Back to Journals » International Journal of General Medicine » Volume 14
Prevalence and Genotype Distribution of Human Papillomavirus Among Attendees at a Sexually Transmitted Diseases Clinic in Urban Tianjin, China
Authors Xiang J, Han L, Fan Y, Feng B, Wu H, Hu C, Qi M, Wang H, Liu Q, Liu Y
Received 25 February 2021
Accepted for publication 21 April 2021
Published 20 May 2021 Volume 2021:14 Pages 1983—1990
DOI https://doi.org/10.2147/IJGM.S308215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Junqiu Xiang, Long Han, Yanfei Fan, Bin Feng, Haoqing Wu, Chunmin Hu, Manli Qi, Huiping Wang, Quanzhong Liu, Yuanjun Liu
Department of Dermatovenereology, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China
Correspondence: Yuanjun Liu
Department of Dermatovenereology, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin, 300052, People’s Republic of China
Tel +86-22-60362902
Fax +86-22-60362917
Email [email protected]
Background: Human papillomavirus (HPV) is linked to various cancers in males and females. The prevalence and genotype distribution of HPV vary depending on geographical region and the immunity provided by vaccines. Investigation of HPV epidemiology is of great meaning for the development of prevention programs.
Methods: From January 2017 to September 2019, using PCR-reverse dot blot hybridisation, we determined the HPV subtypes in 2801 patients 17– 89 years old at the sexually transmitted diseases (STD) clinic of Tianjin Medical University General Hospital.
Results: The HPV infection rate was 50.79% in males and 50.64% in females. The most common HPV genotype in males and females was HPV6 (30.15% and 30.43%), followed by HPV16 (18.76% and 20.65%) and HPV11 (14.61% and 15.67%). Infection with a single HPV subtype predominated in both males and females, and the rate in males was higher than in females. By contrast, the rate of high-risk HPV (hrHPV) and low-risk HPV (lrHPV) mixed infection in females was higher than in males. Most HPV-positive patients were 20– 39 years of age. The prevalence of infection with only hrHPV differed among the age groups; the peak age was 50 to 59 years.
Conclusion: The HPV prevalence was higher among the STD clinic outpatients than the general population. Therefore, a large-scale survey of high-risk populations is needed. It is anticipated that HPV vaccines, regular education and physical examinations may have a significant impact on the prevention of HPV-related diseases in high-risk groups.
Keywords: human papillomavirus, prevalence, genotyping, HPV vaccination
Introduction
Human papillomavirus (HPV) is a small, double-stranded DNA virus that replicates within skin and mucosal epithelial cells, and HPV is mainly transmitted via sexual contact.1,2 The International Human Papillomavirus Reference Center recognises 227 HPV subtypes of 52 species [www.hpvcenter.se, accessed on 2020-03-05]. The two most common low-risk (lr)HPV subtypes are HPV6 and HPV11, which cause genital warts and intraepithelial neoplasia. Persistent infection of hrHPV can lead to carcinogenesis, and more than half of HPV-related cancers are caused by HPV16 and 18. The quadrivalent HPV vaccine covers the four most common HPV types (HPV6, 11, 16, and 18).3–5
HPV imposes an enormous burden on the global healthcare system. Annually, 630,000 cancer cases (4.5% of all cancer cases) can be attributed to HPV, of which 83.0% are cervical cancer. Several million females die from cervical cancer each year, and head-and-neck, anal, penile, vaginal, and vulvar cancers also have high mortality rates.5,6 Furthermore, the aetiology of > 80% of cases of cervical cancer is HPV infection.7 In addition, the incidence of HPV-associated anogenital and oropharyngeal cancers in males has increased, and HPV infection may be associated with prostate cancer.8,9 And males can transmit HPV to females through sexual contact, therefore, HPV infection in males warrants greater attention. It is estimated in 2018 that 106,430 women in China are diagnosed with cervical cancer each year, accounting for about 19.71% of the global total, of which 47,739 women die of cervical cancer each year. The incidence of cervical cancer compared with other cancers ranks sixth among women in china and third among women between the ages of 15 and 44.10 Since 2006, the approval of HPV vaccine is a promising step towards the global eradication of cervical cancer initiated by the World Health Organization (WHO).11 The primary target group for HPV vaccination is females 9–14 years old and those who have yet to engage in sexual activity, as recommended by the WHO in 2017. Epidemiological data on HPV infection in males are sparse, and HPV vaccination is not recommended for males in most countries.12 As of January 2020, 104 countries have incorporated HPV vaccination into their national immunization programmes (NIP).13 In China, universal cervical cancer screening begin in 2009, and the HPV vaccine is not approved until 2016. However, Italy has provided free vaccines to all 11-year-old girls since 2008, and the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV) is now committed to supporting vaccination programmes for children and adolescents, which is very meaningful in reducing the incidence of cervical, vaginal and vulvar lesions. In addition, Some developed countries have initiated gender-neutral immunization programs.14,15 These measures in developed countries have played an important catalytic role in the popularization of HPV vaccine in developing countries. The (CFDA) of the China Food and Drug Administration approved the first HPV vaccine Cervarix (bivalent HPV) in 2016. The other two prophylactic vaccines, Gardasil (quadrivalent HPV) and Gardasil 9 (nonavalent HPV), as well as Cecolin, the the first domestic HPV vaccine, were subsequently approved in Chinese mainland. But these vaccines are expensive and the supply is limited. These vaccines are mainly purchased by women between the ages of 18 and 45, while girls between the ages of 9 and 14 are still largely unvaccinated.16–18 At present, HPV vaccine has not been included in the NIP in China, and people can only be vaccinated at their own expense. Coupled with the lack of vaccination awareness, knowledge and the high price of HPV vaccine, HPV vaccination coverage is low. A survey in 2019 shows that less than 3% of women on Chinese mainland have been vaccinated.14
HPV is one of the most common STDs worldwide, and most sexually active males and females are exposed to it during their lifetime.19 STD clinics enable the collection of HPV epidemiological data. The prevalence and genotype distribution of HPV in China differ geographically.20 Tianjin is a major city in northern China, but there are no HPV epidemiological data on high-risk groups in Tianjin. We investigated the HPV prevalence and subtype distribution among STD outpatients at Tianjin Medical University General Hospital.
Materials and Methods
Study Population
From January 2017 to September 2019, 2801 outpatients at the STD clinic of Tianjin Medical University General Hospital were enrolled. Patients were included if they met one of the following criteria: (1) engaged in sexual practices that may lead to transmission; (2) had a current or recent STD; and (3) had a history of STD or had sexual intercourse with an unstable partner without a condom in the prior 6 months. Exclude those who had been tested positive for HPV in STD clinic of Tianjin Medical University General Hospital between January 2017 and September 2019.
Specimen Collection
The samples were collected by physicians in the STD clinic of Tianjin Medical University General Hospital. Sampling was performed as follows: (1) males—the skin and mucous membrane of the urethral orifice, glans, coronal sulcus, and distal prepuce was scraped several times using male swabs; (2) females—the physician fully exposed the cervix using a vaginal speculum, removed excessive secretions, and used a cervical brush to rotate the cervical orifice three to five times, and placed the brush head in cell preservation solution. Samples were tested on the day of collection whenever possible. If not tested immediately, samples were stored at 4°C and tested within 1 week.
Detection and Typing of HPV
HPV (23 subtypes) genotyping kits (PCR-reverse dot blot hybridisation) were provided by Yaneng BIOscience (Shenzhen) Co., Ltd. The tests were conducted by trained technicians according to the manufacturer’s instructions. The following 23 HPV subtypes were detected: lrHPV6, 11, 42, 43, 81, and 83 and high-risk (hr) HPV16, 18, 3L, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82.
Statistical Analysis
The data were entered into a Microsoft Excel file and subjected to statistical analysis using SPSS software (version 25; IBM). Numerical data are expressed as rates (%), stratified by age and gender, and were analysed by χ2 test. A value of P < 0.05 was considered indicative of statistical significance.
Results
Overall Epidemiology of HPV
The study population consisted of 1711 males of average age 34.73 ± 9.52 years (range, 17–89 years) and 1090 females of average age 32.19 ± 8.71 years (range, 17–68 years). Among them, 1421 (50.73%) subjects were HPV positive, comprising 869 males and 552 females. The rates of HPV infection were 50.79% and 50.64% in male and female subjects, respectively. The HPV infection rate in females was similar to that in males (χ2 = 0.006, P > 0.05).
Genotype Distribution
All 23 HPV genotypes were detected. Among males the rates of infection with HPV6 and HPV16 were 30.15% and 18.76%, respectively, followed by HPV11 (14.61%), HPV42 (12.31%), HPV51 (11.05%), and HPV52 (11.05%). HPV6 and HPV16 also predominated in females, accounting for 30.43% and 20.65%, respectively, followed by HPV11 (16.67%), HPV52 (14.86%), HPV58 (11.23%), and HPV42 (10.69%). There were significant differences in the HPV18 (3.57% vs 5.98%), 52 (11.05% vs 14.86%), 56 (6.56% vs 10.33%), and 73 (1.27% vs 2.72%) infection rates between males and females (all P < 0.05). There was no significant difference in the rate of infection with other HPV subtypes between males and females (Figure 1, Table 1).
 |
Table 1 HPV Genotype Distribution of 1421 Patients |
 |
Figure 1 Comparison of 1421 cases of male and female patients with HPV genotype distributions. |
Single and Multiple HPV Infections
The overall rates of infection with single and multiple HPV subtypes were 28.17% (482/1711) and 22.62% (387/1711) in males and 24.86% (271/1090) and 25.78% (281/1090) in females, respectively. The rate of infection with a single HPV subtype was 55.47% (482/869) and 49.09% (271/552) in males and females, respectively (χ2 = 5.502, P < 0.05). The rate of infection with two HPV subtypes was 24.97% (217/869) and 24.46% (135/552) in males and females, respectively, and that of infection with three HPV subtypes was 11.85% (103/869) and 13.04% (72/552), respectively (χ2 = 0.048, 0.433, P > 0.05). The rate of infection with more than three subtypes was 7.71% (67/869) and 13.41% (74/552) in males and females, respectively (χ2 = 12.253, P < 0.05). A 44-year-old male was infected with 12 HPV subtypes (HPV6, 11, 42, 81, 16, 33, 39, 51, 56, 58, 66, and 68) (Figure 2, Table 2).
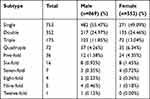 |
Table 2 The Distribution of Single or Multiple Genotypes in Male and Female Patients |
 |
Figure 2 Constituent ratio of single or multiple genotypes in male and female patients. |
Among the 753 patients infected with a single HPV subtype, 357 cases were lrHPV and 396 were hrHPV (χ2 = 2.020, P > 0.05). The rate of infection was 3.52% (50/1421) for multiple lrHPV, 11.40% (162/1421) for multiple hrHPV, and 32.09% (456/1421) for mixed lrHPV and hrHPV (P < 0.01). The rate of mixed hrHPV and lrHPV infection was significantly higher in females (198/552, 35.87%) than in males (258/869, 29.69%) (χ2 = 5.917, P < 0.05) (Table 1).
Prevalence of HPV According to Age
The subjects were classified into six age groups (< 20, 20–29, 30–39, 40–49, 50–59, and ≥ 60 years). The range prevalence of HPV was 40.00–58.33% (χ2 = 5.986, P > 0.05). Male and female patients with HPV infection in the 20–39-year-old group accounted for 74.57% and 80.98%, respectively, of the total. In the < 20-, 50–59-, and > 60-year-old groups, the rate of HPV infection was < 7%. The rate of hrHPV infection was highest in the 50–59-year-old group (29.41%) (χ2 = 12.558, P < 0.05). The < 20-year-old group had the lowest rate of hrHPV infection. There was no significant difference in the lrHPV infection rate (χ2 = 6.700, P > 0.05) or the hrHPV and lrHPV mixed infection rate (χ2 = 5.890, P > 0.05) among the six age groups (Figure 3, Table 3).
 |
Table 3 The Age-Specific Prevalence of High-Risk, Low-Risk and Mixed HPV Infection |
 |
Figure 3 Comparison of the age distribution of HPV infection in male and female patients. |
Discussion
Most prior studies focused on HPV infection among females in the general population; few evaluated the epidemiology of HPV infection in males and females in high-risk groups. This is the first epidemiological survey of HPV infection in a high-risk group in Tianjin, China.
The overall HPV infection rate in females was 50.64%, higher than that in Tianjin Province (14.71%) and Xinjiang Province (14.02%);21,22 this might be because those studies involved only females. In addition, it was higher than the rate of HPV in Sichuan Province (24.1%) among hospitalised female patients tested for HPV.23 Guangzhou Kingmed Diagnostics is the largest independent laboratory in China. This laboratory performed HPV genotyping of 51,345 cervical samples from 364 hospitals, clinics, and physical examination centres in Guangdong (55%) and other provinces (45%). The total HPV infection rate was 26.0%,24 lower than in this work. The HPV infection rate of males was 50.79%, similar to that among male patients at an STD clinic in Beijing (44.44%).25 The HPV infection rates among healthy males in the United States (Tampa, Florida), Brazil (Sao Paulo), and Mexico (Curnahuaca) were reportedly 32.5–34.5%,26 lower than in this study. Therefore, STD outpatients are at high risk of HPV infection because of their greater exposure to STDs.
In this study, HPV6 (30.23%) and HPV11 (15.41%) were the two most common lrHPV subtypes, and the two most common hrHPV subtypes were HPV16 (19.49%) and HPV52 (12.53%). The most commonly detected genotypes were identical in males and females. Among females, the main subtypes of HPV were HPV16, 58, 18, and 66 in Tianjin;21 HPV16, 58, 52, and 56 in Sichuan Province;27 and HPV16, 52, 58, and 53 in Xinjiang Province.22 The main HPV genotypes among females were HPV16, 6, 58, and 11.24 The main genotypes among married females in Zhejiang and Shanxi Provinces were HPV52, 58, 16, and 33 and HPV16, 58, 52, and 33, respectively.28,29 HPV16, HPV58, HPV52, HPV33 and HPV31 were the most common genotypes in the subpopulation of CIN2/3 patients in china.30 Some studies have shown that HPV-16, HPV-33, HPV18, HPV31 and HPV45 were the most common genotypes in Italian CIN3 patients.31–33 HPV6, 11, 31, and 16/18 were the major genotypes among males at an STD clinic in Beijing.25 In Urban Chengdu, Xi’an, Taiyuan, and Shenzhen, the main genotypes among men-who-have-sex-with-men were HPV6, 18, 16, and 11.34,35 HPV6, 11, 52, and 16 were the four most common HPV subtypes among patients with genital warts in Urban Xi’an.36 Therefore, the distribution of HPV genotypes differs among Chinese regions and populations and so epidemiological surveys of HPV are needed. Although the most common HPV subtypes in high-risk populations varied geographically, HPV6, 11, and 16 were consistently among the most common subtypes. The nine-valent HPV vaccine (Gardasil 9) (HPV6, 11, 16, 18, 31, 33, 45, 52, and 58) covers the main genotypes in high-risk groups, but in China is approved only for females 16–26 years old.
Infection with a single HPV subtype predominated in both sexes. The single infection rate in females was lower than that in males. The rate of infection with multiple HPV subtypes in females was 25.78%, higher than that in Tianjin, Sichuan, and Xinjiang Provinces (7.72%, 21.0%, and 19.08%, respectively).21–23 Multiple infections accounted for 46.60% of male patients with HPV infection at STD clinics in Beijing,25 similar to our finding (44.53%). The above results suggest that the rate of infection with multiple HPV subtypes in high-risk populations is higher than that in the general population. Infection with a single HPV subtype increases the risk of cervical cancer in females 19.9-fold, compared to 31.8-fold for multiple subtypes.37 Also, cervical infection with multiple HPV subtypes increases the risk of new cervical HPV infection.38 The above findings suggest that females at high risk are more likely to be infected with multiple HPV subtypes, increasing their risk of cervical cancer.
Most patients with HPV infection were 20–39 years old, indicating that sexually active individuals are the main targets of HPV. Medical workers carry out regular education and physical examinations for HPV-related diseases for the purpose of preventing and detecting HPV infection in high-risk groups.
Our data provide insight into the epidemiology of HPV in a high-risk population in Tianjin. However, this study had several limitations. First, the population was limited to those predisposed to HPV infection attending an STD clinic, which is only part of the population at high risk of HPV infection. Second, the subjects were at high risk of HPV infection, and so the findings cannot be extended to the general population. Third, we did not collect data on other relevant factors—such as age at first sexual intercourse, education, smoking, and immune status—which could have shed further light on the epidemiology of HPV infection.
Conclusions
In conclusion, the rate of HPV infection among STD outpatients in Tianjin was high, indicating a considerable burden of HPV infection in high-risk groups. The Gardasil 9 vaccine may be useful for the high-risk population in Tianjin but is not approved for males in China. Our results will inform efforts to develop HPV prevention programs and facilitate the early detection of HPV-related diseases.
Statement of Ethics
The research was performed in accordance with relevant guidelines/regulations and written informed consent was obtained from all participants or their legal guardians. This study was approved by Ethics Committee of Tianjin Medical University General Hospital (NO. IRB2020-WZ-143) and conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Acknowledgment
We sincerely thank the staff of the Department of Dermatovenereology clinic, Tianjin Medical University General Hospital for their support to sample collection.
Funding
This research received no specific grant from any funding agency in the public, commercial, and not-for-profit sectors.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Yilmaz G, Biswas-Fiss EE, Biswas SB. Genetic variations in the DNA replication origins of human papillomavirus family correlate with their oncogenic potential. Biochim Biophys Acta Gen Subj. 2018;1862:979–990. doi:10.1016/j.bbagen.2017.12.010
2. Zur HH. Papillomavirus infections–a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–F78. doi:10.1016/0304-419x(96)00020-0
3. Georgescu SR, Mitran CI, Mitran MI, et al. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress. J Immunol Res. 2018;2018:1–10. doi:10.1155/2018/5315816
4. Sudenga SL, Ingles DJ, Campbell CMP, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM Study. Eur Urol. 2016;69:166–173. doi:10.1016/j.eururo.2015.05.032
5. Serrano B, Brotons M, Bosch FX, et al. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet. 2018;47:14–26. doi:10.1016/j.bpobgyn.2017.08.006
6. Chibwesha CJ, Stringer JSA. Cervical cancer as a global concern: contributions of the dual epidemics of HPV and HIV. J Am Med Assoc. 2019;322:1558–1560. doi:10.1001/jama.2019.16176
7. de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi:10.1002/ijc.30716
8. Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;461:S20–S26. doi:10.1002/ijc.30716
9. Moghoofei M, Keshavarz M, Ghorbani S, et al. Association between human papillomavirus infection and prostate cancer: a global systematic review and meta-analysis. Asia Pac J Clin Oncol. 2019;15:E59–E67. doi:10.1111/ajco.13124
10. Bruni L, Albero G, Serrano B, et al. ICO/IARC information centre on HPV and Cancer (HPV information centre). Human papillomavirus and related diseases in China. Summary Report 17 June 2019. Available from: https://hpvcentre.net/statistics/reports/CHN.pdf?t=1617614054427.,
11. Brisson M, Drolet M. Global elimination of cervical cancer as a public health problem. Lancet Oncol. 2019;20(3):319–321. doi:10.1016/S1470-2045(19)30072-5
12. World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine. 2017;35(43):5753–5755. doi:10.1016/j.vaccine.2017.05.069
13. World Health Organization. Vaccine introduction slides; 2020.
14. Hu S, Xu X, Zhang Y, et al. A nationwide post-marketing survey of knowledge, attitude and practice toward human papillomavirus vaccine in general population: implications for vaccine roll-out in mainland China. Vaccine. 2021;39(1):35–44. doi:10.1016/j.vaccine.2020.11.029
15. Ciavattini A, Giannella L, De Vincenzo R, et al. HPV vaccination: the position paper of the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV). Vaccines (Basel). 2020;8(3). doi:10.3390/vaccines8030354
16. Qiao YL, Wu T, Li CR, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomised clinical trial. J Natl Cancer Inst. 2019;112:145–153. doi:10.1093/jnci/djz074
17. Zhu FC, Chen W, Hu YM, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18–25 years: results from a randomized controlled trial. Int J Cancer. 2014;135:2612–2622. doi:10.1002/ijc.28897
18. Hu YM, Guo M, Li CG, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Sci China Life Sci. 2020;63:582–591. doi:10.1007/s11427-019-9547-7
19. Trottier H, Franco EL. Human papillomavirus and cervical cancer: burden of illness and basis for prevention. Am J Manag Care. 2006;12S:S462–S472.
20. Wang R, Guo X, Wisman GBA, et al. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. Bmc Infect Dis. 2015;15. doi:10.1186/s12879-015-0998-5
21. Chen X, Wallin K, Duan M, et al. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J Med Virol. 2015;87:1966–1972. doi:10.1002/jmv.24248
22. Wang J, Tang D, Wang K, et al. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. Bmc Womens Health. 2019;19. doi:10.1186/s12905-019-0785-3
23. Li B, Wang H, Yang D, et al. Prevalence and distribution of cervical human papillomavirus genotypes in women with cytological results from Sichuan province, China. J Med Virol. 2019;91:139–145. doi:10.1002/jmv.25255
24. Zeng Z, Yang H, Li Z, et al. Prevalence and genotype distribution of HPV infection in China: analysis of 51,345 HPV genotyping results from China’s largest CAP certified laboratory. J Cancer. 2016;7:1037–1043. doi:10.7150/jca.14971
25. Xin HN, Li HJ, Li Z, et al. Genital HPV infection among heterosexual and homosexual male attendees of sexually transmitted diseases clinic in Beijing, China. Epidemiol Infect. 2017;145:2838–2847. doi:10.1017/S0950268817001698
26. Ingles DJ, Lin H, Fulp WJ, et al. An analysis of HPV infection incidence and clearance by genotype and age in men: the HPV Infection in Men (HIM) Study. Papillomavirus Res. 2015;1:126–135. doi:10.1016/j.pvr.2015.09.001
27. Tao G, Yaling G, Zhan G, et al. Human papillomavirus genotype distribution among HPV-positive women in Sichuan province, Southwest China. Arch Virol. 2018;163:65–72. doi:10.1007/s00705-017-3556-1
28. Hong H, He T, Ni H, et al. Prevalence and genotype distribution of HPV infection among women in Ningbo, China. Int J Gynecol Obstet. 2015;131:96–99. doi:10.1016/j.ijgo.2015.04.027
29. Dai M, Bao YP, Li N, et al. Human papillomavirus infection in Shanxi Province, People’s Republic of China: a population-based study. Br J Cancer. 2006;95:96–101. doi:10.1038/sj.bjc.6603208
30. Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. 2020;302(6):1329–1337. doi:10.1007/s00404-020-05787-w
31. Giannella L, Fodero C, Boselli F, et al. Age-related changes in the diagnostic assessment of women with severe cervical lesions. Climacteric. 2015;18(4):617–623. doi:10.3109/13697137.2015.1005592
32. Giannella L, Fodero C, Boselli F, et al. Age-related changes in pre- and post-conization HPV genotype distribution among women with high-grade cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2017;137(1):72–77. doi:10.1002/ijgo.12106
33. Giannella L, Delli CG, Di Giuseppe J, et al. Age-related changes in the fraction of cervical intraepithelial neoplasia grade 3 related to HPV genotypes included in the nonavalent vaccine. J Oncol. 2019;2019:713789. doi:10.1155/2019/7137891
34. Li X, Li M, Yang Y, et al. Anal HPV/HIV co-infection among men who have sex with men: a cross-sectional survey from three cities in China. Sci Rep. 2016;6. doi:10.1038/srep21368
35. Zhang D, Yin Y, Feng T, et al. HPV infections among MSM in Shenzhen, China. PLoS One. 2014;9. doi:10.1371/journal.pone.0096364
36. Zhu C, Wang Y, Mao W, et al. Prevalence and distribution of HPV types in genital warts in Xi’an, China: a prospective study. BMJ Open. 2019;9:e023897. doi:10.1136/bmjopen-2018-023897
37. Lee SA, Kang D, Seo SS, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip. Cancer Lett. 2003;198:187–192. doi:10.1016/s0304-3835(03)00312-4
38. Goodman MT, McDuffie K, Hernandez BY, et al. The influence of multiple human papillomavirus types on the risk of genotype-concordant incident infections of the Anus and Cervix: the Hawaii HPV Cohort Study. J Infect Dis. 2011;203:335–340. doi:10.1093/infdis/jiq058
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
