. Predictors of HIV virological failure and drug resistance in Chinese patients after 48 months of antiretroviral treatment, 2008–2012: a prospective cohort study. BMJ Open. 2017;7(9):e016012. doi:10.1136/bmjopen-2017-016012
12. Gross R, Yip B, Lo Re V, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194(8):1108–1114. doi:10.1086/507680
13. Huong DT, Bannister W, Phong PT, Kirk O, Peters L. Factors associated with HIV-1 virological failure in an outpatient clinic for HIV-infected people in Haiphong, Vietnam. Int J STD AIDS. 2011;22(11):659–664. doi:10.1258/ijsa.2011.010515
14. Organization WH. Global action plan on HIV drug resistance 2017–2021. 2017.
15. Grabar S, Pradier C, Corfec EL, et al. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS (London, England). 2000;14(2):141–149. doi:10.1097/00002030-200001280-00009
16. Hare AQ, Ordóñez CE, Johnson BA, et al. Gender-specific risk factors for virologic failure in KwaZuluNatal: automobile ownership and financial insecurity. AIDS Behav. 2014;18(11):2219–2229. doi:10.1007/s10461-014-0849-1
17. Thorsteinsson K, Ladelund S, Jensen-Fangel S, et al. Impact of gender on response to highly active antiretroviral therapy in HIV-1 infected patients: a nationwide population-based cohort study. BMC Infect Dis. 2012;12(1):1–11. doi:10.1186/1471-2334-12-293
18. Teshome W, Assefa A, He W. Predictors of immunological failure of antiretroviral therapy among HIV infected patients in Ethiopia: a matched case-control study. PLoS One. 2014;9(12):e115125. doi:10.1371/journal.pone.0115125
19. Ding H, Wilson CM, Modjarrad K, McGwin G, Tang J, Vermund SH. Predictors of suboptimal virologic response to highly active antiretroviral therapy among human immunodeficiency virus infected adolescents. Arch Pediatr Adolesc Med. 2009;163(12):1100–1105. doi:10.1001/archpediatrics.2009.204
20. Nanzigu S, Kiguba R, Kabanda J, et al. Poor immunological recovery among severely immunosuppressed antiretroviral therapy-naïve Ugandans. HIV/AIDS (Auckland, Nz). 2013;5:309–319.
21. Kokeb M, Degu G. Immunological response of HIV-infected children to highly active antiretoviral therapy at Gondar University Hospital, North-Western Ethiopia. Ethiop J Health Sci. 2016;26(1):25–30. doi:10.4314/ejhs.v26i1.6
22. Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS (London, England). 2014;28(14):2097–2107. doi:10.1097/QAD.0000000000000349
23. Bacha T, Tilahun B, Worku A. Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first line anti-retroviral therapy. BMC Infect Dis. 2012;12:197. doi:10.1186/1471-2334-12-197
24. Yirdaw KD, Hattingh S, Paraskevis D. Prevalence and predictors of immunological failure among HIV patients on HAART in Southern Ethiopia. PLoS One. 2015;10(5):e0125826. doi:10.1371/journal.pone.0125826
25. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: 2013 WHO Recommendations for a Public Health Approach. World Health Organization; 2016.
26. Teshome W, Tefera A. Detection of immunological treatment failure among HIV infected patients in Ethiopia: a retrospective cohort study. BMC Immunol. 2015;16(1):1–7. doi:10.1186/s12865-015-0120-1
27. Vanobberghen FM, Kilama B, Wringe A, et al. Immunological failure of first-line and switch to second-line antiretroviral therapy among HIV-infected persons in Tanzania: analysis of routinely collected national data. Trop Med Int Health. 2015;20(7):880–892. doi:10.1111/tmi.12507
28. Bunupuradah T, Puthanakit T, Kosalaraksa P, et al. Immunologic and virologic failure after first-line NNRTI-based antiretroviral therapy in Thai HIV-infected children. AIDS Res Ther. 2011;8(1):1–6. doi:10.1186/1742-6405-8-40
29. Sanne IM, Westreich D, Macphail AP, Rubel D, Majuba P, Rie AV. Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study. J Int AIDS Soc. 2009;12:38. doi:10.1186/1758-2652-12-38
30. Tsuchiya N, Pathipvanich P, Wichukchinda N, et al. Incidence and predictors of regimen-modification from first-line antiretroviral therapy in Thailand: a cohort study. BMC Infect Dis. 2014;14:565. doi:10.1186/s12879-014-0565-5
31. Organization WH. HIV Drug Resistance Report 2019. 2019.
32. Dejene TA, Tegegne AS, Ndlovu P, Zewotir T. Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. PLoS One. 2018;18(1):197.
33. Teshome Yimer Y, Yalew AW, Cameron DW. Magnitude and predictors of anti-retroviral treatment (ART) failure in private health facilities in Addis Ababa, Ethiopia. PLoS One. 2015;10(5):e0126026. doi:10.1371/journal.pone.0126026
34. Bulage L. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014–July 2015. Pharmacol Res Perspect. 2017;17(1):326.
35. Shet A, Neogi U, Kumarasamy N, DeCosta A, Shastri S, Rewari BB. Virological efficacy with first-line antiretroviral treatment in India: predictors of viral failure and evidence of viral resuppression. Trop Med Int Health. 2015;20(11):1462–1472. doi:10.1111/tmi.12563
36. Gesesew HA, Ward P, Woldemichael K, Mwanri L. Immunological failure in HIV-infected adults from 2003 to 2015 in Southwest Ethiopia: a retrospective cohort study. PLoS One. 2018;8(8):e017413.
37. Lenjiso GA, Endale BS, Bacha YD. Clinical and immunological failure among HIV-positive adults taking first-line antiretroviral therapy in Dire Dawa, eastern Ethiopia. BMC Public Health. 2019;19(1):771. doi:10.1186/s12889-019-7078-5
38. Dragsted UB, Mocroft A, Vella S, et al. Predictors of immunological failure after initial response to highly active antiretroviral therapy in HIV-1-infected adults: a EuroSIDA study. J Infect Dis. 2004;190(1):148–155. doi:10.1086/420786
39. Musa BM, Coker M, Bussell S, et al. Long-term outcomes of antiretroviral therapy in an adult HIV program: a 10-year retrospective cohort study in Kano, Nigeria. Ann Saudi Med. 2015;35(4):303–311. doi:10.5144/0256-4947.2015.303
40. Alave J, Paz J, Gonzalez E, et al. [Risk factors associated with virologic failure in HIV- infected patients receiving antiretroviral therapy at a public hospital in Peru]. Rev Chilena Infectol. 2013;30(1):42–48. doi:10.4067/S0716-10182013000100006
41. Haile D, Takele A, Gashaw K, Demelash H, Nigatu D, Ho W. Predictors of treatment failure among adult antiretroviral treatment (ART) clients in Bale Zone Hospitals, South Eastern Ethiopia. PLoS One. 2016;11(10):e0164299. doi:10.1371/journal.pone.0164299
42. Telele NF, Kalu AW, Marrone G, et al. Baseline predictors of antiretroviral treatment failure and lost to follow up in a multicenter countrywide HIV-1 cohort study in Ethiopia. PLoS One. 2018;13(7):e0200505. doi:10.1371/journal.pone.0200505
43. Ferreyra C, Yun O, Eisenberg N, et al. Evaluation of clinical and immunological markers for predicting virological failure in a HIV/AIDS treatment cohort in Busia, Kenya. PLoS One. 2012;7(11):e49834. doi:10.1371/journal.pone.0049834
44. Rajasekarana S, Jeyaseelanb L, Vijilaa S, Gomathia C, Raja K. Predictors of failure of first-line antiretroviral therapy in HIV-infected adults: Indian experience. AIDS (London, England). 2007;21(suppl 4):S47–S53. doi:10.1097/01.aids.0000279706.24428.78
45. Tran DA, Wilson DP, Shakeshaft A, Ngo AD, Doran C, Zhang L. Determinants of virological failure after 1 year’s antiretroviral therapy in Vietnamese people with HIV: findings from a retrospective cohort of 13 outpatient clinics in six provinces. Sex Transm Infect. 2014;90(7):538–544. doi:10.1136/sextrans-2013-051353
46. Bayu B, Tariku A, Bulti AB, Habitu YA, Derso T, Teshome DF. Determinants of virological failure among patients on highly active antiretroviral therapy in University of Gondar Referral Hospital, Northwest Ethiopia: a case-control study. HIV/AIDS (Auckland, Nz). 2017;9:153–159.
47. Ahmed M, Merga H, Jarso H. Predictors of virological treatment failure among adult HIV patients on first-line antiretroviral therapy in Woldia and Dessie hospitals, Northeast Ethiopia: a case-control study. BMC Infect Dis. 2019;19(1):305. doi:10.1186/s12879-019-3924-4
48. Sadashiv MS, Rupali P, Manesh A, et al. Risk factors of clinical and immunological failure in South Indian cohort on generic antiretroviral therapy. J Assoc Physicians India. 2017;65(12):34–39.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
