Back to Journals » Risk Management and Healthcare Policy » Volume 14
Prevalence and Mortality of Hypochloremia Among Patients with Coronary Artery Disease: A Cohort Study
Authors Huang H, Mai Z, Chen L, Li Q, Chen S, Bao K , Tang R, Wei W , Yu Y, Huang Z , Lai W, Wang B, Tan N, Chen J, Liu J, Liu Y
Received 19 February 2021
Accepted for publication 15 June 2021
Published 27 July 2021 Volume 2021:14 Pages 3137—3145
DOI https://doi.org/10.2147/RMHP.S306125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jessica Fleming
Haozhang Huang,1,2,* Ziling Mai,1,3,* Liling Chen,4,* Qiang Li,1 Shiqun Chen,1 Kunming Bao,4 Ronghui Tang,5 Wen Wei,4 Yaren Yu,6 Zhidong Huang,1 Wenguang Lai,1,3 Bo Wang,1 Ning Tan,1– 3 Jiyan Chen,1– 3 Jin Liu,1 Yong Liu1– 3
1Department of Cardiology, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, People’s Republic of China; 2The Second School of Clinical Medicine, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 3Guangdong General Hospital, Affiliated with South China University of Technology, Guangzhou, 510515, People’s Republic of China; 4Department of Cardiology, Longyan First Hospital Affiliated with Fujian Medical University, Longyan, 364000, People’s Republic of China; 5Yunnan Fuwai Cardiovascular Hospital, Department of Ultrasound Imaging, Yunnan, 650000, People’s Republic of China; 6The First People’s Hospital of Foshan, Foshan, Guangdong Province, 528000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Liu; Jin Liu
Department of Cardiology, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, People’s Republic of China
Tel +86 2083827812-10528
Fax +86 2083851483
Email [email protected]; [email protected]
Purpose: Hypochloremia is a predictor for short-term mortality in patients with cardiovascular disease, but its association with coronary artery disease (CAD) is still unclear. We aimed to assess the impact of hypochloremia on all-cause mortality (short-and long-term) among patients with CAD.
Patients and Methods: Based on the registry at Guangdong Provincial People’s Hospital in China, we analyzed data of 49,025 hospitalized patients who underwent coronary angiography (CAG) and were diagnosed with CAD from January 2007 to December 2018. To assess the association between hypochloremia and the study endpoints, a logistic-regression model (for 30-day all-cause mortality) and a Cox regression model (for long-term all-cause mortality) were fitted.
Results: Overall, 4.4% of the study population showed hypochloremia (< 98 mmol/L). During a median follow-up of 5.2 (3.1– 7.8) years, a total of 6486 (13.2%) patients died. Patients with hypochloremia were generally older and at risk for diabetes, cardiorenal dysfunction, and morbidity than those without hypochloremia. After adjustment for confounders, hypochloremia remained a significant predictor of mortality risk (30-day all-cause death: adjusted odds ratio [aOR], 1.99; 95% confidence interval, 1.08– 3.18; P=0.017 and long-term all-cause death: adjusted hazard ratio [aHR], 1.32; 95% confidence interval, 1.19– 1.47; P< 0.001).
Conclusion: Hypochloremia is mildly common in patients with CAD and is associated with increased short-and long-term mortality. Meanwhile, it is necessary to further investigate effective and preventive measures and the potential mechanisms of hypochloremia in patients with CAD.
Keywords: hypochloremia, coronary artery disease, prevalence, short- and long-term mortality
Introduction
Several clinical studies have shown hypochloremia is an independent predictor for short-term mortality among patients with cardiovascular disease (CVD) such as acute or chronic heart failure1–4 and hypertension.5 The association of hypochloremia with short-term mortality is more pronounced in patients with chronic kidney disease (CKD) and postoperative patients with coronary artery disease (CAD) confirmed by coronary angiography (CAG).6–8 The correlation between hypochloremia and long-term mortality is poorly understood, but potential mechanisms are linked to the loss of gastrointestinal tract, fluid-related imbalance of adrenal hormones, and inflammatory response.9,10
CAD forms a major proportion of all CVDs and is the leading cause of global morbidity and mortality, which is often accompanied with heart failure, hypertension, and CKD.11,12 Given the significant role of hypochloremia in patients with CVD, we hypothesized that it may be also an important prognostic factor of short- and long-term mortality in patients with CAD. Therefore, we aimed to assess the prevalence and mortality (short- and long-term) of hypochloremia among CAD patients.
Patients and Methods
Data Collection
From January 2007 to December 2018, data were extracted from the electronic clinical management records system of the Guangdong Provincial People’s Hospital (ClinicalTrials.gov NCT04407936). The baseline information included patient demographics, laboratory test results, mortality, and other clinical variables. Patients’ blood samples were collected early morning after they were fasted overnight for measurement of baseline chloride ion concentration. Drugs and other treatments at admission are judged by experienced clinicians. Data on follow-up information was mainly performed by telephone or clinical visit and partly missing dates will be obtained from the Guangdong Provincial Public Security which was matched to the electronic Clinical Management System of the Guangdong Provincial People’s Hospital records according to the unique ID number of patients.
Study Population
This was a single-center, observational, retrospective cohort study that included patients with CAG-confirmed CAD according to the 10th Revision Codes of the International Classification of Diseases (ICD-10; I20.xx–I25.xx, I50.00001, and I91.40001, Supplemental Table S1) at the Guangdong Provincial People’s Hospital, Guangdong, China, from January 2007 to December 2018. Coronary angiography or percutaneous coronary intervention (PCI) was performed following standard clinical practice guidelines.13,14 We excluded patients with missing data on follow-up evaluation and serum chloride levels and those with cancer. Eventually, 49,025 patients were included for the final analysis (Figure 1).
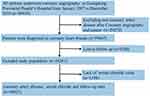 |
Figure 1 The flow of participants through the trial. |
The study protocol was approved by the GDPH ethics committee (No. GDREC2019555H [R1]), and the study was performed according to the tenets of the Helsinki declaration. All identifiable personal information were removed from the analytic dataset to protect patients’ privacy. The ethics committee of Guangdong Provincial People’s Hospital waived the need for informed consent for this study because it was a a retrospective study. We hided all the personal information of patients, and the study would not cause any damage to patients.
Endpoints and Definition
The primary endpoint of this study was the 30-day postadmission all-cause death and long-term all-cause death. Hypochloremia was defined according to the baseline chloride concentration (<98 mmol/L) of venous blood drawn at admission. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation.15 Acute myocardial infarction (AMI), diabetes mellitus (DM), and hypertension were defined by the 10th Revision Codes of the International Classification of Diseases (Supplemental Table 1).
Statistical Analysis
Descriptive statistics are reported as the mean (standard deviation [SD]), or number and percentage when appropriate. Differences between groups were analyzed using the Student’s t-test. Pearson chi-squared tests were used to analyze categorical data.
Time-to-event data were presented graphically using Kaplan–Meier curves (Figure 3 and Supplemental Figure 1). Log rank tests were used to compare survival between groups. Restricted cubic splines were used to investigate the associations of serum chlorine levels with long-term all-cause death and the 30-day all-cause death after adjusting for age and sex (Figure 2). To assess the association between hypochloremia and the study endpoints, a logistic regression model (for 30-day all-cause mortality) and a Cox regression model (for long-term all-cause mortality and 30-day all-cause mortality) was fitted (Table 2 and Supplemental Table 2). Model 1 was unadjusted; model 2 was to adjusted for age (as a continuous variable) and sex; and model 3 included the variables that were associated with mortality according to clinical experience (it included the variables from Models 1 and 2, history of present illness information, and drugs information). Finally, we defined the results of Model 3 as the primary results. We also performed a subgroup analysis among six prespecified subgroups (sex, acute coronary syndromes [ACS], DM, CKD, use of diuretics, and older[age>65 years]) to assess the impact of hypochloremia on long-term all-cause mortality among CAD patients. All P-values were two-sided, and P<0.05 was considered to indicate statistical significance. All statistical analyses were performed using R (ver. 4.0.3).
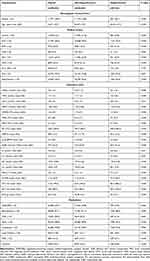 |
Table 1 Baseline Characteristics of the Patients |
 |
Table 2 The Association Between Hypochloremia and the Study Endpoints in Different Models |
 |
Figure 2 Restricted spline curve for the chloride ion hazard ratio. (A) long-term mortality; (B) 30-day mortality; adjusted for age and sex. |
Results
The Characteristics of Patients
According to the cut-off value of the serum chloride level (98 mmol/L), 49,025 patients with CAD were divided into two groups (hypochloremia [<98 mmol/L] vs non-hypochloremia [≥98 mmol/L]). The percentage of patients with hypochloremia was 4.4% (n=2162). The mean age of the cohort was 63.0±10.7 years; 24% patients were male; 9773 had AMI (20.0%); 3074 had pre-MI (6.3%); and 35,755 (73%) underwent PCI.
Serum chloride was normally distributed, and the mean serum chloride level in the hypochloremia and non-hypochloremia groups was 105.0±3.1 mmol/L and 95.1±2.9 mmol/L, respectively. Patients with CAD who had hypochloremia tended to be older and of female sex. Patients with hypochloremia were positively associated with CHF (1.0% vs 0.5%, P=0.006); DM (42.7% vs 26.9%, P<0.001); hypertension (62.6% vs 56.2%, P<0.001); anemia (39.8% vs 31.8%, P<0.001); CKD (37.9% vs 21.4%, P<0.001); and ACS (52.3% vs 44.2%, P<0.001). Some biochemical markers such as high-density lipoprotein cholesterol, sodium ions, albumin, hepatic enzymes, and routine blood index were associated with hypochloremia. In addition, patients with hypochloremia often used more diuretics, either loop diuretics or mineralocorticoid receptor antagonists. More details of the baseline characteristics of patients enrolled are shown in Table 1.
Chloride Levels and Clinical Endpoints
During a median follow-up of 5.2 (3.1–7.8) years, a total of 6486 (13.2%) patients died. In the 30-day follow-up after admission, 538 patients (1.1%) died of all causes. In hypochloremia patients, an inverse association was observed between the hazard ratio (HR)/odds ratio (OR) for the endpoints and chloride level (Figure 2). Kaplan–Meier analysis showed that hypochloremia would have a higher long-term all-cause mortality than non-hypochloremia. (Figure 3, log-rank, p<0.0001) The relationships between all-cause mortality and hypochloremia were evaluated using Cox proportional hazards models, which showed that hypochloremia was associated with a higher risk of 30-day all-cause death even after full adjustment of major confounders (OR: 1.99, 95% CI: 1.08–3.38, P<0.01) than normal serum chloride level (Table 2). Importantly, it suggested that admission chloride level was an independent predictor of long-term all-cause death. Hypochloremia was associated with a higher risk of long-term all-cause death (model 1: HR 1.79, 95% CI 1.64–1.97, P<0.001; model 2: HR 1.65, 95% CI 1.50–1.81, P<0.001; and model 3: HR 1.32, 95% CI 1.19–1.47, P=0.017) than normal serum chloride level (Table 2).
 |
Figure 3 Kaplan–Meier curves for long-term all-cause mortality of hypochloremia. |
In the subgroup analysis, Cox regression analysis revealed that hypochloremia had a relatively consistent risk of mortality across dichotomized subgroups (DM, CKD, age>65 years, sex, ACS, and use of diuretics) (Table 3).
 |
Table 3 Hazard Ratios for the Long-Term All-Cause Mortality in Different Subgroups (Hypochloremia Vs Non- Hypochloremia) |
Discussion
To our knowledge, this is the first study to explore the prevalence of and mortality associated with hypochloremia among CAD patients. Our study indicated that 4.4% CAD patients had hypochloremia, and an inverse association was found between admission chloride levels and short- and long-term mortality among patients with CAD. Further, similar results were observed among the different subgroups.
Chloride is the most abundant anion in the extracellular fluid.10 Some studies have reported that the regulation of macula densa renin secretion in the kidneys may be dependent on chloride.16 Chloride plays an important role in the acid-base balance, muscle activity, and immune regulation.17 The adverse outcomes of hyponatremia have been elucidated in previous studies.18–23 Despite being a counter anion of sodium, chloride has not gained as much attention as sodium, but in recent years, chloride ion studies have gained more focus.1,2,5,6,24–28 An earlier study has shown that hypochloremia observed within 48 h after surgery was not rare and was independently associated with the increased risk of in-hospital mortality.7 However, the evidence of prognostic value for patients with CAD is not sufficient. Our study reveals that chloride could be a prognostic factor for patients with CAD, which is consistent with some previous studies.
The exact mechanisms underlying the link between hypochloremia and CAD are unknown; hence, several hypotheses have been proposed. First, a likely reason for the relation of hypochloremia to short- and long-term mortality is that low chloride levels might upregulate inflammatory cytokines that could promote the inflammation process29 and are well-known predictors of poor outcome and mortality in CAD.30–32 Second, chloride ions have a critical role in regulatory pathways central to physiological stability. For example, low chloride reflects a high anion gap and high renin levels,5,33,34 associated with high blood pressure.35 Additionally, several chloride ion channels with important regulatory functions have been identified or further characterized recently.20,31 Third, according to our results, hypochloremia could be considered a marker of clinical complexity, because it is observed more often in older patients with DM, CKD, hypertension, and anemia than others, which eventually leads to a significantly increased mortality risk.32–36
Given the growing body of literature demonstrating the risk of hypochloremia, we conducted research relevant to the relation between hypochloremia and CAD mortality. We evaluated the impact of outcomes among CAD patients. It is possible that hypochloremia is a therapeutic target that could be amenable to treatment with acetazolamide or chloride supplementation. Our study suggests that hypochloremia is a therapeutic target that is amenable to treatment with chloride supplementation in CAD patients. Moreover, it is also important to prospectively evaluate the mechanism of hypochloremia on the outcome (short- and long-term mortality) in all CAD patients.
Limitations
Our study has some limitations. First, this was a single-center retrospective cohort study with selection bias. For example, patients with more severe CAD may have been recruited into this study than other studies, because our patients were enrolled from the largest cardiovascular center in southern China. However, sizeable data extracted from medical records is allowed to control a variety of confounders in analyses. Second, information about cause-specific death including cardiovascular mortality was not available, and it is difficult to examine the significant correlation between hypochloremia and cause-specific death. Further studies are needed to explore the relationship between hypochloremia and cardiovascular death among CAD patients. Third, data of chloride levels were only extracted at admission without assessment of changes after discharge. Although the status of chloride and the effect of its changes were still unknown after discharge, the follow-up patient information about chloride is being collected for future analysis. In addition, although we have information on the use of diuretics, the information on dose is lacking. Therefore, this could not be analyzed further. Fourth, certain unknown and unmeasured risk factors remained in the analyses after adjusting various confounders to control bias. Last, blood gas analysis is the fundamental approach to study electrolyte disorders. As the diagnosis of hypochloremia in our study was based on venous blood, we ignored arterial chloride levels. Although we included all patients with coronary heart disease, not all data on arterial blood gas. In subsequent studies, it may be possible to analyze the prognostic differences of chloride levels in arterial and venous blood.
Conclusion
Hypochloremia assessment could allow clinicians to identify patients with CAD at elevated risk for short- and long-term mortality. Adequate assessment of hypochloremia status and necessary chloride supplementation may help to improve the prognosis of patients with CAD. This information can have a substantial clinical impact on CAD patients with hypochloremia by prevention and active intervention in the early stage.
Data Sharing Statement
No additional data are available.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Role of the Funders/Sponsors
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Funding
This research was funded and supported by the National Key Research and Development Program of China, Grant (2016YFC1301202); Multi-center study on key techniques for prevention, diagnosis and treatment of high risk coronary artery disease (DFJH2020026).
Disclosure
Ms Ronghui Tang reports grants from Beijing Lisheng Cardiovascular Health Foundation and from Guangdong Provincial People's Hospital Foundation, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Testani JM, Hanberg JS, Arroyo JP, et al. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016;18(6):660–668. doi:10.1002/ejhf.477
2. Grodin JL, Simon J, Hachamovitch R, et al. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66(6):659–666. doi:10.1016/j.jacc.2015.06.007
3. Radulović B, Potočnjak I, Dokoza Terešak S, et al. Hypochloraemia as a predictor of developing hyponatraemia and poor outcome in acute heart failure patients. Int J Cardiol. 2016;212:237–241. doi:10.1016/j.ijcard.2016.03.081
4. Ter Maaten JM, Damman K, Hanberg JS, et al. Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure. Circ Heart Fail. 2016;9(8):Aug. doi:10.1161/circheartfailure.116.003109
5. McCallum L, Jeemon P, Hastie CE, et al. Serum chloride is an independent predictor of mortality in hypertensive patients. Hypertension. 2013;62(5):836–843. doi:10.1161/hypertensionaha.113.01793
6. Mandai S, Kanda E, Iimori S, et al. Association of serum chloride level with mortality and cardiovascular events in chronic kidney disease: the CKD-ROUTE study. Clin Exp Nephrol. 2017;21(1):104–111. doi:10.1007/s10157-016-1261-0
7. Kimura S, Matsumoto S, Muto N, et al. Association of serum chloride concentration with outcomes in postoperative critically ill patients: a retrospective observational study. J Intensive Care. 2014;2(1):39. doi:10.1186/2052-0492-2-39
8. Li Z, Xing C, Li T, Du L, Wang N. Hypochloremia is associated with increased risk of all-cause mortality in patients in the coronary care unit: a cohort study. J Int Med Res. 2020;48(4):300060520911500. doi:10.1177/0300060520911500
9. Cuthbert JJ, Bhandari S, Clark AL. Hypochloraemia in patients with heart failure: causes and Consequences. Cardiol Ther. 2020;9(2):333–347. doi:10.1007/s40119-020-00194-3
10. Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14(4):226. doi:10.1186/cc9052
11. Dai H, Much AA, Maor E, et al. Global, regional, and national burden of ischemic heart disease and its attributable risk factors, 1990–2017: results from the global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2020. doi:10.1093/ehjqcco/qcaa076
12. Khodaminasab A, Reisi M, Vahedparast H, Tahmasebi R, Javadzade H. Utilizing a health-promotion model to predict self-care adherence in patients undergoing coronary angioplasty in Bushehr, Iran. Patient Prefer Adherence. 2019;13:409–417. doi:10.2147/ppa.S181755
13. Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60(7):645–681. doi:10.1016/j.jacc.2012.06.004
14. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-Elevation Myocardial Infarction: an Update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA Guideline for the management of ST-Elevation myocardial infarction. J Am Coll Cardiol. 2016;67(10):1235–1250. doi:10.1016/j.jacc.2015.10.005
15. Aguiar-Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. 2010;139(1):68–74. doi:10.1016/j.ijcard.2008.10.006
16. Lorenz JN, Weihprecht H, Schnermann J, Skøtt O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol. 1991;260(4 Pt 2):F486–93. doi:10.1152/ajprenal.1991.260.4.F486
17. Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23(3):203–211. doi:10.1016/j.ejim.2011.11.013
18. De Luca L, Klein L, Udelson JE, et al. Hyponatremia in patients with heart failure. Am J Cardiol. 2005;96(12):19l–23l. doi:10.1016/j.amjcard.2005.09.066
19. O’Connor CM, Ahmad T. The role of sodium and chloride in heart failure: Does It Take Two to Tango? J Am Coll Cardiol. 2015;66(6):667–669. doi:10.1016/j.jacc.2015.05.070
20. Mapa B, Taylor BE, Appelboom G, Bruce EM, Claassen J, Connolly ES
21. Carcel C, Sato S, Zheng D, et al. Prognostic significance of hyponatremia in acute intracerebral hemorrhage: pooled analysis of the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Crit Care Med. 2016;44(7):1388–1394. doi:10.1097/ccm.0000000000001628
22. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–1026. doi:10.1056/NEJMoa0801209
23. Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(6):1652–1660. doi:10.1053/j.gastro.2006.02.010
24. Zhang Y, Peng R, Li X, Yu J, Chen X, Zhou Z. Serum chloride as a novel marker for adding prognostic information of mortality in chronic heart failure. Clin Chim Acta. 2018;483:112–118. doi:10.1016/j.cca.2018.04.028
25. Ferreira JP, Girerd N, Duarte K, et al. Serum chloride and sodium interplay in patients with acute myocardial infarction and heart failure with reduced ejection fraction: an analysis from the high-risk myocardial infarction database initiative. Circ Heart Fail. 2017;10(2). doi:10.1161/circheartfailure.116.003500
26. Kubota K, Sakaguchi Y, Hamano T, et al. Prognostic value of hypochloremia versus hyponatremia among patients with chronic kidney disease-a retrospective cohort study. Nephrol Dial Transplant. 2020;35(6):987–994. doi:10.1093/ndt/gfy299
27. Grodin JL, Verbrugge FH, Ellis SG, Mullens W, Testani JM, Tang WH. Importance of abnormal chloride homeostasis in stable chronic heart failure. Circ Heart Fail. 2016;9(1):e002453. doi:10.1161/circheartfailure.115.002453
28. Naal T, Abuhalimeh B, Khirfan G, Dweik RA, Tang WHW, Tonelli AR. Serum chloride levels track with survival in patients with pulmonary arterial hypertension. Chest. 2018;154(3):541–549. doi:10.1016/j.chest.2018.04.022
29. Yang H, Huang LY, Zeng DY, et al. Decrease of intracellular chloride concentration promotes endothelial cell inflammation by activating nuclear factor-κB pathway. Hypertension. 2012;60(5):1287–1293. doi:10.1161/hypertensionaha.112.198648
30. Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–589. doi:10.1093/eurheartj/eht367
31. Montecucco F, Liberale L, Bonaventura A, Vecchiè A, Dallegri F, Carbone F. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep. 2017;19(3):11. doi:10.1007/s11883-017-0646-1
32. Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi:10.1056/NEJMoa032804
33. Taylor EN, Forman JP, Farwell WR. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension. 2007;50(2):320–324. doi:10.1161/hypertensionaha.107.092643
34. Kotchen TA, Luke RG, Ott CE, Galla JH, Whitescarver S. Effect of chloride on renin and blood pressure responses to sodium chloride. Ann Intern Med. 1983;98(5 Pt 2):817–822. doi:10.7326/0003-4819-98-5-817
35. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi:10.1093/eurheartj/ehy339
36. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi:10.1093/eurheartj/ehz486
37. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi:10.1093/eurheartj/ehz425
38. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
39. Collet JP, Thiele H, Barbato E, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;29. doi:10.1093/eurheartj/ehaa575
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
