Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Prevalence and Predictors of Neurocognitive Impairment in Ethiopian Population Living with HIV
Authors Salahuddin M , Manzar MD, Hassen HY , Unissa A, Abdul Hameed U, Spence DW , Pandi-Perumal SR
Received 14 July 2020
Accepted for publication 22 September 2020
Published 13 October 2020 Volume 2020:12 Pages 559—572
DOI https://doi.org/10.2147/HIV.S260831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Mohammed Salahuddin,1,2 Md Dilshad Manzar,3 Hamid Yimam Hassen,4,5 Aleem Unissa,6 Unaise Abdul Hameed,7 David Warren Spence,8 Seithikurippu R Pandi-Perumal9
1Department of Pharmacy, College of Medicine and Health Sciences, Mizan-Tepi University (Mizan Campus), Mizan, Ethiopia; 2Pharmacology Division, Department of BioMolecular Sciences, University of Mississippi, Oxford, Mississippi, USA; 3Department of Nursing, College of Applied Medical Sciences, Majmaah University, Al Majmaah 11952, Saudi Arabia; 4Department of Public Health, College of Health Sciences, Mizan Tepi University, (Mizan Campus), Mizan, Ethiopia; 5Department of Primary and Interdisciplinary Care, College of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; 6Malla Reddy College of Pharmacy, Hyderabad, Telangana, India; 7Department of Physiotherapy, Faculty of Medicine, Nursing and Health Sciences, Monash University, Australia; 8Independent Researcher, Toronto, ON M6K 2B4, Canada; 9Somnogen Canada Inc, Toronto, ON, Canada
Correspondence: Mohammed Salahuddin Department of BioMolecular Sciences
University of Mississippi, School of Pharmacy, 331 Faser Hall, P.O. Box 1848, University, MS 38677-1848 Tel +1 662-609-3011
Email [email protected]
Background: Modern antiretroviral therapy has extended the life expectancies of people living with HIV; however, the prevention and treatment of their associated neurocognitive decline have remained a challenge. Consequently, it is desirable to investigate the prevalence and predictors of neurocognitive impairment to help in targeted screening and disease prevention.
Materials and Methods: Two hundred and forty-four people living with HIV were interviewed in a study using a cross-sectional design and the International HIV Dementia Scale (IHDS). Additionally, the sociodemographic, clinical, and psychosocial characteristics of the patients were recorded. Chi-square and binary logistic regression analysis were used to determine the level of significance among the independent risk factors and probable neurocognitive impairment.
Results: The point prevalence of neurocognitive impairment was found to be 39.3%. Participants’ characteristics of being older than 40 years (AOR= 2.81 (95% CI; 1.11– 7.15)), having a history of recreational drug use (AOR= 13.67 (95% CI; 6.42– 29.13)), and being non-compliant with prescribed medications (AOR= 2.99 (95% CI; 1.01– 8.87)) were independent risk factors for neurocognitive impairment.
Conclusion: The identification of predictors, in the Ethiopian people living with HIV, may help in the targeted screening of vulnerable groups during cART follow-up visits. This may greatly help in strategizing and implementation of the prevention program, more so, because (i) HIV-associated neurocognitive impairment is an asymptomatic condition for considerable durations, and (ii) clinical trials on neurocognitive impairment therapies have been unsuccessful.
Keywords: cART, HIV, IHDS, Africa, dementia, Ethiopia, recreational drugs
Background
Human immunodeficiency virus type 1 (HIV-1) continues to be a serious public health concern with over 690,000 infected individuals in Ethiopia with approximately 20,000 new infections since 2010.1 The hot spot clusters in the latest Ethiopian demographic survey included Gambella (4.8%) and Addis Ababa (3.4%) regions.2 In recent decades, the successful development and widespread implementation of anti-retroviral therapies have seen an associated increase in the longevity of HIV-infected patients. However, this important public health achievement has presented new challenges of clinically maintaining the health and quality of life of these patients. One of the major health deficits afflicting HIV-infected individuals is the development of neurocognitive disorders, including progressive symptoms of dementia. The successful implementation of cART regimens has considerably reduced the prevalence of the most severe form of dementia, ie, HIV-associated dementia (HAD).3,4 However, milder forms of this condition, namely, mild neurocognitive disorder (MND) and asymptomatic neurocognitive impairment (ANI), are commonly prevalent and may reduce the quality of life of HIV-infected patients. These clinical manifestations have been an important concern, especially for patients belonging to special clinical categories, including those with a late diagnosis of HIV, untreated adults, pregnant women, patients with low medication adherence, and pediatric patients, associated diagnoses which make affected patients particularly vulnerable to severe forms of dementia (HAD).5,6 Some of the milder and severe forms of associated co-morbid symptomology include, but are not limited to, attention deficits, depression, mood swings, psychomotor disturbances, and HIV- associated myelopathy which is characterized by symptoms like spastic lower limbs, gait abnormalities, and dysfunctional bladder.7,8 An additional symptom is an increase in the alteration in extrapyramidal movements, which in turn is associated with major signs of neurological deterioration, including astrocytosis, microgliosis, demyelination of axons, breaks in the dendritic processes, neuro-degeneration, increased infiltration of inflammatory mediators and lymphocytes, and a chronic increase in markers of oxidative stress.9–12 Moreover, the HIV-infected individuals diagnosed with HIV-associated neurocognitive impairment remain asymptomatic for long durations.13 The neurocognitive impairment could potentially interfere with daily life functioning such as work ineptitude (Individuals possess the knowledge but fail to apply this knowledge accurately), increased driving errors (like traffic light violations which may lead to crashes, and off-road excursions) when compared to control subjects,14 and poorer adherence to treatment, which may indirectly influence the viral load and CD4 lymphocyte status. A lower CD4 count is the hallmark of a weaker immune system and compromises the ability to fight infections, thus increasing the degree of neurodegenerative insult by attacking the microglia and macrophages in the central nervous system. Additionally, HIV-infected persons frequently present with comorbidities such as Hepatitis-C co-infection, drug abuse, or prior head injuries, which can further exacerbate HIV-related effects on the brain. Thus, early diagnosis of the neurocognitive impairment symptoms is important for improved CD4 count and reduced viral load. Post cART era there has been a decrease in the prevalence of HAD but up to 40% still suffer from HAND.15,16 Additional study by Heaton et al group in 2010 revealed greater than 50% of HIV+ patients showed HAND symptomology.17 Nevertheless, it is prudent to mention that unavailability of sophisticated tools to parse the asymptomatic neurocognitive disorder (ANI) from mild neurocognitive disorder (MND) in the present population, IHDS was used as a screening tool for those individuals who are at high risk of neurocognitive impairment only. HIV is associated with a steady loss of mental functioning and physical coordination which may get worse over time and potentially impair the quality of life of already stigmatized HIV infected individuals. Moreover, HIV patients contend with additional comorbid symptoms like depression, opportunistic infections and nutritional deficiencies which may further compromise their activities of daily living.18 Thus, a timely assessment may help identify the extent of neurocognitive impairment and initiate the needed interventional therapy to reduce the viral load and ease dementia symptoms. However, no studies have investigated the prevalence of neurocognitive impairment and its associated conditions in a population of Ethiopians living with HIV in the present health facility setting which provides healthcare to Ethiopian population. Therefore, the present study sought to determine the prevalence and predictors of neurocognitive impairment using the International HIV dementia scale (IHDS). Since IHDS is not the gold standard, further follow-up of patients by robust neuropsychological test batteries before and after the intervention is recommended. To assess the prevalence and predictors of neurocognitive impairment, HIV patients were assessed in a behavioral battery of recall memory task, motor function task, and psychomotor tasks using the IHDS tool. We hypothesize that a history of recreational drug use, non-compliance to medications, and age greater than 40 years predict neurocognitive impairment in HIV patients.
Materials and Methods
Participants and Procedures
The target population was people living with HIV, who were residents of Mizan-Aman, Ethiopia. The accessible population consisted of people living with HIV who were attending the HIV/ART clinic of Mizan-Tepi University Teaching Hospital (MTUTH), Aman, Ethiopia. The study was carried out for a period over two months from February 2018 to April 2018. Out of the total of 384 patients attending the HIV/ART clinic of MTUTH during these months, 250 eligible patients initially agreed and signed an informed consent form prior to the study commencement. Inclusion criteria were patient’s age greater than 18 years and exclusion criteria were any patients who did not satisfy the inclusion criteria and were on self-reported neuropsychiatric medications. Finally, after removing the construct-level missing data, 244 samples were used for quantitative analysis (See Figure 2). Three trained psychiatric nurses from the HIV/ART clinic of MTUTH performed the structured interview. The four words used as a part of the recall memory test were translated into Amharic by a native Amharic language expert. These four words were native to the local Ethiopian community and are used commonly in the Ethiopian cultural context.
The IHDS task comprised 3 tasks that assessed memory recall, motor speed, and psychomotor speed. The first task involved a short-term memory task in which the participants were given four words to recall (dog, hat, bean, red) (translated into Amharic as wusha, kofiya, bakele, keyi) and were provided one second to say each word. The participants were then asked to remember the 4 words and told that they would be asked to recall the words again a bit later. This was followed by a motor speed task in which the patient was asked to tap the first two fingers of the non-dominant hand as quickly and as rapidly as possible. The maximum score for the motor task was 4 points, with specific performance levels being scored as follows: 4 = ≥ 15 taps in 5 seconds; 3 = 11–14 taps in 5 seconds; 2 = 7–10 taps in 5 seconds, 1 = 3–6 taps in 5 seconds; and 0 = 0–2 taps in 5 seconds with the maximum 4 points for motor speed task. Psychomotor speed was further assessed by asking the patient to perform several movements with the non-dominant hand as quickly as possible. Primarily the patients needed to clench their hand into a fist on a flat surface. They were then asked to put their hand flat on the surface with their palm down. Finally, they were asked to place their hand perpendicular to the flat surface while displaying the 5th digit. The whole task was demonstrated once to the patients who were then allowed to practice twice before starting the test. A maximum of 4 points as possible for the psychomotor task, with patient performance being scored as follows: 4 = 4 sequences in 10 seconds; 3 = 3 sequences in 10 seconds; 2 = 2 sequences in 10 seconds; 1 = 1 sequence in 10 seconds; and 0 = unable to perform the task. Finally, the patients were given the follow-up to the memory recall in which they were asked to recall the four words. For the words that were not recalled correctly the patients were prompted with a semantic clue as follows: animal (dog); a piece of clothing (hat); vegetable (bean); color (red). A maximum of 4 points as possible for the memory recall task, with the scoring as follows: one point for each word spontaneously recalled, and 0.5 points for each correct answer after prompting. The final score was a sum of the three tasks, the maximum being 12. Patients who scored 10 points or less were referred for further neuropsychological testing.
Ethics Statement
This cross-sectional study was approved by the Institutional review board of the College of Medicine and Health Sciences, Mizan-Tepi University, Ethiopia. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed written consent was obtained from all participants prior to the commencement of the study.
Measures
Sociodemographic Measures
A questionnaire was used to gather sociodemographic information regarding the participants’ age, gender, marital status, religion, ethnicity, and occupation. The questionnaire also recorded information related to substance use: this included information about participants’ habitual use of commercial and indigenous alcoholic drinks such as tej, tella, areki, shamita, borde, and korefe, as well as about habits such as tobacco smoking, consumption of caffeinated drinks, and khat chewing.19
Clinical Measures
Data regarding participants’ clinical symptoms were also recorded. These included the patients’ current CD4 count, baseline CD4 count, viral load, duration on combination antiretroviral therapy regimen (cART), side effects from cART, opportunistic infections, duration since HIV diagnosis, other neuropsychiatric diagnoses, and duration of hospital stay. The absence of routine virological monitoring and data on HIV viral loads are not available in the present setting. Nevertheless, viral load information would have been important in understanding the correlation between viral load and the symptoms.
Psychosocial Measures
Information related to psychosocial factors such as support from family, perceived stigma accruing from HIV status, and discrimination from society was collected. Further, questions about perceived memory deficits in the past month which might have interfered with daily functioning were assessed by questions such as “Do you experience frequent memory loss?”, “Do you feel you are slower when reasoning or solving problems?”, and “Do you have difficulties in paying attention?”20
The International HIV Dementia Scale (IHDS)
The International HIV dementia scale (IHDS) was used to screen for neurocognitive impairment. This tool has been validated in different African and Caucasian populations and has been shown to have a sensitivity of for HIV dementia as 88 and 80% and specificity scores for HIV dementia as 50 and 55% respectively.21,22 The tool measures three essential components of neurological impairments: these include cognition, motor, and psychomotor deficits. Each component has a maximum score of 4, with a total score of three components summing to 12.21 Hence, in the present study, any value of less than 10 is indicative of neurocognitive impairment, and patients receiving such a score were diagnosed with neurocognitive impairment and referred for further psychiatric follow-up at a higher referral hospital.21 The IHDS does not require proficiency in the English language and is ideal for measuring probable neurocognitive impairment in people living with HIV. However, one of the components of the tool that comprised of recall of words were translated into the Amharic language. Amharic translations of these four words are native to the local Ethiopian community and are used commonly (ie High-frequency words) in the Ethiopian cultural context. Though, it is plausible to think that the word length and number of syllables might play some role in recall memory because the Amharic translation of these words is slightly longer and have a slightly higher number of syllables. However, the associative retrieval mechanism facilitated by the high-frequency nature of these four words in both English and Amharic versions would have somewhat compensated the effect caused by the variation in the word lengths and number of syllables. Most of the words which have been translated are commensurate to general vocabulary use for the Ethiopian population. Other tools like Mini-Mental State Examination (MMSE), Mini-Cog, Montreal cognitive assessment (MOCA) was not used in the present study population as it lacked suitability to administer to subjects who cannot read and write. Given that socio-educational variables may have a considerable effect of on the outcome and limit the effectiveness to detect the neurocognitive impairment. Thus, IHDS has used which screen all the aspects of neurocognitive impairment namely motor, memory, and psychomotor in HIV infected population.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS), version 21 (SPSS Inc., Chicago, IL). Chi-square tests were used to determine the correlation between the independent variables and probable neurocognitive impairment status; those sociodemographic, clinical and psychosocial risk factors with a p-value of less than 0.25 were selected for the multivariate association analysis with the outcome measure. Binary logistic regression was used to assess the multivariate association between the dependent variable, ie, neurocognitive impairment status, and independent variables. Models were adjusted for age, gender, education, marital status, severity of illness (hospital stay), and stigma (indirectly related to increased anxiety and depression) towards people living with HIV. A Mann Whitney test was performed to assess the difference between the mean scores of participants in the probable neurocognitive impairment group and the non- neurocognitive impairment group.
Logistic regression was applied after verifying all its assumptions in the study data. In general, the dependent variable, namely, neurocognitive impairment status was measured as a dichotomous variable. Second, there was the independence of observations as assessed by the Durbin Watson test. No outlier was found, as assessed by Mahalanobis Distances for multivariate outliers and box plot analysis for univariate outliers. Independent variables (continuous) were linearly related to log odds as determined by the absence of significance for the interaction effect. Additionally, the study data also satisfied the minimum sample size requirement. Based on the present prevalence of 39% for neurocognitive impairment, the sample size calculation for 9 independent variables included in the model, and the expected probability of the least frequent outcome being 0.10, it was determined that a minimum sample size of (10*9/.4) =90/.4=225 was needed.
Results
Sociodemographic Characteristics of the Study Population
Table 1 presents the patients’ characteristics. Most of the patients were under 40 years of age (85%). Most of the patients (64%) were female and approximately three-fourths (69%) were married. Most of the patients (70%) reported having completed less than eight years of primary school or had no formal education. Approximately 20% of the study subjects greater than 40 years old were diagnosed to have HIV infection for more than 6 months. One-fourth of the patients admitted that they used recreational drugs (25%). Nearly 65% of the patients belonged to the low and very low-income groups. Most of the patients lived in urban locations (86%). No significant association between education level and gender groups was found; χ2(3) = 4.985, p = 0.173.
 |
Table 1 Participants’ Characteristics and Their Relationship with Neurocognitive Impairment in Mizan Tepi University Teaching Hospital (MTUTH) |
Clinical Characteristics of the Study Population
Table 2 shows the clinical characteristics of the study participants. Approximately 96% (235, 96.4%) of patients had been diagnosed with HIV for greater than 6 months of which 94 (40%) showed symptoms of neurocognitive impairment. Per the WHO recommended criteria; HIV patients in clinical stage 4 may contend with HIV encephalopathy which could potentially lead to signs of neurocognitive impairment.23,24 In the present study, approximately 44% of the HIV diagnosed patients had a current CD4 count <500 cells/mm3. Most of the patients reported that they did not adhere to their prescribed medication regimen (88%). About one-third of the patients were diagnosed with opportunistic infections at the time of participation in the study. None of the clinical characteristics showed any significant statistical association with neurocognitive impairment in the bivariate analysis. Chronic co-morbidities reported in the present study were used as predictors to assess the burden of opportunistic infections on neurocognitive dysfunction. We also found 45.2% of study subjects to have viral load “below the undetectable limit” and all these patients were started on antiretroviral therapy and prescribed different anti-retroviral drug regimens comprising of (TDF/3TC/EFV or ABC/3TC/EFV or AZT/3TC/EFV) and approximately 97% of the patients showed fair to good medication adherence.
 |
Table 2 Clinical Characteristics and Their Relationship with Neurocognitive Impairment in Mizan Tepi University Teaching Hospital (MTUTH) |
Psychosocial Characteristics of the Study Population
Table 3 shows the psychosocial characteristics of the patients enrolled in the study. Most of the patients (87%) reported no social support for medical adherence from their family or friends. Medication adherence data was determined as good, fair, and poor from the patient charts. Most (about three fourths) of the participants reported that they did not experience any stigma and discrimination from society.
 |
Table 3 Psychosocial Characteristics and Their Relationship with Neurocognitive Impairment in Mizan Tepi University Teaching Hospital (MTUTH) |
Bivariate Associations: Neurocognitive Impairment Status with Sociodemographic, Clinical and Psychosocial Covariates
Age was significantly associated with neurocognitive impairment, ie, participants aged 40 years of age or older were more likely than younger participants to show neurocognitive symptoms [χ2(1) = 2.635, p<0.05; Table 1]. Recreational drug use also significantly predicted neurocognitive impairment [χ2(1) = 57.245, p<0.05; Table 1].
Differences in Neurocognitive Impairment Scores Between Neurocognitive impaired and Neurocognitive stable Groups
The prevalence of neurocognitive impairment identified by IHDS was 39.34% with the IHDS total score of 9.57±1.57. Independent mean IHDS recall score was revealed as 3.36±0.80, motor score as 3.32±0.66, and psychomotor score as 2.90±0.72 (Table 4). The difference between those who had a neurocognitive impairment and those who did not was evident in all the three components of IHDS, ie, recall [U = 2732, p = p<0.001], motor performance [U = 2704.500, p<0.001] and psychomotor abilities [U = 1930.500, p<0.001; see Figure 1].
 |
Table 4 IHDS Scores and Their Relationship with Neurocognitive Impairment in Mizan-Tepi University Teaching Hospital (MTUTH) |
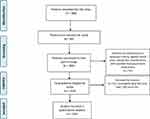 |
Figure 2 Flow chart of the sampling procedure and sample size calculation. |
Factors Associated with the Neurocognitive Impairment: A Multivariate Analysis
Binary logistic regression was performed to measure the effects of age, gender, current CD4 count, medication adherence status, stigma, marital status, hospital stay, recreational drug use, and education on the neurocognitive impairment status. Gender, education, marital status, current CD4 count and hospital stay were included in the model based on previous studies showing them to be consistent predictors for neurocognitive impairment.25–32 The logistic regression model was statistically significant, χ2 (9) = 72.91. The model explained 35.0% (Nagelkerke R2) of the variance in neurocognitive impairment status and correctly classified 75.8% of cases. Participants who were older than 40 years of age were 2.8 times more likely than younger subjects to exhibit neurocognitive impairment (p<0.05, Table 5). Those who admitted to that they did not adhere to their medication regimens were 2.9 times more likely to have symptoms of neurocognitive impairment (p<0.05, Table 5). Study subjects using recreational drugs showed 13.6 times higher odds to have symptoms of neurocognitive impairment than non-recreational drug users (p<0.001, Table 5)
 |
Table 5 Multivariate Logistic Regression Analysis: Association Between Neurocognitive Impairment in HIV Positive Patients in Mizan Tepi University Teaching Hospital (MTUTH) |
Discussion
The present study determined the prevalence and identified predictors for neurocognitive impairment in Ethiopians living with HIV.IHDS and the sociodemographic tool was used to assess the level of neurocognitive impairment and associated factors in the people living with HIV. The present findings confirmed the hypothesis that age greater than 40 years, having a history of recreational drug use, being non-compliant with prescribed medications were independent risk factors for neurocognitive impairment. The present study may lend support to the notion that timely identification of predictors in Ethiopians living with HIV may help in the targeted screening of vulnerable groups during cART follow-up visits and tailor the drug regimens specifically to individual patients. This may greatly help in strategizing and implementation of the prevention program, more so, because: (i) HIV-associated neurocognitive impairment is an asymptomatic condition for considerable durations, and (ii) clinical trials of neurocognitive impairment therapies have been unsuccessful.13
The point prevalence of neurocognitive impairment in this study was 39%. The present prevalence findings were similar to those of a study from Canada (39.4%)33 but higher when compared to other studies from Ethiopia (33.3%, 67.1%),28,34 sub-Saharan Africa (30.9%).35 However, the present findings were less than some of the studies conducted in Nigeria (54.3%, 2012; 66.2%, 2013),36,37 Cameroon (85%),38 Asia (85%)39 and Uganda (64.4%, 2013).40
The majority of the present study sample (96%) had been diagnosed as having had HIV for more than 6 months and thus most of the patients (99%) were already on the cART regimen. Despite being treated with cART medication, some of the patients still showed symptoms of neurocognitive impairment. The possible explanation for this would be that many patients reported having poor medication adherence (88%) or inability of cART to achieve optimum therapeutic concentration in the CNS due to its poor penetration beyond the blood-brain barrier.41
Similar to previous studies, being older than 40 years was found to have a positive association with neurocognitive impairment status in the people living with HIV.42–46 Generally, neurocognitive performance deteriorates with increasing age, and some studies have shown that being in the 40 plus age group is a significant predictor of neurocognitive impairment. One study in Uganda did not show any association between advanced age and neurocognitive impairment.40 This might be because of some moderator variables in their study40 because consistency in the association is suggested by multiple reports.42–46
Recreational drug use is a strong predictor of neurocognitive impairment in the present study. The present study assessed self-reported use of recreational drugs such as khat, alcohol, cigarette, coffee, and other caffeinated drinks in people living with HIV. Drinking indigenous varieties of alcohol and khat chewing is among the most prevalent social habits in Ethiopia.47 There has been a growing body of evidence which points towards neurocognitive decline due to drug misuse.48–50 Social drug misuse frequently involves drug takers in various risk-taking behaviors.51 Some studies have shown that low to moderate alcohol consumption may facilitate cognition;52,53 others have shown that it impairs cognition54 or produces no change.55 Another recreational drug called Khat is abused widely and has been associated with short-term memory deficits and loss of cognitive flexibility in adults.56–58 Additionally, the deleterious effects of khat on neurocognitive performance have been similarly demonstrated in people living with HIV.59 Nevertheless, the association between khat and cognition is complex. Khat has a biphasic response on cognition, wherein low doses may enhance cognition until reaching a peak, at which progressively higher doses begin to produce adverse cognitive effects, and, additionally, are associated with classic signs of addiction similar to psychostimulants.60 Other lines of evidence have tended to suggest the presence of an adverse additive effect, in as much as, drug users without HIV are more vulnerable to neurocognitive impairment than patients who are HIV positive.61–64 It’s worth mentioning that since Khat is a recreational drug being extensively used in the present Ethiopian community setting and clinical and preclinical evidences show that it produces spatial memory, working memory and cognitive flexibility deficits.56 Delineating khat mediated neurocognitive effects from other recreational drugs like coffee, tobacco, alcohol and soft beverages may need a longitudinal follow-up of the HIV+ patients who abused Khat and was not accounted for in the present study. Hence future studies should explore individual recreational drugs on the neurocognitive functioning in HIV populations. Furthermore, recreational drug use may produce synergistic interaction with HIV proteins and in people living with HIV may need future longitudinal to follow up trials and experiments for its confirmation.65,66 Nevertheless, this is consistent to a study in the preclinical population which demonstrated the combined exposure of HIV-1 Tat protein and clinical opioid namely Oxycodone to promote the psychomotor behavioral effects, thus making the drug more rewarding, with the initially perceived potency of the drug being an important predictor of whether the patient would enter into the addiction cycle.67,68
The study sought to identify which clinical factors might be most important for predicting neurocognitive impairment. The candidate predictors included the current CD4 count, duration of time since HIV diagnosed, presence of opportunistic infections, and WHO clinical staging. These factors, however, did not show any significant association with neurocognitive impairment status in our study. Inconsistent results have been reported between different clinical correlates of neurocognitive impairment.69,70 One of the possible associations, ie, between self-reported lapses in medication adherence and neurocognitive impairment, was of interest inasmuch as patients frequently reported that being on multiple drug regimens made it particularly burdensome for them to reliably take their medicines.71 However, this potential association failed to reach statistical significance. The study subjects positive to have neurocognitive impairment should further be confirmed by more robust neurocognitive neuropsychological test batteries, computerized testing, Mini-Mental State Examination (MMSE), and grooved pegboard action fluency.72
There was an apparent contradiction in the findings of bivariate and multivariate analysis with regards to the association of medication adherence with neurocognitive impairment. Poor medication adherence did not show a significant relationship with neurocognitive impairment in the bivariate analysis, but it did have a significant positive association in the multivariate analysis. This is most plausibly explained by statistical consideration that some of the covariates in the multivariate analysis in this study might have mediated the relationship.73
The limitations of assessing the cellular and molecular changes in the brain morphology in the clinical population make it a pressing need for identifying these aspects in the preclinical population for further understanding the interactions of combined expression of the HIV+ proteins and other factors which predict cognitive impairment.
Limitations of the Study
The study did not include any psychosocial correlates such as depression, stress, and anxiety, which could potentially influence the outcome variable. The study also could not include HIV negative control subjects to compare with the HIV infected population as well as patients belonging to special clinical categories, those with a late diagnosis of HIV, untreated adults, and pediatric patients, associated diagnoses that make these HIV patients particularly vulnerable to severe forms of dementia (HAD).5,6 The study also failed to account for the individual effects of the recreational drugs but reported combined use of khat, coffee, tobacco, and alcohol on neurocognitive functions. Hence future studies could explore this aspect in the HIV+ infected population.
Moreover, the IHDS tool comes with limitations. Previous studies signify the use of IHDS to be suboptimal in screening neurocognitive impairment among HIV+ individuals.74 However, in the present limited resource setting where full neuropsychological testing was not possible, IHDS was used as a screening tool for those individuals who are at high risk of neurocognitive impairment. Any patients showing probable neurocognitive symptoms would then be referred to a neurological facility for further diagnosis. However, IHDS is not the gold standard, and further follow-up of patients by robust neuropsychological test batteries who report memory issues is needed. Moreover, future studies may need to explore the psychometric properties of the IHDS scale to determine the sensitivity and specificity scores in the Ethiopian demographics. Moreover, future assessments of the sensitivity and specificity for recall, motor, and psychomotor which are the independent components of the IHDS tool is the need of the hour.
Conclusion
This study showed the point prevalence of probable HIV associated neurocognitive disorder in an Ethiopian population living with HIV as 39.3%. The use of recreational drugs, poor medication adherence, and 40 years of age or older were significant predictors of neurocognitive impairment in people living with HIV. Additional strategies to curb this epidemic would emphasize early screening for the diagnosis of neurocognitive impairment in the resource-poor sections of the Ethiopian community. This would further help to direct the targeted patients for further follow-up in higher psychiatric referral hospitals for assessment and management, given the positive association between people living with HIV and neurocognitive impairment. Furthermore, awareness campaigns about the deleterious effects of khat chewing and drinking excess alcohol on the functional effects of the brain need to be investigated.
Abbreviations
ABC, abacavir; TDF, tenofovir; 3TC, lamivudine; AZT, zidovudine; EFV, efavirenz; IHDS, International HIV Dementia Scale; cART, combinative anti-retroviral therapy; MMSE, Mini-Mental State Examination; MOCA, Mini-Cog, Montreal cognitive assessment.
Data Sharing Statement
The de-identified dataset used and/or analyzed during the current study is available as a supplementary file.
Consent for Publication
Not applicable.
Acknowledgments
We are grateful to the participants of the study and Mizan-Tepi University. The authors extend their appreciation to the Deanship of Scientific Research at Majmaah University for funding this work under Project Number No (RGP-2019-40).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Role of the Sponsor
Mizan Tepi University, and Majmaah University had no role in the design and conduct of the study: collection; management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Funding
The Deanship of Scientific Research at Majmaah University funded this work under Project Number No (RGP-2019-40). There was no formal research funding received, however, later on, one of the authors received funds from through a publication support program. The funder provided support in the form of salaries for authors to cover the cost of statistical software, consultation with statistical experts, access to subscribed referencing software, access to subscribed scientific literature, and access to subscribed English editing services and software, but did not have any additional role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
Disclosure
The authors have read the journal’s policy and have the following potential conflicts: This study was not an industry-supported study. S.R. Pandi-Perumal is a stockholder and the President and Chief Executive Officer of Somnogen Canada Inc., a Canadian Corporation. This does not alter his adherence to all the journal policies. Pandi-Perumal has edited several academic volumes for which he receives occasional annual royalties. He declares that he has no competing interests that might be perceived to influence the content of this article.
Other remaining authors declare that they have no proprietary, financial, professional, nor any other personal interest of any nature or kind in any product or services and/or company that could be construed or considered to be a potential conflict of interest that might have influenced the views expressed in this manuscript.
The views expressed in this article are those of the authors and do not necessarily represent the official views of their affiliated institutions.
References
1. UNAIDS, [Internet]. Ethiopia; 2018. Available from: https://www.unaids.org/en/regionscountries/countries/ethiopia.
2. EDHS. HIV Prevalence report in Ethiopia data from the Ethiopia demographic and health survey; 2016. Available from: https://dhsprogram.com/pubs/pdf/FR328/FR328.HIV.pdf.
3. Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry. 2000;69(3):376–380. doi:10.1136/jnnp.69.3.376
4. Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8(2):136–142. doi:10.1080/13550280290049615
5. McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–2252. doi:10.1212/wnl.43.11.2245
6. Zhang YL, Ouyang YB, Liu LG, Chen DX. Blood-brain barrier and neuro-AIDS. Eur Rev Med Pharmacol Sci. 2015;19(24):4927–4939.
7. Kopstein M, Mohlman DJ. HIV-1 encephalopathy and aids dementia complex. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020 June 30.
8. Petito CK, Navia BA, Cho E-S, Jordan BD, George DC, Price RW. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;312(14):874–879. doi:10.1056/NEJM198504043121402
9. Shapshaka P, Kangueane P, Fujimurae RK, et al. Editorial NeuroAIDS review. spAIDS. 2011;25(2):123–141.
10. Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204(5):753–760. doi:10.1093/infdis/jir387
11. Klunder AD, Chiang MC, Dutton RA, et al. Mapping cerebellar degeneration in HIV/AIDS. Neuroreport. 2008;19(17):1655–1659. doi:10.1097/WNR.0b013e328311d374
12. Cole MA, Castellon SA, Perkins AC, et al. Relationship between psychiatric status and frontal–subcortical systems in HIV-infected individuals. J Int Neuropsychol Soc. 2007;13(3):549–554.
13. Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder - Pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–248.
14. Cheng S, Klein H, Bartsch DU, Kozak I, Marcotte TD, Freeman WR. Relationship between retinal nerve fiber layer thickness and driving ability in patients with human immunodeficiency virus infection. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1643–1647. doi:10.1007/s00417-011-1735-4
15. Nabha L, Duong L, Timpone J. HIV-associated neurocognitive disorders: perspective on management strategies. Drugs. 2013;73(9):893–905. doi:10.1007/s40265-013-0059-6
16. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi:10.1212/01.WNL.0000287431.88658.8b
17. Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: charter Study. Neurology. 2010;75(23):2087–2096.
18. McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157(12):3–10. doi:10.1016/j.jneuroim.2004.08.042
19. Manzar MD, Salahuddin M, Sony P, et al. Sleep disturbances and memory impairment among pregnant women consuming khat: an under‑recognized problem. Ann Thorac Med. 2017;12(4):247–251. doi:10.4103/atm.ATM_24_17
20. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi:10.1097/QAD.0b013e3283354a7b
21. Sacktor NC, Wong M, Nakasujja N, et al. The International HIV Dementia Scale: A new rapid screening test for HIV dementia. Aids. 2005;19(13):1367–1374.
22. Singh D, Sunpath H, John S, Eastham LGR. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts- a preliminary report. Afr J Psychiatry. 2008;11(4):282–286.
23. World Health Organization. Interim WHO clinical staging of HVI/AIDS and HIV/AIDS case definitions for surveillance: african Region; 2005. Available from: https://apps.who.int/iris/handle/10665/69058.
24. Kumar S, Himanshu D, Tandon R, Atam V, Sawlani KK, Verma SK. Prevalence of hiv associated neurocognitive disorder using modified mini mental state examination and its correlation with CD4 counts and anti-retroviral therapy. J Assoc Physicians India. 2019;67(4):47–51.
25. Bhaskaran K, Mussini C, Antinori A, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63(2):213–221. doi:10.1002/ana.21225
26. Yakasai AM, Gudaji MI, Muhammad H, et al. Prevalence and correlates of HIV-associated neurocognitive disorders (HAND) in Northwestern Nigeria. Neurol Res Int. 2015;2015.
27. Alford K, Banerjee S, Nixon E, et al. Assessment and management of HIV-associated cognitive impairment: experience from a multidisciplinary memory service for people living with HIV. Brain Sci. 2019;9(2):1–13.
28. Animut MD, Sorrie MB, Birhanu YW, Teshale MY. High prevalence of neurocognitive disorders observed among adult people living with HIV/AIDS in Southern Ethiopia: A cross-sectional study. PLoS One. 2019;14(3):1–15. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2001727802%0Ahttp://dx.doi.10.1371/journal.pone.0204636
29. Yusuf AJ, Hassan A, Mamman AI, Muktar HM, Suleiman AM, Baiyewu O. Prevalence of HIV-Associated Neurocognitive Disorder (HAND) among Patients Attending a Tertiary Health Facility in Northern Nigeria. J Int Assoc Provid AIDS Care. 2017;16(1):48–55. doi:10.1177/2325957414553839
30. Thai TT, Jones MK, Harris LM, Heard RC. Prevalence and correlates of probable HIV-associated dementia in HIV outpatients in Ho Chi Minh City, Vietnam. J Int Assoc Provid AIDS Care. 2017;16(4):366–375. doi:10.1177/2325957417701195
31. Kinai E, Komatsu K, Sakamoto M, et al. Association of age and time of disease with HIV-associated neurocognitive disorders: a Japanese nationwide multicenter study. J Neurovirol. 2017;23(6):864–874. doi:10.1007/s13365-017-0580-6
32. Gandhi NS, Moxley RT, Creighton J, et al. Comparison of scales to evaluate the progression of HIV-associated neurocognitive disorder. HIV Ther. 2010;4(3):371–379. doi:10.2217/hiv.10.23
33. Skinner S, Adewale AJ, Deblock L, Gill MJ, Power C. Neurocognitive screening tools in HIV/AIDS: comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009;10(4):246–252.
34. Belete T, Medfu G, Yemiyamrew E. Prevalence of HIV associated neurocognitive deficit among hiv positive people in ethiopia: a cross sectional study at ayder referral hospital. Ethiop J Health Sci. 2017;27(1):67–76. doi:10.4314/ejhs.v27i1.9
35. Habib AG, Yakasai AM, Owolabi LF, et al. Neurocognitive impairment in HIV-1-infected adults in Sub-Saharan Africa: a systematic review and meta-analysis. Int J Infect Dis. 2013;17(10):e820e831. doi:10.1016/j.ijid.2013.06.011
36. Oshinaike OO, Akinbami AA, Ojo OO, Ojini IF, Okubadejo UN, Danesi AM. Comparison of the minimental state examination scale and the international hiv dementia scale in assessing cognitive function in nigerian HIV patients on antiretroviral therapy. AIDS Res Treat. 2012;2012.
37. Ogunrin. The burden of HIV-associated dementia in acquired immunodeficiency syndrome: a case-control study. J Neurol Epidemiol. 2013.
38. Atashili J, Gaynes BN, Pence BW, et al. Prevalence, characteristics and correlates of a positive-dementia screen in patients on antiretroviral therapy in Bamenda, Cameroon: A cross-sectional study. BMC Neurol. 2013:13(1); 1. doi:10.1186/1471-2377-13-86
39. Achappa B, Priyadarshni S, Madi D, et al. Neurocognitive dysfunction among HIV positive patients using International HIV dementia scale. Asian J Med Sci. 2014;5(4):61–64. doi:10.3126/ajms.v5i4.8724
40. Nakku J, Kinyanda E, Hoskins S. Prevalence and factors associated with probable HIV dementia in an African population: A cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatry. 2013;13:126. doi:10.1186/1471-244X-13-126
41. Miller G. Drug targeting. Breaking down barriers. Science. 2002;297(5584):1116–1118. doi:10.1126/science.297.5584.1116
42. Childs EA, Lyles RH, Selnes EA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52(3):607. doi:10.1212/WNL.52.3.607
43. Wong MH, Robertson K, Nakasujja N, et al. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 2007;68(5):350–355. doi:10.1212/01.wnl.0000252811.48891.6d
44. Becker JT, Lopez OL, Dew MAAH. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(1):S11–8. doi:10.1097/00002030-200401001-00003
45. Nakasujja N, Skolasky R L, Musisi S, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatry. 2010;10.
46. Tozzi V, Balestra P, Lorenzini P, et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol. 2005;11(3):265–273. doi:10.1080/13550280590952790
47. Manzar MD, Salahuddin M, Maru TT, et al. Sleep correlates of substance use in community-dwelling Ethiopian adults. Sleep Breath. 2017;21(4):1005–1011. doi:10.1007/s11325-017-1567-5
48. Juárez-Portilla C, Molina-Jiménez T, Morin J, Roldán-Roldán G, Zepeda R. Influence of drugs on cognitive function. Intech. 2016.
49. Gould TJ. Addiction and cognition. Addict Sci Clin Pract. 2010;5(2):4–14.
50. Hulse GK, Lautenschlager NT, Tait RJAO. Dementia associated with alcohol and other drug use. Int Psychogeriatr. 2005;17(Suppl 1):109–127. doi:10.1017/S1041610205001985
51. Bezinović P, Malatestinić D. Perceived exposure to substance use and risk-taking behavior in early adolescence: cross-sectional study. Croat Med J. 2009;50(2):157–164. doi:10.3325/cmj.2009.50.157
52. Sklar AL, Gilbertson R, Boissoneault J, Prather R, Nixon SJ. Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcohol Clin Exp Res. 2012;36(12):2150–2156. doi:10.1111/j.1530-0277.2012.01833.x
53. Gilbertson R, Ceballos NA, Prather R, Nixon SJ. Effects of acute alcohol consumption in older and younger adults: perceived impairment versus psychomotor performance. J Stud Alcohol Drugs. 2009;70(2):242–252. doi:10.15288/jsad.2009.70.242
54. Friedman TW, Robinson SR, Yelland GW. Impaired perceptual judgment at low blood alcohol concentrations. Alcohol. 2011;45(7):711–718doi:10.1016/j.alcohol.2010.10.007
55. Dry MJ, Burns NR, Nettelbeck T, Farquharson AL, White JM. Dose-related effects of alcohol on cognitive functioning. PLoS One. 2012;7(11):1–8. doi:10.1371/journal.pone.0050977
56. Berihu BA, Asfeha GG, Welderufael AL, Debeb YG, Zelelow YBBH. Toxic effect of khat (Catha edulis) on memory: systematic review and meta-analysis. J Neurosci Rural Pr. 2017;8(1):30–37. doi:10.4103/0976-3147.193524
57. Colzato LS, Ruiz MJ, van den Wildenberg WPM, Hommel B. Khat use is associated with impaired working memory and cognitive flexibility. PLoS One. 2011;6(6):1–6. doi:10.1371/journal.pone.0020602
58. Hoffman R, Al’Absi M. Working memory and speed of information processing in chronic khat users: preliminary findings. Eur Addict Res. 2013;19(1):1–6. doi:10.1159/000338285
59. Yideg Yitbarek G, Mossie Ayana A, Bariso Gare M, Garedew Woldeamanuel G. Prevalence of cognitive impairment and its predictors among HIV/AIDS patients on antiretroviral therapy in Jimma University Medical Center, Southwest Ethiopia. Psychiatry J. 2018;2018:1–7.
60. Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: A continuum of behavioral and cognitive activation. Pharmacol Rev. 2014;66(1):193–221. doi:10.1124/pr.112.007054
61. Chiesi A, Vella S, Dally LG, et al. Epidemiology of AIDS dementia complex in Europe. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11(1):39–44.
62. Goodkin K, Shapshak P, Metsch LR, et al. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83(1–2):88–101. doi:10.1016/S0165-5728(97)00225-7
63. Tyor WR, Middaugh LD. Do alcohol and cocaine abuse alter the course of HIV-associated dementia complex? J Leukoc Biol. 1999;65(4):475–481. doi:10.1002/jlb.65.4.475
64. Nath A, Hauser KF, Wojna V, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S6269. doi:10.1097/00126334-200210012-00006
65. Soontornniyomkij V, Kesby JP, Morgan EE, et al. Effects of HIV and methamphetamine on brain and behavior: evidence from human studies and animal models. J Neuroimmune Pharmacol. 2016;11(3):495–510. doi:10.1007/s11481-016-9699-0
66. Attonito JM, Dévieux JG, Lerner BD, Hospital MM, Rosenberg R. Exploring substance use and HIV treatment factors associated with neurocognitive impairment among people living with HIV/AIDS. Front Public Health. 2014;2:105. doi:10.3389/fpubh.2014.00105
67. Koob GF, Schulkin J. Addiction and stress: an allostatic view. Neurosci Biobehav Rev. 2019;106(September2018):245–262. doi:10.1016/j.neubiorev.2018.09.008
68. Salahuddin MF, Qrareya AN, Mahdi F, et al. Combined HIV-1 Tat and oxycodone activate the hypothalamic-pituitary-adrenal and -gonadal axes and promote psychomotor, affective, and cognitive dysfunction in female mice. Horm Behav. 2020;119:104649. doi:10.1016/j.yhbeh.2019.104649
69. Clifford DB, Ances BM. HIV-Associated Neurocognitive Disorder (HAND). Lancet Infect Dis. 2013;13(11):976–986.
70. Nightingale S, Winston A, Letendre S, et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014;13(11):1139–1151. doi:10.1016/S1474-4422(14)70137-1
71. Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010;74(15):1217–1222. doi:10.1212/WNL.0b013e3181d8c1ca
72. Bloch M, Kamminga J, Jayewardene A, et al. A screening strategy for HIV-associated neurocognitive disorders that accurately identifies patients requiring neurological review. Clin Infect Dis. 2016;63(5):687–693.
73. Baron RM, Kenny D. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Personality Soc Psychol. 1986;51(6):1173–1182. doi:10.1037/0022-3514.51.6.1173
74. Milanini B, Paul R, Bahemana E, et al. AST. Limitations of the international HIV dementia scale in the current era. AIDS. 2018;32(17):2477–2483. doi:10.1097/QAD.0000000000001968
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

