Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Prevalence and Factors Associated with Microalbuminuria in Pediatric Patients with Type 1 Diabetes Mellitus at a Large Tertiary-Level Hospital in Botswana
Authors Ramaphane T, Gezmu AM , Tefera E , Gabaitiri L, Nchingane S, Matsheng-Samuel M, Joel D
Received 6 August 2021
Accepted for publication 16 October 2021
Published 2 November 2021 Volume 2021:14 Pages 4415—4422
DOI https://doi.org/10.2147/DMSO.S322847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Tshireletso Ramaphane,1 Alemayehu M Gezmu,1 Endale Tefera,1 Lesego Gabaitiri,2 Seeletso Nchingane,3 Motlalekgomo Matsheng-Samuel,3 Dipesalema Joel1
1Department of Pediatrics and Adolescent Health, Faculty of Medicine, University of Botswana, Gaborone, Botswana; 2Department of Statistics, Faculty of Social Sciences, University of Botswana, Gaborone, Botswana; 3Princess Marina Hospital, Department of Pediatrics, Gaborone, Botswana
Correspondence: Alemayehu M Gezmu
Department of Pediatrics and Adolescent Health, Faculty of Medicine, University of Botswana, Bag UB00713, Gaborone, Botswana
Email [email protected]
Introduction: Microalbuminuria is considered the earliest sign of diabetic nephropathy among patients with type 1 diabetes mellitus (T1DM). The prevalence of microalbuminuria among African children with T1DM is reported to be high, yet its prevalence and population-specific risk factors in Botswana are not known.
Aim: This study aimed to determine the prevalence of microalbuminuria among children and young adults with T1DM in Botswana and identify factors associated with microalbuminuria in this population.
Methods: A retrospective cross-sectional study was conducted on 127 T1DM patients aged < 24 years followed at a pediatric endocrinology clinic in Botswana from 2010 to 2017. Clinical, laboratory, and demographic data were collected using chart review and patient surveys. Descriptive statistics were reported as mean and standard deviation for continuous variables, and frequency and percentage for categorical variables. Prevalence of microalbuminuria was calculated as a simple proportion. Group comparison was done using two-sample independent t-test, X2-test, or Fisher’s exact test and logistic regression to assess for associations. Level of significance was set at p< 0.05.
Results: There were a total of 71 (55.9%) females. The mean age was 18.7 (± 5) years and mean duration of T1DM was 6.6 (± 4.6) years. Most study participants were of African descent. The prevalence of microalbuminuria was 28.3%. Group comparison revealed gender (p= 0.040), duration of diabetes (p= 0.002), systolic blood pressure (p=0.003), baseline glycated hemoglobin (HbA1c) (p=0.009) and Tanner’s stage (p=008) to be significantly associated with microalbuminuria. On binary logistic regression, only gender (p=0.039) and baseline HbA1c (p=0.039) were independently associated with the presences of microalbuminuria.
Conclusion: This study identified a high prevalence of microalbuminuria among children and young adults with T1DM in Botswana and reaffirms the importance of early detection, glycemic control, and regular screening to prevent diabetic nephropathy.
Keywords: microalbuminuria, diabetic nephropathy, type 1 diabetes mellitus, pediatrics diabetes
Introduction
Worldwide, diabetic nephropathy accounts for the majority of morbidity and mortality due to type 1 diabetes mellitus (T1DM).1,2 Diabetic nephropathy is associated with end-stage renal disease (ESRD) requiring renal replacement therapy, cardiovascular diseases, escalating health care costs, and premature death.3 A two-year prospective study conducted among American T1DM patients aged 8–18 years found an incidence rate of 10% and 9% for children and adolescents, respectively, but the incidence may be even higher among children in African countries.2
The development of diabetic nephropathy consists of several stages, the earliest being microalbuminuria which can progress to overt proteinuria and ultimately ESRD.11,12 It invariably precedes overt diabetic nephropathy and can be an early sign of vascular damage in both kidneys and heart.13,14 Although microalbuminuria may regress spontaneously in a proportion of cases, it remains the best documented predictor for high risk of development of diabetic nephropathy in T1DM.15
The incidence of microalbuminuria among children with T1DM varies depending on study setting. According to the Oxford Regional Prospective study, the cumulative prevalence of microalbuminuria was 25.7% after 10 years of diabetes and 50.7% after 19 years of diabetes and 5182 patient years of follow-up.4 The prevalence of microalbuminuria reported from African settings is highly variable, ranging from 12% in Tanzania to 51% in Ethiopia, but this variability may be due to diversity of study design and small sample sizes.5–10
Hypertension and poor glycemic control have been found to be the most significant factors associated with microalbuminuria and ultimately ESRD.17 Additionally, the incidence of microalbuminuria in T1DM increases at puberty, a time of exaggerated physiologic insulin resistance and higher androgen and growth hormone production.18 Other risk factors for microalbuminuria identified in previous studies include duration of diabetes, female gender, obesity, dyslipidemia, and genetic predisposition.12,16,19–21
Racial and ethnic differences in the prevalence of diabetic nephropathy and ESRD have also being reported. American patients of African ancestry have been found to be more affected by diabetic nephropathy and complications of ESRD.5 A study conducted in South Africa showed that microalbuminuria was significantly more prevalent in black than in white patients, despite a 50% shorter duration of diabetes for black patients.14 Several factors could have contributed to these differences, including genetic predisposition and/or racial disparities resulting in differential access to care leading to late diagnosis and poor glycemic control.
In Botswana, there are no published studies on T1DM and its associated complications. More studies are needed to ascertain the burden of T1DM in this setting, to characterize its short- and long-term complications, as well as identify risk factors associated with an increased risk of complications and progression to ESRD. Hence, in this study, we aimed to define the prevalence of and identify factors associated with microalbuminuria in pediatric patients with TIDM to better inform prevention measures in this population.
Materials and Methods
A retrospective cross-sectional study was conducted in a Pediatric Endocrinology clinic based in Botswana’s largest tertiary referral hospital. The clinic was established in 2010 and is staffed by two board-certified pediatric endocrinologists and a diabetic nurse. The clinic sees an average of 20 patients per week, the majority of which are diabetic patients. The clinic has access to basic and advanced laboratory services to conduct a range of clinical tests, including urine protein, glycated hemoglobin (HbA1C), renal and liver function tests.
Study inclusion criteria included the following: children and young adults <24 years of age with T1DM and followed at the Pediatric Endocrinology clinic between 2010 and 2017 and had at least two urine chemistry tests completed during that period. Those patients with known causes of proteinuria other than T1DM were excluded. Demographic characteristics, duration of T1DM, HbA1c level, and systolic blood pressure, were collected as exposure variables. Presence and frequency of microalbuminuria, as well as reversion to normoalbuminuria from microalbuminuria, were outcome variables.
Screening and Diagnosis of Microalbuminuria
Using international consensus guidelines from the International Society for Pediatrics and Adolescent Diabetes (ISPAD), patients were initially screened for albuminuria 2–5 years after the diagnosis of T1DM, or at the onset of puberty, whichever came first.22 Diagnosis of microalbuminuria was defined as two or more urine albumin sample values of 30–300 mg/day, and macroalbuminuria was defined as two or more urine albumin sample values of >300 mg/day.
Medical records of all patients who fulfilled the inclusion criteria were reviewed to obtain laboratory results, blood pressure, and glycemic control as well as insulin and/or antihypertensive medication use. A data extraction form was used to capture demographic and clinical characteristics of the participants.
Data Analysis
Data were analysed using Statistical Package for Social Sciences version 27 for Mac (Chicago, USA). Continuous variables were expressed as means ± standard deviation (SD) whereas categorical variables were expressed as frequencies and percentages. The prevalence of microalbuminuria was described in simple proportion. Continuous variables between groups were compared using two-sample independent t-test and categorical variables by Chi Square or Fisher’s exact tests. Factors that demonstrated an association with microalbuminuria were entered into binary logistic regression model to control for confounders. Level of significance (p-value) was set at 0.05.
Results
A total of 180 patients were diagnosed with T1DM from 2010 to 2017 at the study site. Fifty-three patients were excluded from the analysis; 29 did not have urine results in their records while 24 of the patients were not yet eligible for screening according to the inclusion criteria. A total of 127 patients were used for the final analysis (Figure 1).
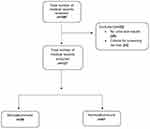 |
Figure 1 Flow diagram of study participants selection. |
There were 71 (55.9%) females. The majority (123; 96.9%) of study participants were of black race. The mean age of the study participants was 18.7 (±5) years with the mean duration of T1DM disease of 6.6 (±4.6) years. Family history of T1DM was found in 66 (52%) of the study participants. Most of the study participants were in the pubertal and post-pubertal stage of development. Only 12 (9.4%) were on angiotensin-converting enzyme inhibitors/Angiotensin II receptor blockers (ACEI/ARB) treatment (Table 1).
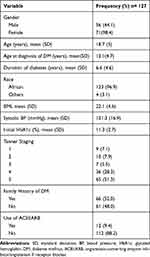 |
Table 1 Sociodemographic Characteristics of Study Participants |
One hundred and seven (84.3%) of the study participants had no concurrent conditions. In the remaining 15.7% of the participants, documented concurrent conditions included cerebrovascular accident (n=2), epilepsy (n=2), hypercholesterolemia (n=2), pulmonary tuberculosis (n=2), asthma (n=1), Bardet–Biedl syndrome (n=1), Beckwith–Wiedemann syndrome (n=1), developmental delay (n=1), Graves’ disease (n=1), human immunodeficiency virus infection (n=1), hypothyroidism (n=1), obesity (n=1), Prader–Willi syndrome (n=1) and Down syndrome (n=1). All the study participants were on insulin treatment, while three of the participants were using both insulin and oral hypoglycemic agents for their obesity treatment.
Microalbuminuria was present in 36 out of 127 study participants giving a prevalence of 28.3% for this cohort. No study participant experienced macroalbuminuria. Gender (p=0.04), duration of T1DM disease (p=0.002), systolic blood pressure (p=0.003), baseline HbA1c (0.009), Tanner stage (p=0.009) and use of ACEI/ARB (p=0.001) had shown statistically significant difference between groups on simple group comparison (Table 2). After adjusting for potential confounders on binary logistic regression model, female gender (p=0.039) and higher initial HgbA1c (p=0.039) were found to be independently associated with the presence of microalbuminuria (Table 3).
 |
Table 2 Clinical Characteristics of Study Participants and Their Association to the Primary Outcome (Microalbuminuria) |
 |
Table 3 Risk Factors Associated with Development of Microalbuminuria Among Patients with T1DM |
Four patients (3%) had remission of microalbuminuria to normoalbuminuria. Three patients had missing information on the use of ACEI/ARB medication. Group comparisons were not made due to the very small number of patients who experienced remission.
Discussion
In this eight-year cross-sectional survey of children and young adults with T1DM in Botswana, we identified a high prevalence of microalbuminuria. Furthermore, female gender and elevated initial HbA1C were found to be independently associated with microalbuminuria in this setting.
The high prevalence of microalbuminuria identified in this study is comparable to other studies carried out in Africa in recent years23, including in neighboring South Africa, where the prevalence of microalbuminuria among patients with T1DM is reported to be 39.7% among those of black race.14 High prevalence in Botswana may be due to genetic predisposition, or barriers to accessing care, leading to late diagnosis and poor glycemic control, which is corroborated by the finding that higher baseline HbA1C was independently associated with the presence of microalbuminuria. This finding re-affirms the well-documented association between poor glycemic control and progression to microalbuminuria and reinforces the importance of glycemic control as the most important modifiable factor to prevent renal dysfunction in patients with both T1DM and Type 2 diabetes mellitus.21,24,26
This study also identified female gender as an independent risk factor associated with the presence of microalbuminuria. This phenomenon has been described in several other studies, including the Oxford Regional Prospective Study.27,28 Factors hypothesized to be driving this difference include changes in growth hormone release and low insulin-like growth factor-1 levels, more commonly reported in girls with T1DM. Furthermore, onset of puberty is earlier in girls and, therefore, adolescent girls may have had higher cumulative exposure to puberty hormones, as compared to adolescent boys.28
Development of microalbuminuria has also been shown to be linked to longer duration of T1DM,12,25 with a peak duration of 10–15 years, according to a 10-year observational study conducted in Denmark.15 Although our study demonstrated a statistically significant association with duration of illness on univariate analysis, this association weakens during the adjusted analysis, potentially due to small sample size as well as the relatively short duration of illness experienced among children and adolescents.
Hypertension and microalbuminuria have been shown to commonly coexist, but it is unclear whether hypertension contributes to the development of microalbuminuria.21 More recent studies have demonstrated this association displays diurnal variation.29 In our study, systolic blood pressure was not found to be statistically significant on logistic regression analysis.
According to the Joslin study on the regression of microalbuminuria in type 1 diabetes mellitus patients, regression of microalbuminuria was common with a 6-year cumulative incidence of 58% among patients aged 15–44 years.30 ACEI/ARB therapy was found to be protective against the development of diabetic nephropathy.30 Our study however showed that only 4 (3%) of the patients during the study period regressed to normoalbuminuria. This may be explained by the fact that, of the 36 patients microalbuminuria, only 12 (33.3%) were on ACEI/ARB therapy. Interestingly though, the 4 patients who regressed to normoalbuminuria were not on ACEI/ARB therapy. It was therefore difficult to identify factors associated with regression due to the small number of patients in this group as well as infrequent use of ACEI’s.
This study had several limitations. First, its cross-sectional nature does not account for sequence of events and any associations detected and thus attributable risk could not be estimated. Second, because a convenience sample was used, we may have lacked sufficient power to detect associations that were present. Additionally, many patients did not have urine testing performed with the frequency, which is necessary to adequately detect microalbuminuria, due to limitations in laboratory capacity.
Bias may have played a role at various parts of this study, starting from enrolment. Patients who were compliant with attending clinic appointments, and thus eligible for enrollment, may have been more likely to adhere to treatment regimens, thus leading to a selection bias and an underestimation of the true prevalence of microalbuminuria among children with T1DM in Botswana. Since this study relied on consistent documentation by healthcare workers, information bias may have been introduced if extra effort was made to search for risk factors in patients who were noted to have microalbuminuria, potentially introducing an information bias. There may also be other unmeasured confounders that might have influenced these results including nocturnal blood pressure, serum lipid levels, and barriers to healthcare access (such as health literacy and household income).
Conclusion
We identified a high prevalence of microalbuminuria among children and young adults with T1DM in Botswana, comparable to most studies done in Africa. The association detected between baseline HbA1c and microalbuminuria in this setting reaffirm the importance of early detection, excellent glycemic, and regular screening in the prevention of diabetic nephropathy.
Ethics Approval and Consent to Participate
All procedures performed and data collected in this study were adhered to the guidelines of Declaration of Helsinki and the ethical standards of institutional ethics review committees. The Institutional Review Boards (IRBs) of the University of Botswana (UBR/RES/IRB/BIO/GRAD/030), the Botswana Ministry of Health and Wellness (HPDME 13/18/1) and research ethics committee of Princess Marina Hospital (PMH 5/79(418-1-2018) granted approval to conduct this research. Waiver for consent was given to collect data from patients’ medical record as this study explores patient information retrospectively. To keep patient confidentiality, all identified medical records were stored in a locked filing cabinet which was accessed only by the investigators. All information collected from the medical records were coded and all patient identifiers were removed.
Acknowledgment
The authors would like to acknowledge Dr Jonathan Strysko for copy editing the manuscript and the parents and patients who took part in this study. The authors would like to acknowledge the support of Children’s Hospital of Philadelphia-Global Health centre (CHOP-Global) for payment of article publishing charges.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348(23):2285–2293. doi:10.1056/NEJMoa021835
2. Alleyn CR, Volkening LK, Wolfson J, Rodriguez‐Ventura A, Wood JR, Laffel LMB. Occurrence of microalbuminuria in young people with type 1 diabetes: importance of age and diabetes duration. Diabet Med. 2010;27(5):532–537. doi:10.1111/j.1464-5491.2010.02983.x
3. Noubiap JJN, Naidoo J, Kengne AP. Diabetic nephropathy in Africa: a systematic review. World J Diabetes. 2015;6(5):759. doi:10.4239/wjd.v6.i5.759
4. Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ. 2008;336(7646):697–701. doi:10.1136/bmj.39478.378241.BE
5. Tesfaye S, Gill G. Chronic diabetic complications in Africa. African J Diabetes Med. 2011;19(1):4–8.
6. Majaliwa ES, Munubhi E, Ramaiya K, et al. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Dar Es Salaam, Tanzania. Diabetes Care. 2007;30(9):2187–2192. doi:10.2337/dc07-0594
7. Lutale JJK, Thordarson H, Abbas ZG, Vetvik K. Microalbuminuria among type 1 and type 2 diabetic patients of African origin in Dar Es Salaam, Tanzania. BMC Nephrol. 2007;8(1):1–8. doi:10.1186/1471-2369-8-2
8. Gill G, Gebrekidan A, English P, Wile D, Tesfaye S. Diabetic complications and glycaemic control in remote North Africa. QJM: Int J Med. 2008;101(10):793–798. doi:10.1093/qjmed/hcn096
9. Macky TA, Khater N, Al-Zamil MA, El Fishawy H, Soliman MM. Epidemiology of diabetic retinopathy in Egypt: a hospital-based study. Ophthalmic Res. 2011;45(2):73–78. doi:10.1159/000314876
10. Elbagir MN, Eltom MA, Mahadi EO, Berne C. Pattern of long-term complications in Sudanese insulin-treated diabetic patients. Diabetes Res Clin Pract. 1995;30(1):59–67. doi:10.1016/0168-8227(95)01146-3
11. Warram JH, Scott LJ, Hanna LS, et al. Progression of microalbuminuria to proteinuria in type 1 diabetes: nonlinear relationship with hyperglycemia. Diabetes. 2000;49(1):94–100. doi:10.2337/diabetes.49.1.94
12. De Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171(5):412–420. doi:10.1001/archinternmed.2011.16
13. Koroshi A. Microalbuminuria, is it so important? Hippokratia. 2007;11(3):105.
14. Kalk WJ, Raal FJ, Joffe BI. The prevalence and incidence of and risk factors for, micro-albuminuria among urban Africans with type 1 diabetes in South Africa: an inter-ethnic study. Int J Diabetes Mellit. 2010;2(3):148–153. doi:10.1016/j.ijdm.2010.10.003
15. Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328(7448):1105. doi:10.1136/bmj.38070.450891.FE
16. Stone ML, Craig ME, Chan AK, Lee JW, Verge CF, Donaghue KC. Natural history and risk factors for microalbuminuria in adolescents with type 1 diabetes: a longitudinal study. Diabetes Care. 2006;29(9):2072–2077. doi:10.2337/dc06-0239
17. Moore THM, Shield JPH. Prevalence of abnormal urinary albumin excretion in adolescents and children with insulin dependent diabetes: the MIDAC study. Arch Dis Child. 2000;83(3):239–243. doi:10.1136/adc.83.3.239
18. Williamson JR, Rowold E, Chang K, et al. Sex steroid dependency of diabetes-induced changes in polyol metabolism, vascular permeability, and collagen cross-linking. Diabetes. 1986;35(1):20–27. doi:10.2337/diab.35.1.20
19. The Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. J Am Med Assoc. 2003;290(16):2159. doi:10.1001/jama.290.16.2159
20. Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29(7):1486–1490. doi:10.2337/dc06-0293
21. Giorgino F, Laviola L, Perin PC, Solnica B, Fuller J, Chaturvedi N. Factors associated with progression to macroalbuminuria in microalbuminuric Type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia. 2004;47(6):1020–1028. doi:10.1007/s00125-004-1413-8
22. Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD clinical practice consensus guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2018;19:262–274.
23. Ahmed H, Elshaikh T, Abdullah M, Wallner M. Early diabetic nephropathy and retinopathy in patients with type 1 diabetes mellitus attending Sudan childhood diabetes centre. J Diabetes Res. 2020;2020:1–8. doi:10.1155/2020/7181383
24. Mogensen CE, Poulsen PERL. Microalbuminuria, glycemic control, and blood pressure predicting outcome in diabetes type 1 and type 2. Kidney Int. 2004;66:S40–S41. doi:10.1111/j.1523-1755.2004.09210.x
25. Rossing P, Hougaard P, Parving HH. Progression of microalbuminuria in type 1 diabetes: ten-year prospective observational study. Kidney Int. 2005;68(4):1446–1450. doi:10.1111/j.1523-1755.2005.00556.x
26. Svensson M, Eriksson JW, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care. 2004;27(4):955–962. doi:10.2337/diacare.27.4.955
27. Amin R, Schultz C, Ong K, et al. Low IGF-I and elevated testosterone during puberty in subjects with type 1 diabetes developing microalbuminuria in comparison to normoalbuminuric control subjects: the Oxford Regional Prospective Study. Diabetes Care. 2003;26(5):1456–1461. doi:10.2337/diacare.26.5.1456
28. Gallego PH, Bulsara MK, Frazer F, Lafferty AR, Davis EA, Jones TW. Prevalence and risk factors for microalbuminuria in a population‐based sample of children and adolescents with T1DM in Western Australia. Pediatr Diabetes. 2006;7(3):165–172. doi:10.1111/j.1399-543X.2006.00164.x
29. Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347(11):797–805. doi:10.1056/NEJMoa013410
30. Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi:10.1681/ASN.2006080872
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
