Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Prevalence and Predictors of Anemia in HIV-Infected Persons in Nepal
Authors Sah SK , Dahal P, Tamang GB , Mandal DK , Shah R, Pun SB
Received 6 January 2020
Accepted for publication 13 May 2020
Published 2 June 2020 Volume 2020:12 Pages 193—200
DOI https://doi.org/10.2147/HIV.S244618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Shiv Kumar Sah,1 Prastuti Dahal,1 Gyan Bahadur Tamang,1 Dipendra Kumar Mandal,2 Rajesh Shah,2 Sher Bahadur Pun2
1Purbanchal University, Little Buddha College of Health Science, Kathmandu, Nepal; 2Sukraraj Tropical and Infectious Disease Hospital, Teku, Kathmandu, Nepal
Correspondence: Shiv Kumar Sah
Purbanchal University, Little Buddha College of Health Science, Minbhawan, Kathmandu, Nepal
Email [email protected]
Background: Anemia is the commonest hematological complications in HIV patients, and has a significant impact on quality of life, morbidity, and mortality. However, little is known about the epidemiology of anemia in this population in a Nepalese setting. Therefore, the present study aimed at assessing the prevalence of anemia in patients living with HIV and further to determine the independent predictors associated with it.
Methods: This cross-sectional study was conducted in patients diagnosed with HIV at Sukraraj Tropical and Infectious Disease Hospital, Teku, Kathmandu from November 2016 to August 2017. Anemia was considered a core variable, and covariates used for analysis were age, sex, CD4 count, antiretroviral therapy regimen, history of intravenous drug use, marital status, religion, geography, employment status, hypertension, and diabetes mellitus. Prevalence of anemia and its independent predictors were evaluated. Fisher’s exact and χ2 tests were performed to determine the significance of differences among categorical variables and t-tests for continuous variables. Binary logistic regression was modeled to assess predictors associated with anemia.
Results: Of the total 210 patients analyzed, median age was 37.50± 10.57 years, and 110 (52.6%) were male. The estimated prevalence of anemia overall was 66.7% (95% CI 60.64%– 73.35%): mild anemia 14.3% (95% CI 8.25%– 19.74%), moderate anemia 40.5% (95% CI 31.88%– 48.11%), and severe anemia 11.9% (95% CI 6.61%– 17.30%). Prevalence of anemia increased significantly with decreasing CD4 count: 5.71%, 12.85%, and 48.09% among patients with CD4 counts > 500, 200– 499, and < 200 cells/mm3, respectively (P=0.019). Severity of anemia was significantly associated with immunostatus (< 200, 200– 499, and > 500; P=0.048). Female sex was significantly associated with increased odds of anemia (OR 2.27, P=0.007).
Conclusion: The present study demonstrated a high rate of anemia in a substantial number of HIV individuals. Therefore, early detection and timely management of anemia, especially in females and those with decreased immunostatus, are crucial to prevent anemia progression and improve quality of life.
Keywords: anemia, HIV, risk factors
Introduction
Anemia is a extensive global health burden affecting developing countries more than developed countries,1 and can cause severe impacts on quality of life, morbidity, mortality, and the social and economic development of individuals.2 Hematologic complications have been identified as the commonest cause of morbidity and mortality among HIV-seropositive patients, with considerable impact on quality of life and clinical outcomes.3–5 Several causes of anemia have been reported in HIV patients, among which the most commonly reported are deficiencies in minerals, iron, and vitamin B12,6 In addition to hookworm infestation, malaria infection, vitamin A deficiency, genetic defects, and chronic infections, such as TB and HIV.7–9
In recent epidemiological studies, anemia has appeared to be the commonest clinical burden in people living with HIV/AIDs,10–12 and its severity increases with declining CD4 count13 and progression of the HIV/AIDs to the advanced stage.14 Moreover, among those with HIV, anemia influences the natural history of the disease, leading to disease progression, and is an independent predictor of death, irrespective of CD4 count or viral load.15–17 A systematic review of the literature suggests that anemia in patients with HIV in a number of subpopulations tends to becommoner in developing countries than developed countries,18 with estimates of up to 80%19–21 depending on region and threshold used to define anemia. Factors often contributed to the risk of developing anemia in HIV infection include antiretroviral therapy (ART) regimen, presence of opportunist infections, sex,22–25 low CD4+ T-lymphocyte count,20,25,26 increased viral load,27 being pregnant,18 intravenous (IV)drug use,18,28 and increased age.29 Importantly, the documented rate of anemia and its potential causes is different in different settings and times. According to recent estimates, Nepal is home to approximately 50,200 people living with HIV.30 Despite the high rate, the magnitude of anemia and its contributing factors in this populations has been largely ignored. Therefore, the present study aimed to determine the prevalence of anemia and examine factors associated with it.
Methods
Study Design and Setting
This was a cross-sectional study conducted at Sukraraj Tropical and Infectious Disease Hospital, Teku, Kathmandu from November 2016 to August 2017. It is one of the most renowned hospitals in the country, containing 100 beds in Kathmandu Valley, and is specifialized in the treatment of HIV and providing health services to patients from all parts of the country. This hospital not only provides health-related services to HIV individuals but also provides motivation to fight against HIV and AIDS. It enables individuals and communities affected by HIV to protect themselves, care for others, advocate for better services, and challenge stigma and discrimination.
Study Population and Selection
Patients visiting the hospital from November 2016 to August 2017 who met the enrollment criteria for study were included. A total of 210 HIV-seropositive patients were eligible and included for analysis. Patients were eligible if aged 16 years or above and showed willingness to participate. Patients with incomplete demographic and clinical information, hemolytic anemia, and active gastrointestinal bleeding and pregnant women were excluded from the study.
Variable of Interest
The core variable of interest of this study was anemia. Covariates used for analysis were age, sex, CD4 count, ART regimen, history of IV-drug use, marital status, religion, domicile, employment status, hypertension, and diabetes mellitus.
Data Collection
Standardized data-collection forms were completed. Sociodemographic and relevant clinical data from patient interviews and medical record files were assessed. Demographic information — age, sex, religion, marital status, employment status, geographical location, and history of IV-drug use — was gathered. Clinical information, ie, ART status, hypertension, diabetes mellitus, and opportunistic infections, were assessed from patients’ medical records, and laboratory profiles, eg, hemoglobin (HB) and CD4 counts, were assessed.
Assessment of Anemia
Anemia for men was defined as Hb concentration <13 g/dL (mild 11–12 g/dL, moderate 8–10.9 g/dL, severe <8 g/dL), whereas for nonpregnant women it was defined as <12.0 g/dL (mild 11–11.9 g/dL, moderate 8–10.9 g/dL, severe<8 g/dL).31
Assessment of Immunostatus
Immunostatus was categorized into mild immunodeficiency, advanced immunodeficiency, and no significant immunodeficiency using CD4 counts of <200 cells/mm3, 200–499 cells/mm3, and ≥500 cells/mm3, respectively.32 A Sysmex automated hematology analyzer (XN-L series XN-330) was used for Hb estimation (cyanide-free sodium lauryl sulfate method). In this machine, Hb conversion of oxy-Hb method is fast, as blood Hb is instantly converted into oxy-Hb. In addition, it does not use poisonous substances, such as cyanide, and thus is a suitable method for performing automatic analysis. A BD FACSCount was used for determination of CD4 count. When whole blood is added to the reagent tube, fluorochrome-labeled antibodies in the reagents bind specifically to white blood–cell surface antigens and a fluorescent nuclear dye binds to the nucleated blood cells. After a fixative solution is added, the sample is run on the instrument. During sample acquisition, cells pass through the laser light, which causes labeled cells to fluoresce. This fluorescent light provides the information necessary for the instrument to identify and count lymphocytes and CD4 T lymphocytes. In addition, the reagent tubes contain a known number of fluorescent reference beads, to which a precise volume of whole blood is added. The software automatically identifies lymphocyte populations of interest and calculates CD4 counts (cells/µL) by comparing cellular events to bead events. Results include CD4 counts and CD4 percentages.
Statistical Analysis
Continuous data are expressed as means ± SD. Categorical variables are presented as numbers and percentages in each category. Prevalence of anemia is reported as percentages with corresponding 95% CIs. Fisher’s exact and χ2 tests were performed to determine the significance of differences among categorical variables and t-tests for continuous variables. Binary logistic regression yielding ORs) with 95% CIs were used to determine predictors associated with anemia among people living with HIV. To fit the binary logistic model, multicollinearity for each independent predictor was checked. Statistical tests were two-tailed, and P<0.05 was considered significant. All statistical analysis was performed with SPSS version 11.5.
Ethical Issues
The study protocol was approved by the ethical research committee of Sukraraj Tropical and Infectious Disease, Teku, Kathmandu. Hospital permission was granted prior to data collection. Patients were well aware of the study protocol, and written consent from those who showed willingness to participate was sought prior to commencing the study. The study was conducted in accordance with the Declaration of Helsinki. Patient confidentiality was assured.
Results
Table 1 presents sociodemographic and health characteristics of the study population. Of the total 210 HIV patients, the median age was 37.50±10.57 (16–66) years, and 110 (52.4%) were female. A total of 145 (69%) study subjects were married and eight (3.8%) widowed. Eighteen (8.6%) subjects had a history of IV-drug use. The mean Hb level was 11.03±10.80 (8–17.4) mg/dL. About two-thirds 141, 67.1%) of the population had CD4 counts <200, followed by 53 (25.2%) with 200–499 and 16 (7.6%) with >500.
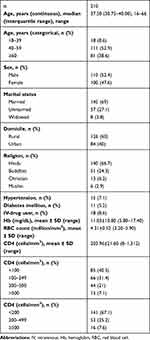 |
Table 1 Sociodemographic and Clinical Characteristics of Participants |
The prevalence of anemia overall was 66.7% (95% CI 60.64%–73.35%): mild anemia 14.3% (95% CI 8.25%–19.74%), moderate anemia 40.5% (95% CI 31.88%–48.11%), and severe anemia 11.9% (95% CI 6.61–17.30%) (Table 2).
 |
Table 2 Prevalence of Anemia (n=210) |
Table 3 shows associations between anemia and sociodemographic/clinical parameters. Age (continuous), age (categorical) marital status, employment status, domicile, religion, IV-drug use, disease transmission, hypertension, diabetes mellitus, ART status, and CD4 count (continuous variable) were not significantly associated with occurrence of anemia (P>0.05). Mean CD4 counts were insignificantly lower among anemic patients compared to those among nonanemic patients (187.21±228.46 vs 237.46±185.94, P=0.11). However, anemia prevalence increased with decreasing CD4 count (5.71%, 12.85%, and 48.09%) among patients with CD4 counts >500, 200–499, and <200 cells/mm3, respectively, and the difference was statistically significant (P=0.019). In the sex category, anemia was more prevalent in females (36.19%) than males (30.47%), a significant difference (P=0.04). Severity of anemia (mild/moderate/severe) was significantly associated with immunostatus (<200/200–499/>500, P=0.048; Figure 1).
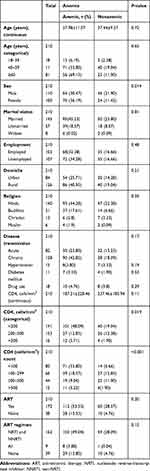 |
Table 3 Associations Between Anemia and Sociodemographic/Clinical Parameters |
Table 4 shows independent predictors associated with anemia. Bivariate logistic regression was modeled to illustrate factors associated with anemia in HIV patients. Age (continuous), marital status, geographic diversity, religion, IV drug-use,ART status, and CD4 count (<200, 200–500, and >500) were taken into account for the model. Sex remained an independent factor associated with anemia, and the odds of having anemia were 2.27 times higher in females than males (OR 2.27, 95% CI 1.25–4.12;P=0.007). After adjustment for age (continuous), marital status, geographic diversity, religion, IV-drug use, ART status, and CD4 count (<200, 200–500, and >500), sex remained an independent predictor of anemia (OR 2.48, 95% CI 1.24–4.96; P=0.01).
 |
Figure 1 Association between severity of anemia and CD4 count (χ2). |
 |
Table 4 Bivariate Logistic Regression Analysis of Factors Associated with Anemia Among HIV+ Patients |
Discussion
Anemia is a growing complication of infection with HIV1 and could be clinically important to public health. The etiology of anemia is multifactorial and thus it is difficult to know its original cause, consequently complicating its proper management.33 In different study settings, the prevalence of anemia varies, and has been estimated at up to 85%.20,26 The overall prevalence of anemia in this study was 66.7%, in agreement with several earlier studies from various locations also indicating high rates: 64% in Nigeria,34 71% in Isfahan, Iran,20 69.7% in Benin, Nigeria,35 77.4% in Tanzania,36 and 85% among ART-naïve patients in India.26 However, our figure is higher than findings from many other Asian and non-Asian regions: Iran (46%),37 China (51.9%),29 South Africa (25.8%),15 Ethiopia (34.6%),24 northwest Ethiopia (34%),23 northern India (16.2%),38 and Ghana (23.8%).39
Our study indicated that 14.3%, 40%, and 11.9% of patients had mild, moderate, and severe anemia, respectively. The mild-anemia prevalence (14.3%) noted in this study corresponds well with results of another study (14%).20 However, this figure was lower than results (32.4%) from a study in China.29 In this study, 40% of anemic patients had moderate anemia. In comparison, lower rates of moderate anemia have been observed in earlier studies: 17% in China29 and 15.6% in Ethiopia.24 The rate of severe anemia (11.9%) reported in this study is higher than previous studies: 2.55% in China,29 5% in Ethiopia,24 and 4% in Iranian HIV patients.20
The variations reported in the current study compared to other studies could be due to disparities in sociodemographic characteristics and status of immunity of the participants. For instance, in this study a majority (67.1%) of subjects had CD4 counts <100. However, in northwest Ethiopia, the prevalence of CD4 counts <200 cells/mm3 was 14.8%,23 with anemia 34%. Similarly, most of the study participants (40.9%) in China had CD4 counts <50 cells/mm3, with anemia 51.9%.29
Other factors that could have contributed to the varying rates of anemia seen among studies include presence/absence and/or degree of opportunistic infection, enrollment criteria, varying nutritional status, as malnutrition has a significant effect on anemia,40,41 different ART regimens, dissimilar methodologies, and the heterogeneity of study populations. Moreover, lower Hb-concentration thresholds used to characterize anemia in some previous studies might have contributed to the noncomparability in documented prevalence. For some of these studies, anemia was defined as Hb <12 for men and <11 for women g/dL.29 As such, the lower cutoffs could have considerably lowered the prevalence of anemia and underestimated the magnitude of the problem.
In this study, the median ages was 37.50±10.85 years, in agreement with an earlier study.24 Numbers of anemic patients aged 18–39, 40–59, and ≥60 yearswere 13, 71, and 56, respectively. Categorical analysis revealed no significant association between anemia and age (strata). However, arithmetically the prevalence of anemic subjects in the age-group ≥60 years appeared to be higher (69.13%) than any other (33.80% for 40–59 and 6.9% for 16–39 years). This nonsignificant association is also supported by results of a previous study.42
There are no firm conclusions regarding the association between sex and anemia in HIV infection. Previous studies demonstrated that sex was not associated with anemia; however, arithmetically the prevalence of anemia was higher in women than men.43 In comparison, our study demonstrated a significant association between sex and anemia, and the odds of developing anemia were significantly higher in females (OR 1.98, P=0.014). Moreover, this association is well supported by several earlier findings in different study settings.22–25 The high prevalence of anemia and its higher risk in HIV-seropositive patients could be explained by the fact that women in childbearing years are more likely to have blood loss from menstruation and increased blood-supply requirements, and in turn an increased rate of anemia.44
In our subjects, CD4 counts in anemic patients were found to be insignificantly lower compared to nonanemic patients (187.21±228.46 vs 237.46±185.94, P=0.11). However, categorical analysis revealed a significant association between CD4 cell count (<200, 200–499 >500) and anemia (P=0.019). Moreover, prevalence of anemia increased with decreasing CD4 count (5.71%, 12.85%, and 48.09%) among patients with CD4 counts >500, 200–499, and <200 cells/mm3 respectively. This corresponds well with results from earlier studies.20,25,26 Zerihin et alrevealed that patients with CD4 counts <200 cells/mm3 were more likely to be anemic than those with CD4 counts ≥500 cells/mm3.23 Similarly,Shen et al29 noted that the prevalence of anemia increased with decreasing CD4 cell count (14.0%, 22.4%, 50.7%, and 74.6% among patients with CD4 counts ≥350, 200–349, 50–199, and <50 cells/mm3 respectively).
In this study, the severity of anemia was significantly correlated with CD4 cell count, and the proportion of severe anemia significantly increased with decreasing CD4 count. This may be an important biological implication in our findings whereby lower CD4 count was associated with increased risk of anemia severity. A limitation is that this was a cross-sectional study, which only allows evaluation of associations at a specific point, but cannot draw conclusions about causal relationships over time. Therefore, a longitudinal study in a large representative population is needed to identify the causal relationship between anemia and various sociodemographic and clinical risk factors.
Conclusion
Our study revealls that anemia is a common comorbidity in patients living with HIV in Nepal. In particular, patients (especially with decreased immunostatus and female sex) are at greater risk of developing anemia, and a particular emphasis on careful evaluation to alleviate immune-system problems in these populations is necessary. Moreover, there is also a need to focus on routine screening and timely management of anemia to prevent disease progression and improve quality of life. In addition, increasing awareness of people living with HIV about the benefits of adhering to a nutritional diet consistently could be useful in managing anemia, as an appropriate diet helps the body in proliferating sufficient red blood cells and other granulocytes in the body.
Abbreviations
ART, antiretroviral therapy; Hb, hemoglobin; NRTI, nucleoside reverse-transcriptase inhibitor; NNRTI, non-NRTI.
Acknowledgments
We express our sincere thanks to Sukraraj Tropical and Infectious Disease Hospital, Teku for granting us permission for the research, and also thank the staff of the deparmtent of tropical and infectious disease, Teku for their assistance during the study. We sincerely thank all participants who voluntarily agreed to be involved in the study and helped during data collection.
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
1. McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi:10.1017/S1368980008002401
2. Horton S, Levin C. Commentary on evidence that iron deficiency anemia causes reduced work capacity. J Nutr. 2001;131(2):691S–6S. doi:10.1093/jn/131.2.691S
3. Redig AJ, Berliner N. Pathogenesis and clinical implications of HIV-related anemia in 2013. ASH Educ Program Book. 2013;2013(1):377–381.
4. Moore RD. Human immunodeficiency virus infection, anemia, and survival. Clin Infect Dis. 1999;29(1):44–49. doi:10.1086/520178
5. Anastos K, Shi Q, French AL, et al. Total lymphocyte count, hemoglobin, and delayed-type hypersensitivity as predictors of death and AIDS illness in HIV-1–infected women receiving highly active antiretroviral therapy. JAIDS J Acquir Immune Defic Syndr. 2004;35(4):383–392. doi:10.1097/00126334-200404010-00008
6. Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309–1316. doi:10.1136/gut.2010.228874
7. Kashinkunti M, Alevoor S. Clinical hematological and coagulation profile in malaria. Sch J App Med Sci. 2014;2(2B):584–588.
8. Semba RD, Bloem M. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr. 2002;56(4):271. doi:10.1038/sj.ejcn.1601320
9. Lee SW, Kang Y, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21(6):1028–1032. doi:10.3346/jkms.2006.21.6.1028
10. Volberding PA, Levine AM, Dieterich D, et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–1463. doi:10.1086/383031
11. Martin C, Poudel-Tandukar K, Poudel KC, Tang JW. HIV symptom burden and anemia among HIV-positive individuals: cross-sectional results of a community-based positive living with HIV (POLH) study in Nepal. PLoS One. 2014;9(12):e116263. doi:10.1371/journal.pone.0116263
12. Ezechi O, Kalejaiye O, Gab-Okafor C, et al. The burden of anaemia and associated factors in HIV positive Nigerian women. Arch Gynecol Obstet. 2013;287(2):239–244. doi:10.1007/s00404-012-2573-2
13. Dikshit B, Wanchu A, Sachdeva RK, Sharma A, Das R. Profile of hematological abnormalities of Indian HIV infected individuals. BMC Hematol. 2009;9(1):5. doi:10.1186/1471-2326-9-5
14. Alamdo AG, Fiseha T, Tesfay A, Deber MK, Tirfe ZM, Tilahun T. Anemia and its associated risk factors at the time of antiretroviral therapy initiation in public health facilities of Arba Minch Town, Southern Ethiopia. Health. 2015;7(12):1657. doi:10.4236/health.2015.712179
15. Takuva S, Maskew M, Brennan AT, Sanne I, MacPhail AP, Fox MP. Anemia among HIV-infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. J Trop Med. 2013;2013:1–6. doi:10.1155/2013/162950
16. O’Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. JAIDS J Acquir Immune Defic Syndr. 2005;40(2):219–225. doi:10.1097/01.qai.0000166374.16222.a2
17. Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. Aids. 1999;13(8):943–950. doi:10.1097/00002030-199905280-00010
18. Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(7):27–43. doi:10.1016/j.amjmed.2003.12.010
19. Ahumareze RE, Rankin J, David A, et al. Prevalence of anaemia and the relationship between haemoglobin concentration and CD4 count in HIV positive children on highly active antiretroviral therapy (HAART) in Lagos, Nigeria. Curr Pediatr Res. 2016.
20. Meidani M, Rezaei F, Maracy MR, Avijgan M, Tayeri K. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17(2):138.
21. Erhabor O, Ejele O, Nwauche C, Buseri F. Some haematological parameters in human immunodeficiency virus (HIV) infected Africans: the Nigerian perspective. Niger J Med. 2005;14(1):33–38. doi:10.4314/njm.v14i1.37132
22. Ferede G, Wondimeneh Y. Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Blood Disord. 2013;13(1):8. doi:10.1186/2052-1839-13-8
23. Zerihun KW, Bikis GA, Muhammad EA. Prevalence and associated factors of anemia among adult human immune deficiency virus positive patients on anti-retroviral therapy at Debre tabor Hospital, Northwest Ethiopia. BMC Res Notes. 2019;12(1):168. doi:10.1186/s13104-019-4214-3
24. Gebremedhin KB, Haye TB. Factors associated with anemia among people living with HIV/AIDS taking ART in Ethiopia. Adv Hematol. 2019;2019:1–8. doi:10.1155/2019/9614205
25. Jam S, Ramezani A, SABZEVARI D, et al. A Cross-Sectional Study of Anemia in Human Immunodeficiency Virus-Infected Patients in Iran. 2009.
26. Parinitha S, Kulkarni M. Haematological changes in HIV infection with correlation to CD4 cell count. Australas Med J. 2012;5(3):157. doi:10.4066/AMJ.2012.100
27. Levine AM, Berhane K, Masri-Lavine L, et al. Prevalence and correlates of anemia in a large cohort of HIV-infected women: women’s Interagency HIV study. J Acquir Immune Defic Syndr. 2001;26(1):28–35. doi:10.1097/00126334-200101010-00004
28. Semba RD, Shah N, Strathdee SA, Vlahov D. High prevalence of iron deficiency and anemia among female injection drug users with and without HIV infection. J Acquir Immune Defic Syndr. 2002;29(2):142–144. doi:10.1097/00042560-200202010-00005
29. Shen Y, Wang Z, Lu H, et al. Prevalence of anemia among adults with newly diagnosed HIV/AIDS in China. PLoS One. 2013;8(9):e73807. doi:10.1371/journal.pone.0073807
30. USAID. HIV/AIDs. [
31. Organization WH. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. World Health Organization; 2011.
32. Organization WH. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance: African Region. Geneva: World Health Organization; 2005.
33. Kreuzer K-A, Rockstroh J. Pathogenesis and pathophysiology of anemia in HIV infection. Ann Hematol. 1997;75(5–6):179–187. doi:10.1007/s002770050340
34. Pennap GR, Abubakar K. Prevalence of anemia among human immunodeficiency virus infected patients accessing healthcare in federal medical center Keffi, Nigeria. Int J Trop Dis Health. 2015;10(3):1–7. doi:10.9734/IJTDH/2015/19657
35. Omoregie R, Omokaro E, Palmer O, et al. Prevalence of anaemia among HIV-infected patients in Benin City, Nigeria. Tanzan J Health Res. 2009;11(1). doi:10.4314/thrb.v11i1.43242.
36. Johannessen A, Naman E, Gundersen SG, Bruun JN. Antiretroviral treatment reverses HIV-associated anemia in rural Tanzania. BMC Infect Dis. 2011;11(1):190. doi:10.1186/1471-2334-11-190
37. Ramezani A, Aghakhani A, Sharif MR, Banifazl M, Eslamifar A, Velayati AA. Anemia prevalence and related factors in HIV-infected patients: a cohort study. Iran J Pathol. 2008;3(3):125–128.
38. Kulkarni D, Bhalerao MM, Mungal S, Dube S. Anemia in people living with HIV/AIDS: a cross sectional study from India. IOSR J Dent Med Sci. 2015;14(2):04–8.
39. Obirikorang C, Issahaku RG, Osakunor DNM, Osei-Yeboah J. Anaemia and iron homeostasis in a cohort of HIV-infected patients: a cross-sectional study in Ghana. AIDS Res Treat. 2016;2016:1–8. doi:10.1155/2016/1623094
40. Osazuwa F, Ayo OM. Contribution of malnutrition and malaria to anemia in children in rural communities of Edo state, Nigeria. N Am J Med Sci. 2010;2(11):532. doi:10.4297/najms.2010.2532
41. Thakur N, Chandra J, Pemde H, Singh V. Anemia in severe acute malnutrition. Nutrition. 2014;30(4):440–442. doi:10.1016/j.nut.2013.09.011
42. Kiragga AN, Castelnuovo B, Nakanjako D, Manabe YC. Baseline severe anaemia should not preclude use of zidovudine in antiretroviral-eligible patients in resource-limited settings. J Int AIDS Soc. 2010;13(1):42. doi:10.1186/1758-2652-13-42
43. Denue BA, Kida IM, Hammagabdo A, Dayar A, Sahabi MA. Prevalence of anemia and immunological markers in HIV-infected patients on highly active antiretroviral therapy in Northeastern Nigeria. Infect Dis Res Treat. 2013;6(IDRT):S10477.
44. Pala K, Dundar N. Prevalence & risk factors of anaemia among women of reproductive age in Bursa, Turkey. Indian J Med Res. 2008;128(3):282.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
