Back to Journals » International Journal of General Medicine » Volume 13
Prevalence and Predictors of Thyroid Dysfunction Among Type 2 Diabetic Patients: A Case–Control Study
Authors Khassawneh AH, Al-Mistarehi AH , Zein Alaabdin AM, Khasawneh L , AlQuran TM , Kheirallah KA , Saadeh NA , Beni yonis O , Shawkat M, Obeidat N
Received 25 July 2020
Accepted for publication 14 September 2020
Published 12 October 2020 Volume 2020:13 Pages 803—816
DOI https://doi.org/10.2147/IJGM.S273900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Adi H Khassawneh,1 Abdel-Hameed Al-Mistarehi,1 Anas M Zein Alaabdin,1 Laith Khasawneh,2 Thekraiat M AlQuran,1 Khalid A Kheirallah,1 Nesreen A Saadeh,3 Othman Beni yonis,1 Mohamid Shawkat,1 Nail Obeidat4
1Department of Public Health and Family Medicine, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Surgery, Faculty of Medicine, The Hashemite University, Zarqa, Jordan; 3Department of Internal Medicine, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 4Department of Obstetrics and Gynecology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
Correspondence: Adi H Khassawneh Department of Public Health and Family Medicine, Faculty of Medicine
Jordan University of Science and Technology, P.O.Box 3030, Irbid 21110, Jordan
Tel +962799199565
Email [email protected]
Background: Type 2 diabetes mellitus (T2DM) and thyroid disorders are common endocrine disorders. This case–control study aims to determine the prevalence and predictors of thyroid disorders in T2DM patients.
Methods: A total of 998 T2DM patients attending a tertiary hospital were included and underwent investigations for thyroid function: thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3); and glycated hemoglobin (HbA1c). They were compared with 343 non-diabetic subjects as controls.
Results: A total of 1341 participants were included in the study. The mean age ± SD was 60.14 ± 12.21, and 47.9% were females. Among T2DM patients, 140 (14%) were known to have thyroid disorders; and as a direct result of screening, 126 (12.6%) new cases of thyroid disorder were diagnosed. Thus, the overall prevalence of thyroid disorders was found to be 26.7% in T2DM patients which significantly higher than the controls (13.7%), (p˂0.001). Subclinical hypothyroidism was the most common one. Using logistic regression, after adjusting for age, gender, obesity, smoking, anemia, presence of goiter, disease duration, and poorly controlled, the risk factors for thyroid dysfunction among T2DM patients were an age of ≥ 50 years with an adjusted OR of 3.895 (95% CI 2.151– 7.052, p< 0.001); female gender (OR 1.757, 95% CI 1.123– 2.747, p=0.013); goiter (OR 2.904, 95% CI 1.118– 7.547, p=0.029), and HbA1c> 7% (OR 2.553, 95% CI 1.472– 4.429, p=0.001). However, there were no significant associations between thyroid disorders and complications or duration of diabetes (p> 0.050).
Conclusion: A high prevalence of thyroid disorders was reported in T2DM patients. Therefore, we suggest that diabetic patients should be routinely screened for thyroid dysfunction. Old age, female gender, goiter, and poorly controlled diabetes found to be risk factors for thyroid dysfunction among T2DM patients. Consequently, appropriate management and control of diabetes may lower the risk of thyroid dysfunction and vice versa.
Keywords: type 2 diabetes mellitus, DM, thyroid dysfunction, thyroid disorders, hypothyroidism, hyperthyroidism, poorly controlled diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most common chronic endocrine disorders and constitutes a major growing public health problem particularly in developing countries.1 A previous study has found that the prevalence of diabetes was up to 17.1% in Jordan and is increasing.2 On the other hand, thyroid dysfunction is commonly reported in the general population. Many studies have shown that the prevalence of hypothyroidism range between 0.2% and 4.8% whereas of hyperthyroidism between 0.5% and 3.0%.3–5 The association between diabetes and thyroid dysfunction has been known for decades.6,7 Most of the researchers have studied the prevalence of thyroid disease among type 1 diabetic patients, due to the autoimmune nature of this disease.8–10
On the contrary, the association between type 2 diabetes and thyroid disorders has been understudying. Some studies have shown that the prevalence of thyroid dysfunction is higher in patients with type 2 diabetes than in the general population where exact prevalence varies among studies ranging between (5.4–31.4%).11–14
This case–control study aims to determine the prevalence and incidence of thyroid disorders in T2DM patients attending a tertiary referral hospital in the north of Jordan. Besides, evaluating the predictors of thyroid dysfunction in this such population.
Methods
Participants, Study Design, and Data Collection
Jordan is a Middle Eastern country with a population of 11 million. This single-center, case–control study was conducted in King Abdullah University Hospital (KAUH), Jordan between the period from March 2017 to March 2019. KAUH is a tertiary educational hospital that serves a population exceeding 2 million in the North of Jordan. In our hospital, we follow the American Diabetes Association (ADA) criteria for the diagnosis of diabetes which includes: a fasting plasma glucose (FPG) level of 126 mg/dL or higher, or a 2-hour plasma glucose level of 200 mg/dL or higher during a 75-g oral glucose tolerance test, or a random plasma glucose of 200 mg/dL or higher in the presence of classic symptoms of hyperglycemia or hyperglycemic crisis, or a glycated hemoglobin (HbA1c) level of 6.5% or higher.15 Patients were eligible to be included in the study if they were Jordanians and diagnosed with T2DM based on archived records of a) having the disease for at least 12 months, b) having at least two HbA1c readings ≥6.5, c) diagnosed with an age >25 years, d) without insulin use in the first year after diagnosis, e) without a history of ketosis or ketonuria, and f) C-peptide was normal to high. These strict inclusion criteria were conducted to ensure T2DM diagnosis, and rule out type 1 diabetes and latent autoimmune diabetes in adults (LADA). Patients diagnosed with type 1 diabetes mellitus (T1DM), or LADA or those who refused to participate were excluded from the study.
Out of 5735 diabetic patients attending KAUH, Jordan between the period from March 2017 to March 2019, 998 (17.4%) T2DM patients were included in the study as a case group (Figure 1). On the other hand, a total of 343 subjects were included as an age- and gender-matched control group. This group was: a) neither diabetic nor impaired glucose tolerant as assessed by the history of normal HbA1c readings <5.7 or/and FPG levels of 99 mg/dL or lower, and having at least two documented readings; b) no history of taking anti-diabetic drugs; c) no history of any disease or medication that may affect the thyroid function. The control group was collected from the same hospital.
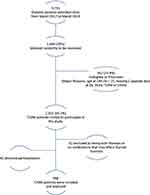 |
Figure 1 Type 2 diabetes mellitus patients flow chart. |
For both groups, venous blood samples were obtained from each patient to assay thyroid function: thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3), also, to measure HbA1c in T2DM patients. Serum TSH, T4, and T3 were measured by using the enzyme-linked immunosorbent assay (ELISA) method. HbA1c was assayed by using the turbidimetric inhibition immunoassay (TINIA) method. Demographic data, medical history, underlying comorbidities, medications, and laboratory findings were extracted from the electronic medical records. The normal range of values for TSH was 0.27–4.64 mIU/L, free T4 was 12–22 pmol/L, and free T3 was 3.1–6.8 pmol/L. Based on these reference ranges, overt hyperthyroidism was diagnosed when TSH< 0.27 mIU/L and free T4 >22 pmol/L and/or free T3>6.8 pmol/L. Overt hypothyroidism when TSH>4.64 mIU/L and free T4<12 pmol/L and/or free T3<3.1 pmol/L. Subclinical hyperthyroidism when TSH<0.27 mIU/L with normal free T4 and free T3. Subclinical hypothyroidism when TSH>4.64 mIU/L with normal free T4 and free T3. The subject that was found to have any of these thyroid function abnormalities or already diagnosed with thyroid disorder based on the medical records, was considered as a patient with thyroid dysfunction. Serum thyroid peroxidase antibodies (anti-TPO) (normal range <10U/L) was also assayed by ELISA. Among T2DM patients, the goal HbA1c was determined to be 7% according to ADA.16 Thus, HbA1c >7% was defined as poorly controlled Diabetes Mellitus. Also, the albumin to creatinine ratio in an early morning spot urine sample that equals 3–29 mg/mmol was defined as microalbuminuria and ≥30 mg/mmol as macroalbuminuria.17 Anemia was typically defined as a hemoglobin (Hb) level of less than 13.5 g/dl in males and as Hb of less than 12.0 g/dl in females.
Informed consent was obtained from the recruited participants. This study was approved by the institutional review board (IRB), and the research and ethics committee at Jordan University of Science and Technology and KAUH with an identified number (50/134/2020).
Statistical Analysis
The characteristics of participants were described using frequency and percentage for categorical variables and mean ± standard deviation for continuous variables. A chi-square test or Fisher’s exact test was used to assess the association between categorical variables, whereas continuous variables were analyzed by the Student’s t-test or ANOVA. These tests were used to figure out the differences between the T2DM patients’ group and the non-diabetic subjects’ group. To investigate factors associated with thyroid dysfunction among T2DM patients, chi-square test or Fisher’s exact test, and Student’s t-test or ANOVA were conducted to compare differences between groups with and without thyroid dysfunction among T2DM patients. A binary logistic regression model was used to assess the risk factors for the development of thyroid dysfunction among T2DM patients after adjusting for age, gender, obesity, smoking, anemia, goiter, T2DM disease duration, and glycemic control. Odds ratio (OR) and their 95% confidence intervals (95% CI) were reported. A p-value of less than 0.05 was considered statistically significant. The IBM Statistical Package for Social Sciences Software (SPSS) for Windows, version 25.0 was used for data processing and analysis.
Results
A total of 1341 participants were included in the study. The mean age ± SD was 60.14 ± 12.21 and ranged from 30 to 93 years old, 642 (47.9%) were females. The T2DM patients’ group consisted of 998 (74.4%) patients with a mean age ± SD was 60.13 ± 11.80 and ranged from 30 to 92 years old with 465 (46.6%) were females. T2DM patients were selected randomly, and following inclusion criteria, out of a total of 5735 diabetic patients with a mean age ± SD was 49.41 ± 17.41 and ranged from 2 to 100 years old with 2822 (49.2%) were females. On the other hand, the age- and gender-matched non-diabetics’ group consisted of 343 (25.6%) subjects with a mean age ± SD was 60.15 ± 13.34 and ranged from 30 to 91 years old with 177 (51.6%) were females. The BMI of T2DM patients (31.14 ± 6.67 kg/m2) was significantly higher compared with that of control subjects (28.57 ± 5.47 kg/m2) (p˂0.001). About one-quarter of participants were ex- or current smokers with higher prevalence (9.5%; 16.5%, respectively) among T2DM patients in comparison to control subjects (4.7%; 14.6%, respectively), (p=0.009). Comorbidities such as hypertension, dyslipidemia, ischemic heart disease, anemia, chronic kidney disease, and stroke were more prevalent in T2DM patients compared to non-diabetic subjects (p<0.050). Diabetic nephropathy and retinopathy were the most common comorbidities and complications in T2DM patients in this study. Serum creatinine and blood urea nitrogen were significantly higher in T2DM patients as compared to non-diabetic subjects (p=0.001). On the other hand, hemoglobin, and mean cell volume were significantly lower among T2DM patients as compared to non-diabetic subjects (p<0.001). Table 1 summarizes the demographic features, comorbidities, medications, clinical characteristics, and laboratory findings of the whole cohort.
 |
Table 1 Demographics, Clinical Characteristics, and Laboratory Findings of Cohort and the Differences Between the Two Groups |
Among the T2DM patients’ group, 140 (14%) T2DM patients had a previous thyroid disease; hypothyroidism was identified in 125 patients: 76 (60.8%) with idiopathic hypothyroidism, 26 (20.8%) with Hashimoto thyroiditis, 18 (14.4%) post-thyroidectomy, four with panhypopituitarism, and one with Riedel’s thyroiditis. Ten T2DM patients have hyperthyroidism, most of them (six patients) were on Thioamide treatment, two of them were diagnosed as Grave’s disease, three were still having active disease post-RAI treatment, and five with idiopathic hyperthyroidism. Five euthyroid T2DM patients: 3 had thyroidectomy for large goiter, and two post-RAI treatment. The prevalence of established thyroid disease was higher in females comprised of 105 (75%) patients than in males with 35 (25%) patients (p<0.001). One hundred and twenty-six (12.6%) new cases of thyroid disease were diagnosed in the studied T2DM patients: subclinical hypothyroidism was diagnosed in 76 (7.6%) patients, overt hypothyroidism in 19 (1.9%) patients, subclinical hyperthyroidism in 20 (2%) patients, and overt hyperthyroidism in 11 (1.1%) patients. The overall prevalence of already known and newly discovered thyroid disorders among T2DM patients was 266 (26.7%) with a higher prevalence in females consisted of 166 (62.4%) than in males with 100 (37.6%), (p<0.001).
In the age- and gender-matched control group, thyroid disease was already known in 35 (10.2%) subjects, with hypothyroidism was the most common one (31 subjects, 9%), followed by 2 subjects (0.6%) had hyperthyroidism, and two (0.6%) euthyroid subjects; one post-thyroidectomy for large goiter, and one post-RAI treatment. In such a group, a new diagnosis of thyroid disease was made in 12 subjects, with a prevalence of 3.5%; subclinical hypothyroidism was discovered in 7 (2%) subjects, subclinical hyperthyroidism in 4 (1.2%), and overt hyperthyroidism in one (0.3%). No subjects were newly diagnosed with overt hypothyroidism. Among the control group, females were found to have a higher prevalence of thyroid disorders consisted of 33 (70.2%) than males 14 (29.8%), (p=0.006).
The univariate logistic analysis showed that thyroid dysfunction was significantly more prevalent in the T2DM patients’ group composed of 266 (26.7%) compared to 47 (13.7%) among non-diabetic subjects with unadjusted OR of 2.289 (95% CI = 1.632–3.210, p<0.001). There was a lower serum-free T3 level and a higher level of serum-free T4 in T2DM patients as compared to the control group (p<0.050). On the other hand, the mean serum TSH levels were comparable between the two groups (p=0.119) (Table 2).
 |
Table 2 Thyroid Disorders and Function in the Whole Cohort, T2DM Patients, and Non-Diabetic Subjects |
Thyroid peroxidase antibodies (Anti-TPO) were available for 90 (9%) T2DM patients and 15 (4.4%) subjects in the control group. Of the 90 T2DM patients, 64 (71.2%) had thyroid dysfunction, and anti-TPO was positive in 31 (34.4%); 16 had subclinical hypothyroidism, 13 had overt hypothyroidism, and 2 had overt hyperthyroidism. Of the 15 subjects in the control group, 10 (66.7%) had thyroid dysfunction, and anti-TPO was positive in 8 (53.3%); 6 with subclinical hypothyroidism, and 2 with overt hypothyroidism. No subclinical hyperthyroidism cases were found in both groups with positive anti-TPO.
Table 3 shows the associations between the presence of thyroid dysfunction and different variables among T2DM patients. The majority of thyroid dysfunction patients were females (62.4%) with unadjusted OR of 2.4 (95% CI = 1.801–3.208, p<0.001). Approximately half of the T2DM patients with thyroid dysfunction (47%) found to have anemia in comparison to 37.8% anemic T2DM patients with normal thyroid function (p=0.009). 4.9% of T2DM patients with thyroid dysfunction had a presence of goiter compared with 1.9% of T2DM patients with normal thyroid function (p=0.010). A significant association between the presence of thyroid dysfunction and glycemic control is noted. Poorly controlled T2DM patients had a significantly higher prevalence of thyroid disorders (78.2%) compared with those controlled T2DM patients (21.8%), (p<0.001). There were no significant associations between the presence of thyroid dysfunction and the complications or duration of diabetes (p>0.050).
 |
Table 3 Univariate Analysis of Factors Associated with Thyroid Dysfunction in T2DM Patients |
Among the whole cohort, participants’ age was divided into four groups of ≤40 years, 41–50 years, 51–60 years, and >60 years. Prevalence of thyroid disorders was found to be significantly increased as the age increased with the lowest prevalence (6.4%) in the young age group and highest prevalence in patients older than 60 years old (49.8%), (p=0.008) (Figure 2). Among T2DM patients, the duration of diabetes was divided into 4 groups based on the quartiles as: ≤5 years, 6–10 years, 11–16 years, and ≥17 years. Diabetic patients with or without thyroid dysfunction did not differ regarding the duration of diabetes (p=0.141) (Figure 3).
 |
Figure 2 Age-specific prevalence of thyroid dysfunction in the whole cohort (p=0.008). |
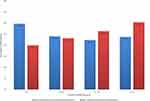 |
Figure 3 Prevalence of thyroid dysfunction according to duration of T2DM in quartiles (p=0.141). |
Using binary logistic regression while adjusting for confounding factors including: age ≥50 years, gender, obesity (BMI ≥ 30 kg/m2), active smoking, anemia, presence of goiter, disease duration, and poorly controlled diabetes mellitus among T2DM patients. The risk factors for developing thyroid dysfunction among T2DM patients were the age of 50 years or older with adjusted OR of 3.895 (95% CI 2.151–7.052, p<0.001); female gender with adjusted OR of 1.757 (95% CI 1.123–2.747, p=0.013); the presence of goiter with adjusted OR of 2.904 (95% CI 1.118–7.547, p=0.029), and poorly controlled diabetes with adjusted OR of 2.553 (95% CI 1.472–4.429, p=0.001) (Table 4).
 |
Table 4 Predictors of Thyroid Dysfunction in T2DM Patients Using Binary Logistic Regression Test |
Discussion
This case–control study shows a significantly higher prevalence of thyroid disorders among T2DM patients (26.7%) as compared to non-diabetic subjects (13.7%), with subclinical hypothyroidism was the most common one followed by overt hypothyroidism and hyperthyroidism. Besides, this study revealed that thyroid disorders were underdiagnosed in this population with 126 (12.6%) thyroid disorder cases were newly diagnosed during the conduction of this study. Old age, female gender, presence of goiter, and poorly controlled diabetes were found to be predictors of thyroid dysfunction among T2DM patients.
Previously, it has been observed that there were variations in prevalence rates of thyroid disorders among T2DM patients12,13,18–26 (Table 5). Zhu et al27 have reported a high prevalence of thyroid disorders in T2DM patients which is consistent with the findings of this study. On the contrary, Smithson12 has reported lower prevalence rates for thyroid disorders in T2DM patients. The high prevalence of thyroid disorders in this study shows that there is a need for focusing on thyroid disorders screening in this target population. However, in the present study, the prevalence of thyroid disorders among T2DM patients was higher than the occurrence rates in healthy elderly populations,28,29 and in the general population.3–5
 |
Table 5 Comparison of the Present Study Findings with Other Studies from Different Countries on Thyroid Disorders Among T2DM Patients |
It has been observed that the extent of T2DM patients who were incidentally diagnosed with thyroid disease during the conduction of this study was relatively high (12.6%) and approximate the prevalence of T2DM patients who were already known to have thyroid disorder before this study (14%). This finding was also consistent with a previous study on the Jordanian population.19 The majority of the cases were already diagnosed with thyroid disorders because of the evident symptoms of their thyroid disorders, and additional frequent medical reviews with a physician. On the contrary, the lower diagnosis extent for subclinical hypothyroidism might be because of its asymptomatic nature and with non-certain complaints, which make patients inadequate for identifying subclinical hypothyroidism with the absence of routine thyroid dysfunction screening. However, the incidence of thyroid disorders among T2DM patients during the conduction of this study was relatively high (12.6%) with subclinical hypothyroidism was the most common one (7.6%) and revealed that thyroid disorders were underdiagnosed in this population. Therefore, we recommend regular monitoring among T2DM patients for thyroid function that might be significant to enhance health consequences as well as the quality of life in elderly patients with T2DM.24,30–32
In our study, we found a lower level of serum-free T3 and a higher level of serum-free T4 in T2DM patients as compared to the control group. This finding could be attributed to the effects of long-term hyperglycemia on the peripheral conversion of T4 to T3 and on the nocturnal peak of TRH secretion in T2DM patients.33–36 Besides, insulin as an anabolic hormone, it boosts the levels of FT4 while it suppresses the levels of T3 by inhibiting the hepatic conversion of T4–T3.37 On the other hand, the serum TSH level was not significantly different in T2DM patients and non-diabetic subjects which is consistent with Uppal et al findings.38 However, elderly persons have more often elevated TSH Levels without illness value.39
T2DM patients in this study had a high mean (± SD) of HbA1c = 8.18 (±2.00). This reflects the poor glycemic status of most diabetic patients in our environment as more than half of the T2DM patients in this study did not achieve the glycemic goal, which is concordant with the pattern in earlier studies.40–42 Poor drug adherence and financial constraints may be the major contributing factors.
Among T2DM patients, anemia was associated with thyroid disorders which is concordant with the previous knowledge that thyroid disorders such as hypothyroidism and anemia can occur simultaneously as thyroid hormones are responsible for the proliferation of erythrocyte precursors both directly and via stimulating erythropoietin production and iron-deficient anemia negatively influences thyroid hormone status. Thus, different forms of anemia might develop during thyroid dysfunction, and adding iron to thyroxine therapy improves both conditions compared to thyroxine therapy alone.43–45
In the present study, the high prevalence of diabetes complications such as nephropathy and retinopathy can be attributed to the advanced age of the study participants. The average age of the enrolled T2DM patients was 60 years old with 74.5% cases having hypertension and 31.7% having hyperlipidemia. Therefore, the occurrence of diabetes complications were high. However, in the present study, there were no associations between diabetes complications and thyroid disorders. Previously, the occurrence of diabetes comorbidities has been evaluated in several studies in patients with thyroid disorders.46–49
The association between aging and thyroid dysfunction has been already known for decades.50–52 In this study, we found that the prevalence of thyroid disorders was significantly increased as the age increased independent of type 2 diabetes, and old age was a significant predictor for thyroid dysfunction among T2DM which is concordant with the findings of previous studies.18,20 This can be explained by that elderly patients might have had undetected diabetes for a longer time. However, we did not find any significant association between thyroid dysfunction and the duration of diabetes. Al-Geffari et al 201313 found that the duration of diabetes >10 year is a risk factor for developing hypothyroidism in diabetic patients, in comparison to Diez et al, 201153 and Jali et al18 that found no association between thyroid disorders and duration of diabetes which is concordant with our findings.
As shown in many other studies,13,18,19,54 our study found that T2DM patients with thyroid dysfunction had a significant predominance of females with two times were more affected by thyroid disorders than males. Our study found that poorly controlled diabetes was associated with thyroid dysfunction among T2DM patients without establishing a causality relationship, as it was a cross-sectional study, which is consistent with the findings by previous studies.14,18,55 Jain et al found that most diabetic patients who had thyroid disorder (81.25%) had HbA1c ≥7 in compared with 18.75% had HbA1c <7.55 Also, Celani et al reported a high frequency of thyroid dysfunctions in acute hospital admissions of patients with poorly controlled diabetes.14 Also, thyroid dysfunctions improved with the correction of plasma glucose levels in most of these patients.14 Bagchi et al showed that the thyroid gland secretory response to large doses of TSH is decreased with poorly controlled diabetes among T2DM patients and strict glycemic control improves the response.56 Several studies showed an inverse relationship between serum FT3 and HbA1c, and a positive relationship between TSH and HbA1c in the T2DM patients that could be attributed to the effect of prolonged hyperglycemia on the peripheral deiodination of T4 to T3.33,34,57 These studies indicate that blood glucose, particularly HbA1c, can affect thyroid hormones. Thus, strict glycemic control can improve thyroid function and even correct thyroid hormone abnormalities without the need for thyroid dysfunction treatment. Besides, multiple antidiabetic drugs can affect thyroid function and impact the hypothalamic-pituitary axis by several mechanisms.58
On the other hand, several studies suggest that thyroid hormones can affect blood glucose. A large case–control study was conducted by Ardekani et al found that HbA1c was significantly higher in T2DM patients with thyroid disorder as compared to euthyroid patients (8.9 ± 1.99 vs 7.1 ± 1.02).59 TSH may affect metabolic parameters and cause hyperglycemia by multiple mechanisms, including: stimulating leptin secretion,60,61 increasing hepatic glucose production,62 reducing insulin production and secretion from pancreatic β cells.63 In a longitudinal study conducted by Jun et al64 including a 6-year follow-up of a large sample of euthyroid subjects without diabetes (n = 17,061), to assess the association between the incidence of T2DM and the consecutive changes in serum TSH showed that the risk of incidence of T2DM was significantly increased with each 1 mIU/L serum TSH increment. Thus, the relationship between thyroid hormones and blood glucose is complex as each one can affect the other. This present study also demonstrated the presence of goiter as a risk factor for thyroid dysfunction in T2DM patients which has been well known as a risk factor in diabetic patients,53 as well as non-diabetics.65
This study had several strengths. The prospective nature of thyroid function and HbA1c tests were conducted in this study. The risk of selection bias was reduced by including a random sample of diabetic cases and compared with non-diabetic controls. The control group was included to see the viability of thyroid dysfunction in the studied population. All subjects were submitted to the same study protocol. We used a convenience sample of T2DM patients (n = 998). However, the studied T2DM patients were older than the whole cohort of diabetic patients. This could be explained by the inclusion criteria which include the age of diabetes diagnosis should be 26 years old or more in try to exclude T1DM patients. On the contrary, there were several limitations to this study. First, this study did not include individual changes over time and did not include follow-up of patients. Second, a lot of patients’ information was obtained from electronic hospital records. Thereby, potential confounders were not adjusted in this study as a family history of thyroid disorders and dietary iodine. Third, we did not measure Glutamic Acid Decarboxylase (GAD) or islet cell autoantibodies. However, we rely on the age, insulin history, and C-peptide test result at the time of diabetes diagnosis to exclude the probability of T1DM, and LADA among our patients. Fourth, some types of selection bias may have occurred because the subjects of this study were attending a tertiary referral hospital and already under medical care. Fifth, the representativeness of our sample may be limited, since this was a local, hospital-based study in one center and conducted on the Jordanian population. Hence, our findings may not be applicable to diabetic patients receiving care in the primary care community and populations other than Jordanian. Sixth, the average age of the enrolled patients was 60.14 which may cause oversampling of older people.
Conclusion
A high prevalence of thyroid disorders was reported in patients with type 2 diabetes. Therefore, it was concluded that screening should be routinely performed for thyroid disorders among this such population to identify these disorders initially. This will reduce the morbidity among them and enhance their quality of life. Old age, female gender, presence of goiter, and poorly controlled diabetes found to be the risk factors for developing thyroid dysfunction among T2DM patients. Consequently, appropriate management and control of diabetes may lower the risk of developing thyroid disorders, and vice versa. Future studies with a larger sample size from different centers should be obtained for confirming these findings and more studies are needed to identify the underlying mechanism.
Data Sharing Statement
The datasets generated and analyzed during the current study are available with the corresponding author.
Compliance with Ethical Standards
All procedures performed in this study involving human participants were reviewed and ethically approved by the Institutional Review Board (IRB) of Jordan University of Science and Technology and King Abdullah University Hospital with an identified number (50/134/2020). This study was conducted following the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Funding
No Funding was received for this study.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice. 2011;94(3):311–21.
2. Ajlouni K, Khader YS, Batieha A, Ajlouni H, El-Khateeb M. An increase in prevalence of diabetes mellitus in Jordan over 10 years. J Diabetes Complications. 2008;22(5):317–324. doi:10.1016/j.jdiacomp.2007.01.004
3. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. doi:10.1210/jcem.87.2.8182
4. Aghini-Lombardi F, Antonangeli L, Martino E, et al. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. J Clin Endocrinol Metab. 1999;84(2):561–566. doi:10.1210/jcem.84.2.5508
5. Bjoro T, Holmen J, Krüger O, et al. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT). Eur J Endocrinol. 2000;143(5):639–647. doi:10.1530/eje.0.1430639
6. Hecht A, Gershberg H. Diabetes mellitus and primary hypothyroidism. Metabolism. 1968;17(2):108–113. doi:10.1016/0026-0495(68)90136-4
7. Feely J, Isles TE. Screening for thyroid dysfunction in diabetics. Br Med J. 1979;1(6179):1678. doi:10.1136/bmj.1.6179.1678
8. Abrams P, De Leeuw I, Vertommen J; Belgian Diabetes Registry. In new‐onset insulin‐dependent diabetic patients the presence of anti‐thyroid peroxidase antibodies is associated with islet cell autoimmunity and the high risk haplotype Diabetic medicine. Diabet Med. 1996;13(5):415–419. doi:10.1002/(sici)1096-9136(199605)13:5<415::aid-dia96>3.0.co;2-x
9. Franzese A, Buono P, Mascolo M, Leo AL, Valerio G. Thyroid autoimmunity starting during the course of type 1 diabetes denotes a subgroup of children with more severe diabetes. Diabetes Care. 2000;23(8):1201. doi:10.2337/diacare.23.8.1201
10. Kordonouri O, Klinghammer A, Lang EB, Grüters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care. 2002;25(8):1346–1350. doi:10.2337/diacare.25.8.1346
11. Han C, He X, Xia X, et al. Subclinical hypothyroidism and Type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0135233. doi:10.1371/journal.pone.0135233
12. Smithson MJ. Screening for thyroid dysfunction in a community population of diabetic patients. Diabet Med. 1998;15(2):148–150. doi:10.1002/(SICI)1096-9136(199802)15:2<148::AID-DIA540>3.0.CO;2-H
13. Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, Alnaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol. 2013;2013:417920. doi:10.1155/2013/417920
14. Celani MF, Bonati ME, Stucci N. Prevalence of abnormal thyrotropin concentrations measured by a sensitive assay in patients with type 2 diabetes mellitus. Diabetes Res. 1994;27(1):15–25.
15. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020 Jan;43(Suppl 1):S14-S31. doi: 10.2337/dc20-S002. PMID: 31862745.
16. Al-Azzam SI, Alomari M, Khader YS, Almahasneh FA, Muflih SM, Altawalbeh S. Effects of pioglitazone add-on to gliclazide and metformin on glycemic control in patients with type 2 diabetes. Endocr Res. 2012;37(1):7–11. doi:10.3109/07435800.2011.566238
17. Khatami Z, McIlveen DW, Nesbitt SG, Young IS. Screening for microalbuminuria by use of microproteinuria. East Mediterr Health J. 2005;11(3):358–365.
18. Jali MV, Kambar S, Jali SM, Pawar N, Nalawade P. Prevalence of thyroid dysfunction among type 2 diabetes mellitus patients. Diabetes Metab Syndr. 2017;11(Suppl 1):S105–S108. doi:10.1016/j.dsx.2016.12.017
19. Radaideh AR, Nusier MK, Amari FL, et al. Thyroid dysfunction in patients with type 2 diabetes mellitus in Jordan. Saudi Med J. 2004;25(8):1046–1050.
20. Demitrost L, Ranabir S. Thyroid dysfunction in type 2 diabetes mellitus: A retrospective study. Indian J Endocrinol Metab. 2012;16(Suppl 2):S334–S335. doi:10.4103/2230-8210.104080
21. Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Kardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res. 2010;2(2):75–78. doi:10.4021/jocmr2010.03.281w
22. Chubb SA, Davis WA, Inman Z, Davis TM. Prevalence and progression of subclinical hypothyroidism in women with type 2 diabetes: the Fremantle Diabetes Study. Clin Endocrinol (Oxf). 2005;62(4):480–486. doi:10.1111/j.1365-2265.2005.02246.x
23. Díez JJ, Iglesias P. An analysis of the relative risk for hypothyroidism in patients with Type 2 diabetes. Diabet Med. 2012;29(12):1510–1514. doi:10.1111/j.1464-5491.2012.03687.x
24. Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med. 1995;12(7):622–627. doi:10.1111/j.1464-5491.1995.tb00553.x
25. Elgazar EH, Esheba NE, Shalaby SA, Mohamed WF. Thyroid dysfunction prevalence and relation to glycemic control in patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13(4):2513–2517. doi:10.1016/j.dsx.2019.07.020
26. Palma CC, Pavesi M, Nogueira VG, et al. Prevalence of thyroid dysfunction in patients with diabetes mellitus. Diabetol Metab Syndr. 2013;5(1):58. doi:10.1186/1758-5996-5-58
27. Zhu Y, Xu F, Shen J, et al. Prevalence of thyroid dysfunction in older Chinese patients with type 2 diabetes—A multicenter cross-sectional observational study across China. PLoS One. 2019;14(5):e0216151. doi:10.1371/journal.pone.0216151
28. Runnels BL, Garry PJ, Hunt WC, Standefer JC. Thyroid function in a healthy elderly population: implications for clinical evaluation. J Gerontol. 1991;46:B39–44. doi:10.1093/geronj/46.1.B39
29. Kussmaul T, Greiser KH, Haerting J, Werdan K, Thiery J, Kratzsch J. Thyroid analytes TSH, FT3 and FT4 in serum of healthy elderly subjects as measured by the Roche modular system: do we need age and gender dependent reference levels? Clin Lab. 2014;60:1551–1559. doi:10.7754/Clin.Lab.2014.130328
30. Tirkey A, Ahirwar D, Tandia K. Original article: study of thyroid dysfunction in diabetic patients. Int J Sci Res. 2015;4:938–940.
31. Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract. 2010;64:1130–1139.
32. Tereshchenko IV, Suslina AA. Thyroid dysfunction in patients with type 2 diabetes mellitus. Ter Arkh. 2014;86:119–123.
33. Schlienger JL, Anceau A, Chabrier G, North ML, Stephan F. Effect of diabetic control on the level of circulating thyroid hormones. Diabetologia. 1982;22:486–488. doi:10.1007/BF00282596
34. Bazrafsham HR, Ramezani A, Salehu A. Thyroid dysfunction and its relation with diabetes (NIDDM). J Gorgan Univ Med Sci. 2002;2:5–11.
35. Bartalena L, Cossu E, Grasso L, et al. Relationship between nocturnal serum thyrotropin peak and metabolic control in diabetic patients. J Clin Endocrinol Metab. 1993;76(4):983–987.
36. Naeije R, Golstein J, Clumeck N, Meinhold H, Wenzel KW, Vanhaelst L. A low T3 syndrome in diabetic ketoacidosis. Clin Endocrinol (Oxf). 1978;8:467–472. doi:10.1111/j.1365-2265.1978.tb02183.x
37. Udiong CEJ, Udoh AE, Etukudoh ME. Evaluation of thyroid function in diabetes mellitus in Calabar, Nigeria. Indian J Clin Biochem. 2007;22:74–78. doi:10.1007/BF02913318
38. Uppal V, Vij C, Bedi GK, Vij A, Banerjee BD. Thyroid disorders in patients of type 2 diabetes mellitus. Indian J Clin Biochem. 2013;28(4):336–341. doi:10.1007/s12291-012-0293-9
39. Magri F, Muzzoni B, Cravello L, et al. Thyroid function in physiological aging and in centenarians: possible relationships with some nutritional markers. Metabolism. 2002;51(1):105–109. doi:10.1053/meta.2002.28968
40. Jammal H, Khader Y, Alkhatib S, Abujbara M, Alomari M, Ajlouni K. Diabetic retinopathy in patients with newly diagnosed type 2 diabetes mellitus in Jordan: prevalence and associated factors. J Diabetes. 2013;5(2):172–179. doi:10.1111/1753-0407.12015
41. Al Omari M, Khader Y, Dauod AS, et al. Glycaemic control among patients with type 2 diabetes mellitus treated in primary care setting in Jordan. Prim Care Diabetes. 2009;3(3):173–179. doi:10.1016/j.pcd.2009.08.004
42. Al Sifri S, Basiounny A, Echtay A, et al. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract. 2011;65(11):1132–1140. doi:10.1111/j.1742-1241.2011.02797.x
43. Szczepanek-Parulska E, Hernik A, Ruchała M. Anemia in thyroid diseases. Pol Arch Intern Med. 2017;127(5):352–360. doi:10.20452/pamw.3985
44. Soliman AT, De Sanctis V, Yassin M, Wagdy M, Soliman N. Chronic anemia and thyroid function. Acta Biomed. 2017;88(1):119–127. doi:10.23750/abm.v88i1.6048
45. Fein HG, Rivlin RS. Anemia in thyroid diseases. Med Clin North Am. 1975;59(5):1133–1145. doi:10.1016/s0025-7125(16)31963-0
46. Kim B-Y, Kim C-H, Jung C-H, Mok J-O, Suh K-I, Kang S-K. Association between subclinical hypothyroidism and severe diabetic retinopathy in Korean patients with type 2 diabetes. Endocr J. 2011;58:1065–1070. doi:10.1507/endocrj.EJ11-0199
47. Qi Q, Zhang Q-M, Li C-J, et al. Association of thyroid-stimulating hormone levels with microvascular complications in type 2 diabetes patients. Med Sci Monit Int Med J Exp Clin Res. 2017;23:2715–2720.
48. Seo C, Kim S, Lee M, et al. Thyroid hormone replacement reduces the risk of cardiovascular diseases in diabetic nephropathy patients with subclinical hypothyroidism. Endocr Pract. 2018;24(3):265–272. doi:10.4158/EP-2017-0017
49. Vadivelan M, Sahoo J, Bobby K, Vinod KV, Harichandra Kumar KT. Thyroid dysfunction in patients with type 2 diabetes mellitus and its association with diabetic complications. J Assoc Physicians India. 2016;64:91.
50. Rubenstein HA, Butler VP
51. Davis PJ. Ageing and endocrine function. J Clin Endocrinol Metab. 1979;8:603–619. doi:10.1016/S0300-595X(79)80033-X
52. Rae P, Farrar J, Beckett G, Toft A. Assessment of thyroid status in elderly people. BMJ. 1993;307:177–180. doi:10.1136/bmj.307.6897.177
53. Díez JJ, Sánchez P, Iglesias P. Prevalence of thyroid dysfunction in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(4):201–207. doi:10.1055/s-0031-1271691
54. Vikhe VB, Kanitkar SA, Tamakuwala KK, Gaikwad AN, Kalyan M, Agarwal RR. Thyroid dysfunction in patients with type 2 diabetes mellitus at tertiary care centre. Natl J Med Res. 2013;3(4):377–380.
55. Jain G, Marwaha TS, Khurana A, Dhoat PS. Prevalence of thyroid disorders in patients of type 2 diabetes mellitus. Int J Med Dent Sci. 2013;2(2):153–161.
56. Bagchi N, Palaniswami N, Desai H, Felicetta J, Brown TR. Decreased thyroidal response to thyrotropin in type II diabetes mellitus. Metabolism. 1988;37(7):669–671. doi:10.1016/0026-0495(88)90088-1
57. Ogbonna SU, Ezeani IU, Okafor CI, Chinenye S. Association between glycemic status and thyroid dysfunction in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2019;12:1113–1122. doi:10.2147/DMSO.S204836
58. Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. 2019;40(3):789–824. doi:10.1210/er.2018-00163
59. Ardekani MA, Rashidi M, Shojaoddiny A. Effect of thyroid dysfunction on metabolic response in type 2 diabetic patients. Iran J Diabetes Obes. 2010;2(1):20–26.
60. Duntas LH, Biondi B. The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin. Thyroid. 2013;23(6):646–653. doi:10.1089/thy.2011.0499
61. Menendez C, Baldelli R, Camiña JP, et al. TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol. 2003;176(1):7–12. doi:10.1677/joe.0.1760007
62. Tian L, Song Y, Xing M, et al. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010;52(4):1401–1409. doi:10.1002/hep.23800
63. Santini F, Marzullo P, Rotondi M, et al. Mechanisms in endocrinology: the crosstalk between thyroid gland and adipose tissue: signal integration in health and disease. Eur J Endocrinol. 2014;171(4):R137–R152. doi:10.1530/EJE-14-0067
64. Jun JE, Jin SM, Jee JH, et al. TSH increment and the risk of incident type 2 diabetes mellitus in euthyroid subjects. Endocrine. 2017;55(3):944–953. doi:10.1007/s12020-016-1221-1
65. Vanderpump MPJ, Tunbridge WMG, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. doi:10.1111/j.1365-2265.1995.tb01894.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
