Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 11
Prevalence and predictors of anemia among adult HIV infected patients at the University of Gondar Hospital, Northwest Ethiopia
Authors Yesuf T, Muhie OA , Shibru H
Received 20 March 2019
Accepted for publication 24 August 2019
Published 6 September 2019 Volume 2019:11 Pages 211—217
DOI https://doi.org/10.2147/HIV.S209446
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Tesfaye Yesuf, Oumer Abdu Muhie, Habtewold Shibru
Department of Internal Medicine, University of Gondar, Gondar, Ethiopia
Correspondence: Oumer Abdu Muhie
Department of Internal Medicine, University of Gondar, P.O Box 196, Gondar, Ethiopia
Tel +251 92 163 8680
Email [email protected]
Background: Anemia is the leading hematologic complication of HIV infection occurring in approximately 30% of patients with an asymptomatic infection and in as many as 75–80% of those with AIDS. Anemia increases morbidity and mortality among HIV infected patients.
Objective: This study aimed to determine the prevalence of anemia in adult HIV infected patients and the associated factors.
Method: Retrospective record review was conducted for a total of 404 HIV infected adult patients who were started on HAART from January 2010 up to September 2015 at the University of Gondar Hospital adult ART clinic. Descriptive analysis, bivariate and multivariate logistic regression was used to compute the different rates, proportions, and associations.
Results: The prevalence of anemia was 32.9%, 14.4%, and 9.4% at baseline, after 6 and 12 months of HAART. Lower CD4 count (AOR=1.9; 95% CI: 1.1–3.2) and opportunistic infections (AOR=3.0; 95% CI: 1.8–5.0) were associated with the odds of being anemic at baseline. Baseline anemia was a predictor for being anemic after 6 months (AOR=3.6; 95% CI: 2.0–6.7). Similarly, being anemic after 6 months of HAART predicted the odds of being anemic after 12 months on HAART (AOR=9.1; 95% CI: 4.1–19.9). Zidovudine based regimen was also found to be a predictor of anemia at three months after HAART (AOR=6.0; 95% CI: 2.5–14.3).
Conclusion: Anemia is a common problem in HIV patients at the University of Gondar Hospital, nearly a third of the HIV patients had anemia. A lower CD4 count and opportunistic infection were predictors of being anemic. Anemia during the early periods of HAART initiation was also a predictor of subsequent anemia in HIV patients.
Keywords: HIV, AIDS, anemia, Gondar, Ethiopia
Introduction
Ethiopia is one of the countries that is severely affected by HIV and its associated health and economic impact. According to the 2014 HIV estimates, the national HIV prevalence is 1.14%. The estimated number of people living with HIV was 769 600 with 15 700 new HIV infections and 35 600 AIDS-related deaths.1 Currently, 339 043 adults are receiving ART.1
One of the complications of HIV infection is anemia, occurring in approximately 30% of patients with an asymptomatic infection and in as many as 75–80% of those with AIDS.2
Anemia in HIV infected individuals has multifactorial etiologies that make etiologic diagnosis challenging.3 The virus itself, immune dysregulation, opportunistic infections, and medications result in anemia.4 In a study of adverse drug reactions associated with HAART among 228 adult HIV infected patients at Tikur Anbessa Hospital, in Ethiopia, severe hematological complications were anemia (4.8%), neutropenia (2.6%), and thrombocytopenia (0.9%). Anemia occurred early, in the first 4 weeks of HAART treatment.5 In another study of adverse drug reactions to antiretroviral therapy during the early HAART period at a tertiary hospital in Ghana, anemia was the commonest adverse drug reaction.6
Hematological complications have been documented to be common causes of morbidity and mortality in HIV positive individuals.7 In the Euro SIDA cohort, patients with severe anemia at baseline had a 13 times greater risk of death during the first year of HAART than patients with a normal hemoglobin concentration.8 Independent of CD4 and viral load counts, anemia can predict HIV progression to acquired immune deficiency syndrome (AIDS), with poor survival.4,7 Time-updated moderate or severe anemia during HAART was the strongest independent predictor of both incident tuberculosis and death. Moreover, it had greater predictive value than CD4 cell counts.9 In a study of causes and outcome of hospitalization among HIV infected adults receiving antiretroviral therapy in Mulago Hospital, Uganda, anemia was the third commonest cause for admission next to tuberculosis and cryptococcal meningitis.10 In a study done in Nigeria, anemia was associated with virologic failure and immuno-virologic discordance.11
HAART decreases the incidence of anemia, as demonstrated in different Ethiopian studies; from 52.6% before HAART to 37.4% after HAART at Menelik II Hospital;12 42.9% at baseline to 20.9% and 14.3% at 6 months and 12 months after HAART initiation at Zewditu Memorial Hospital.4
The prevalence of anemia in HIV patients varies considerably, ranging from 1.3% to 95%. Several factors including the stage of HIV, age, and sex are said to account for the variations in anemia prevalence in HIV patients.4
The prevalence of anemia was 16.2% among children having follow-up at the ART (antiretroviral therapy) clinic of the University of Gondar Hospital.13 In a study done at Jimma University Specialized Hospital, the overall prevalence of anemia was 23.1%. The prevalence of anemia in HAART naive and those receiving HAART was 29.9% and 16.2%, respectively.7
In a study done by the International Epidemiologic Databases to Evaluate AIDS (IeDEA) collaboration in 6 regions, it was found that at HAART initiation, anemia was found in 45% of Western Africa patients, 29% of Eastern Africa patients, 21% of Southern Africa patients, 36% of Central Africa patients, 15% of patients in Asia-Pacific, and 14% of patients in Caribbean and Central and South America.13
Male sex, clinical stage III/IV and TB co-infection were independent risk factors for anemia pre-HAART.4 Presence of opportunistic infection, CD4 count <200 cells/µL, and rural residence were found to be predictors of anemia.7 Eating green leafy vegetables and being on cotrimoxazole treatment were also reported to be associated with anemia in HIV patients.14
In a retrospective cohort study among 1061 patients at Zewditu Memorial Hospital, in Ethiopia, male sex, clinical stage III/IV, and TB co-infection were independently associated with increased odds of being anemic before HAART.4
In a study done in China among 2252 HIV infected patients, Uyghur ethnicity, female gender, lower CD4 count, lower body mass index value, self-reported tuberculosis infection, and oral candidiasis were associated with a higher prevalence of anemia at the initiation of HAART.15
In a cross-sectional observational study of 102 HIV-positive women, aged 19 or older, in Vancouver, British Columbia, CD4 cell count <200 cells/µL, a regular menstrual pattern, and African ethnicity were associated with an increased risk of iron deficiency anemia.16
In a retrospective cohort study among 1061 patients on HAART at Zewditu Memorial Hospital, in Ethiopia; anemia at baseline, TDF based HAART regimen, and low CD4 count were independently associated with increased odds of being anemic after 6 and 12 months on HAART [25] (1).4 However, in a cross-sectional study among 117 HAART experienced patients at Jimma University Hospital, in Ethiopia, HAART regimen (AZT/3TC/NVP) and duration of HAART were predictors of anemia.7
Studies done in South Africa, Uganda, Tanzania, and DR. Congo demonstrated that low MCV, baseline anemia, and AZT based HAART were important predictors of persistent anemia after 12 months of HAART.9,17–19 Similarly in the DART trial, women and those with lower hemoglobin, CD4+ T-cell count, and BMI at baseline were predictors of severe anemia in patients started on AZT containing regimens.20
In a study done in the USA in 21 HIV care centers and in Women’s Interagency HIV Study (WIHS), African American race, MCV <80 fl, CD4 count <200 per microliter, higher HIV RNA in plasma, current use of AZT, and history of clinical AIDS were all independent predictors of anemia.2,21
In the study done by IeDEA collaboration in its 6 regions and 34 HIV treatment cohorts, female sex, low baseline hemoglobin level, low baseline CD4 count, more advanced disease stage, and initial HAART containing zidovudine were the predictors of developing anemia within the first year of HAART.14
Anemia in adult HIV patients has been addressed in different studies, however its prevalence and predictors vary from setup to setup and from population to population. In our setup, its prevalence and predictors have not been addressed previously.
Objectives of the study
To determine the prevalence of anemia at the start of HAART and after HAART initiation in adult HIV infected patients.
To assess the predictors of anemia in adult HIV infected patients.
Methods
Study design, study setting, study period, and study population
This study was an institution based retrospective record review conducted at the University of Gondar Hospital. Gondar is a city in North West Ethiopia about 728 km away from the capital, Addis Ababa. There is only one referral hospital and two district hospitals serving around 5 million people. There is large scale farming in the lowlands of the hospital catchment area which makes people migrate from the highlands to the deserts where there is high prevalence of HIV.
University of Gondar Referral Hospital has an ART clinic which was opened on March 2003 GC (Gregorian calendar) which charged fees, and free ART was started on 2005 GC. The clinic is run by full time nurses, general practitioners, internal medicine residents, and Internists. There is a laboratory service for biochemical tests like renal function test, liver function and enzymes test, urinnalysis, stool microscopy, complete blood count, CD4 and viral load determination. Currently, 4890 patients are on HAART. In the last 12 years, 9898 patients were started on ART and 13,330 patients were enrolled in the ART clinic. The data were collected from February, 2016 to August, 2016 from adult HIV infected patients who were started on HAART since January, 2010 until September, 2015 GC.
Sample size and sampling procedure
Sample size was calculated using the formula to estimate a single proportion with level of confidence of 95% and with desired precision of the estimate ±0.05 using the following formula:
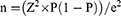
Where Z=value from standard normal distribution corresponding to desired confidence level (Z=1.96 for 95% CI).
P is expected true proportion which is 50% taken from a study done at Zewditu Memorial Hospital, Addis Ababa.
e is desired precision (half desired CI width).
So with this calculation and adding 5% non-response for incomplete charts, the total sample size was 404.
We retrieved medical record number of patients started on HAART at University of Gondar ART clinic from January, 2010 up to September, 2015 GC. Then, by systematic random sampling, the first case was selected randomly and every patient at the sampling interval was included.
Inclusion, exclusion criteria, and study variables
We included HIV infected adult patients ≥18 years of age who were started on HAART from January 2010 up to September 2015 GC. We excluded those medical records with incomplete data, pregnant ladies, transferred-in patients, and transferred-out patients.
Dependant variables
- Anemia
Independent variables
- Age
- BMI
- ART regimen
- CPT
- OI
- CD4 count
- Parasites
Operational definition of variables
- Anemia is defined according to WHO definition when it is <12 mg/dL for females and, <13 mg/dL for males.
Data collection procedure
The dependent and independent variables were collected by medical record review filling well structured pretested questionnaires. Data were entered into IBM SPSS Statistics version 20 for analysis. Descriptive analysis was done to assess prevalence of the outcome. Bivariate analysis was done to assess association between dependant and independent variables. Factors that show association in bivariate association with P-value less than 0.2 were entered into multiple logistic regression models. Confidence interval of 95% and P-value <0.05 was considered as significant.
Ethical clearance
Ethical approval was obtained from University of Gondar ethical board.
All the data were confidentially handled. Each record was coded and every potential identifier was excluded from the entire data analysis document. In conclusion, patient data were treated with confidentiality or anonymized, in accordance with the Declaration of Helsinki.
Results
A total of 404 HIV infected adults who were started on HAART at University of Gondar Hospital from January, 2010 up to September, 2015 GC (Gregorian calendar) were included in the analysis. Females accounted for 247 (61.1%) and males for 157 (38.9%). The majority of patients were in the age category 31–49 with 18–30 constituting 56.2% (227) and 37.9% (153) respectively, with a median age of 34.8 (±8.8) with minimum age 18 and maximum age 75. Most of the patients (62.4%) had BMI in the range of 18.5–24.9 and 38.9% of the patients were underweight. The sociodemographic data are summarized in Table 1.
 |
Table 1 Baseline sociodemographic and clinical characteristics |
The prevalence of anemia was 32.9%, 14.4%, and 9.4% at baseline, after HAART initiation at 6th and 12th month respectively and the anemia was mild (≥10 gm/dL) in the majority of patients. After 3 months of HAART hemoglobin was determined only for 50 patients. Twenty of the 39 patients had hemoglobin level of ≤7 g/dL. These values are depicted in detail in Table 2.
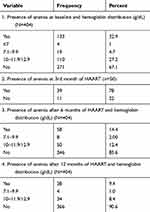 |
Table 2 Prevalence of anemia |
MCV was determined for 173 patients at the start of HAART and 89% had normal MCV. MCV was determined for 34 patients at the 3rd month of HAART; of which 18 had normal MCV and 11 had macrocytosis. After 6th month of HAART, MCV was determined for 154 patients of which 79 had normal MCV and 73 had macrocytosis. After 12 months of HAART, MCV was determined for 164 patients of which 97 patients had macrocytosis and 65 had normal MCV.
The minimum CD4 count was 8 and the maximum was 498 with mean 178 (±105) at the start of HAART. The majority of patients 64.6% (261) had CD4 count less than 200 cells/µL. The proportion of patients having CD4 count less than 200 cells/µL was 31.4% (127) and 24.8% (100) at 6th and 12th month of HAART respectively.
Opportunistic infections were diagnosed in 19.6% (79) of patients; the majority (73 patients) was diagnosed with tuberculosis.
The minimum time patients stayed without being started on HAART was 1 month and the maximum time was 78 months with a mean of 7 months (±14). Most of the patients, 74.8% (302), were started on HAART within 6 months of HIV diagnosis. Most patients, 99.3%, were started on CPT at the start of HAART.
Most of the patients, 47.8% and 50%, were started on TDF and AZT regimen respectively.
Hookworm was identified during episode of anemia only in two patients.
Predictors of anemia before HAART initiation
The strongest predictors of anemia at baseline were CD4 count <200 cells/µL and the presence of opportunistic infection. Both of them increased the odds of being anemic before HAART initiation two and three times compared to those having CD4 count ≥200 cells/µL and those who did not have opportunistic infection respectively. Table 3 shows the predictors of anemia before HAART initiation.
 |
Table 3 Predictors of anemia before HAART initiation |
Predictors of anemia after HAART initiation
AZT based regimen was associated with anemia at the third month of HAART (P=0.000; AOR=6.0; 95% CI: 2.5–14.3). Documented opportunistic infections at any time during follow-up prior to initiation of HAART was also associated with anemia at the third month of HAART initiation (P=0.002; AOR=3.4; 95% CI: 1.6–7.3). But CD4 count at baseline was not associated with anemia at third month (p=0.48; AOR=1.3; 95% CI: 0.6−2.9).
The presence of anemia at baseline was the only significant predictor of anemia after 6 months of HAART. Association of the different variables with the presence of anemia at the 6th month after HAART initiation is shown in Table 4.
 |
Table 4 Predictors of anemia after 6 months on HAART |
Only the presence of anemia after 6 months of HAART was associated with the odds of being anemic after 12 months of HAART. It increased the development of anemia at 12 months by 9-fold compared to those who did not have anemia after 6 months of HAART. The details of the association of the variables with the presence of anemia after 12 months of HAART initiation is presented in Table 5.
 |
Table 5 Predictors of anemia after 12 months on HAART |
Discussion
The prevalence of anemia before HAART initiation was 32.9% in our study, which is in line with a study done in Jimma Specialized Hospital (29.9%)7 but lower than studies done elsewhere (Menelik II Hospital - 52.6%, Zewditu Memorial Hospital - 42.9% and Ghana - 63%).4,12,22
After HAART initiation, the prevalence of anemia decreased to 14.4% and 9.4% after 6 and 12 months on HAART respectively. This was also seen in studies done in Jimma Specialized Hospital (from 29.9 to 16.2%), Menelik II Hospital (from 52.6% to 37.4%), and Zewditu Memorial Hospital (42.9% at baseline to 20.9% and to 14.3% at 6 months and 12 months after HAART initiation).4,7,12 A similar finding was also seen from the Ghanaian study (from 63% to 46%).22
In this study, CD4 count <200 cells/µL and documented opportunistic infection at any time during follow-up prior to the initiation of HAART were associated with increased odds of being anemic. This was in line with the study done in Zewditu Memorial Hospital that revealed World Health Organization (WHO) stage III and IV, TB co-infection, and male sex as predictors of being anemic before HAART initiation.4 The cross-sectional study from Jimma University Hospital, in Ethiopia, showed that opportunistic infections and CD4 count <200 cells/µL were predictors of anemia, similar to our study.7
Sex was not found to be a predictor of being anemic in our study. However, female gender was mentioned as a risk in a few other studies.14,15
In our study, baseline anemia was a significant predictor of being anemic after 6 months of HAART. Anemia after 6 months of HAART initiation was a significant predictor of being anemic after 12 months on HAART. This is similar to the study from Zewditu Memorial Hospital. However, in contrast to our study, male sex and TDF based HAART were found to be predictors of being anemic after 6 months of HAART.4
Similar to our study, studies done in South Africa, Uganda, Tanzania, and DR. Congo demonstrated that baseline anemia was an important predictor of persistent anemia after 12 months of HAART.9,17–19 This was also evident in the study done by International Epidemiologic Databases to Evaluate AIDS (IeDEA) collaboration, where low baseline hemoglobin level was a predictor of developing anemia within the first year of HAART.14
AZT based regimen was found to be a predictor of anemia at the third month of HAART in our study. However, AZT based regimen was not found to be an important predictor of anemia afterward in this study.
Conclusion
About a third of HIV infected adults have anemia at baseline which decreased with HAART. The presence of opportunistic infection and low CD4 count were found to be the most important predictors of anemia at baseline. AZT based regimen was associated with occurrence of anemia at third month of HAART. Baseline anemia was an important predictor for HIV infected patients to be anemic in their first year of HAART.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Retrospective data analysis was approved by the review board of the University of Gondar. Patient informed consent was waived because of the retrospective characteristics of the study.
Abbreviations
3TC, lamivudine; AIDS, acquired immunodeficiency syndrome; ART, antiretroviral treatment; BMI, body mass index; CD4, cluster differentiation 4; CPT, cotrimoxazole preventive therapy; DART, development of antiretroviral therapy in Africa; Fl, femtoliter; HAART, highly active antiretroviral treatment; HIV, human immunodeficiency virus; IeDEA, International Epidemiologic Databases to Evaluate AIDS; MCV, mean cell volume; NVP, nevirapine; OI, opportunistic infection; TDF, tenofovir; AZT, zidovudine.
Acknowledgment
We would like to acknowledge Dr. Ermias Diro who has contributed a lot to the accomplishment of this study.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests to report in this work.
References
1. WHO. ETHIOPIA Update Sheet on HIV/AIDS Programme 2014. ETHIOPIA Update sheet on HIV/AIDS programme 2014; 2015.
2. Levine AM, Berhane K, Masri-Lavine L, et al. Prevalence and correlates of anemia in a large cohort of HIV-infected women: women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001. doi:10.1097/00042560-200101010-00004
3. Ndlovu Z, Chirwa T, Takuva S. Incidence and predictors of recovery from anaemia within an HIV-infected South African cohort, 2004–2010. Pan Afr Med J. 2014. doi:10.11604/pamj.2014.19.114.3600
4. Assefa M, Abegaz WE, Shewamare A, Medhin G, Belay M. Prevalence and correlates of anemia among HIV infected patients on highly active anti-retroviral therapy at Zewditu Memorial Hospital, Ethiopia. BMC Hematol. 2015. doi:10.1186/s12878-015-0024-6
5. Abdissa SG, Fekade D, Feleke Y, Seboxa TDE. Adverse drug reactions associated with antiretroviral treatment among adult Ethiopian patients in a tertiary hospital. Ethiop Med J. 2012;50(2):107–113.
6. Lartey M, Essel A, Asante-Quarshie A, Kenu E, Ganu V, Neequaye A. Dverse drug reactions to antiretroviral therapy during the early art period at a tertiary hospital in Ghana. Pan Afr Med J. 2014. doi:10.11604/pamj.2014.18.25.3886
7. Gedefaw L, Yemane T, Sahlemariam Z, Yilma D, Diemert DJ. Anemia and risk factors in HAART Naïve and HAART experienced HIV positive persons in South West Ethiopia: a comparative study. PLoS One. 2013. doi:10.1371/journal.pone.0072202
8. Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS. 1999. doi:10.1097/00002030-199905280-00010
9. Kerkhoff AD, Wood R, Cobelens FG, Gupta-Wright A, Bekker LG, Lawn SD. The predictive value of current haemoglobin levels for incident tuberculosis and/or mortality during long-term antiretroviral therapy in South Africa: a cohort study. BMC Med. 2015. doi:10.1186/s12916-015-0320-9
10. Namutebi AMN, Kamya MRK, Byakika-Kibwika P. Causes and outcome of hospitalization among HIV-infected adults receiving antiretroviral therapy in Mulago hospital, Uganda. Afr Health Sci. 2013;13(4):977–85. doi: 10.4314/ahs.v13i4.17
11. Anude CJ, Eze E, Onyegbutulem HC, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infect Dis. 2013. doi:10.1186/1471-2334-13-113
12. Adane A, Desta K, Bezabih A, Gashaye AKD. HIV-associated anaemia before and after initiation of antiretroviral therapy at ART centre of Minilik II Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50(1):13–21.
13. Zhou J, Jaquet A, Bissagnene E, et al. Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific, and central and South America. J Int AIDS Soc. 2012. doi:10.1186/1758-2652-15-5
14. Enawgaw B, Alem M, Melku M, Addis Z, Terefe B, Yitayew G. Prevalence and associated risk factors of anemia among HIV infected children attending Gondar University Hospital, Northwest Ethiopia: a cross sectional study. BMC Hematol. 2015. doi:10.1186/s12878-015-0032-6
15. Mijiti P, Yuexin Z, Min L, Wubuli M, Kejun P, Upur H. Prevalence and predictors of anaemia in patients with HIV infection at the initiation of combined antiretroviral therapy in Xinjiang, China. Int J STD AIDS. 2015. doi:10.1177/0956462414531935
16. Renouf LS, Sheps S, Hubley A, Pick N, Johansen D, Tyndall MW. The role of diet in predicting: iron deficiency anemia in HIV-positive women. Can J Diet Pract Res. 2012. doi:10.3148/73.3.2012.128
17. Akilimali PZ, Kashala-Abotnes E, Musumari PM, et al. Predictors of persistent anaemia in the first year of antiretroviral therapy: a retrospective cohort study from Goma, the Democratic Republic of Congo. PLoS One. 2015. doi:10.1371/journal.pone.0140240
18. Kiragga AN, Castelnuovo B, Nakanjako D, Manabe YC. Baseline severe anaemia should not preclude use of zidovudine in antiretroviral-eligible patients in resource-limited settings. J Int AIDS Soc. 2010. doi:10.1186/1758-2652-13-42
19. Johannessen A, Naman E, Gundersen SG, Bruun JN. Antiretroviral treatment reverses HIV-associated anemia in rural Tanzania. BMC Infect Dis. 2011;11:190. doi:10.1186/1471-2334-11-208
20. Ssali F, Stöhr W, Munderi P, et al. Prevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trial. Antivir Ther. 2006;11(6):741–9.
21. Curkendall SM, Richardson JT, Emons MF, Fisher AE, Everhard F. Incidence of anaemia among HIV - Infected patients treated with highly active antiretroviral therapy. HIV Med. 2007. doi:10.1111/j.1468-1293.2007.00500.x
22. Owiredu WKBA, Quaye L, Amidu N, Addai-Mensah O. Prevalence of anaemia and immunological markers among Ghanaian HAART-naïve HIV-patients and those on HAART. Afr Health Sci. 2011;11(1):2–15.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
