Back to Journals » Clinical Ophthalmology » Volume 16
Prevalence and Characteristics of Cytomegalovirus Ocular Disease in Children: A Multi-Center Study
Authors Mercado CL, Froines CP, Gaier ED , Wang Q, Indaram M , Wan MJ, Shah AS , Koo EB
Received 12 March 2022
Accepted for publication 23 June 2022
Published 8 July 2022 Volume 2022:16 Pages 2209—2217
DOI https://doi.org/10.2147/OPTH.S364741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Carmel L Mercado,1,2 Colin P Froines,3 Eric D Gaier,4– 7 Qinyun Wang,8 Maanasa Indaram,8 Michael J Wan,9 Ankoor S Shah,4– 6 Euna B Koo10
1Department of Ophthalmology, University of Washington, Seattle, WA, USA; 2Department of Ophthalmology, Seattle Children’s Hospital, Seattle, WA, USA; 3School of Medicine, University of Washington, Seattle, WA, USA; 4Department of Ophthalmology, Boston Children’s Hospital, Boston, MA, USA; 5Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Boston, MA, USA; 6Department of Ophthalmology, Harvard Medical School, Boston, MA, USA; 7Picower Institute for Learning and Memory, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA, USA; 8Department of Ophthalmology, University of California San Francisco, San Francisco, CA, USA; 9Department of Ophthalmology and Vision Sciences, SickKids, Toronto, ON, Canada; 10Department of Ophthalmology, Stanford University School of Medicine, Palo Alto, CA, USA
Correspondence: Euna B Koo, Department of Ophthalmology, Stanford University School of Medicine, 2452 Watson Ct, Palo Alto, CA, 94303, USA, Tel +1 650-723-6995, Email [email protected]
Purpose: The objective of this study was to identify the prevalence of CMV ocular disease in children and to identify associated risk factors for ocular involvement.
Design: Retrospective multicenter, cross-sectional study.
Methods: Setting: Hospitalized patients screened for CMV viremia by PCR between 2005 and 2018 at four pediatric referral centers. Participants: Seven-hundred and ninety-three children showed CMV viremia (> 135 copies/mL by polymerase chain reaction; PCR). Main Outcomes and Measures: (1) Occurrence of ophthalmologic examination. (2) Presence of CMV ocular disease, defined as retinitis, vasculitis, hemorrhage, optic nerve atrophy, or anterior uveitis in the setting of CMV viremia without other identifiable causes.
Results: A total of 296/793 (37%) underwent ophthalmologic examination following CMV viremia. A total of23/296 patients (8%) had ocular symptoms prompting evaluation while the rest had eye exams for baseline screening unrelated to CMV viremia. Of these, 13 cases (4% of those with an eye exam) with ocular disease were identified (three congenital CMV, five severe combined immunodeficiency disorder (SCID) status post-stem cell transplantation, three hematologic malignancy status post-stem cell transplantation for two of them, one Evans syndrome status post-stem cell transplantation, and one medulloblastoma status post-bone marrow transplantation). No patients with solid organ transplantation developed CMV ocular disease in our cohort.
Conclusion: CMV ocular disease was a rare occurrence in this cohort without an identifiable pattern across sub-groups. Excluding the three congenital CMV cases, nine out of ten patients with CMV ocular disease were status post-stem cell transplantation. We provide integrated screening guidelines based on the best available evidence for this rare condition.
Keywords: cytomegalovirus, retinitis, CMV, screening guidelines, pediatrics
Introduction
Cytomegalovirus (CMV) infection is the most common congenital infection in the United States. Congenital CMV infections are reported in 30,000–40,000 infants per year in the country with rates higher in developing countries.1 The Centers for Disease Control and Prevention2 estimates that by age 40, >50% of the population has been exposed to CMV. Individuals with a functioning immune system experience minimal flu-like symptoms, if any. In those patients who are immunocompromised, CMV infection can manifest with severe end organ damage secondary to inflammation of the lungs, kidneys, heart, pancreas, liver, spleen, central nervous system and eyes. With congenital CMV viremia, many are asymptomatic but among newborns who have symptomatic CMV viremia, 40–60% develop cognitive, auditory, visual, or motor impairment.3–10
Reported incidence of pediatric CMV ocular disease has varied in the literature. For congenital CMV, the earliest studies estimated 1–15% of infected newborns developed CMV ocular disease.11–14 The most recent incidence study by Jin et al15 found 4% (10/237) developed disease. For other risk factor subgroups: 3.4–6% with immunodeficiency secondary to HIV infection,16–20 0.1% in liver transplant recipients (adults included in this study),21 3% following organ and bone marrow transplantation (2/74),22 and 11–23% who developed CMV viremia following stem cell transplant23,24 developed ocular disease. The rarity of ocular involvement with CMV viremia, the transition in diagnostic testing from viral urine culture to polymerase chain reaction (PCR),25,26 and the lack of previous multicenter analyses necessitate further studies on the epidemiology of this potentially blinding complication.
The remaining uncertainty about the prevalence of CMV ocular disease in children are at least in part to blame for the lack of universal guidelines on how to screen viremic children for CMV-related eye disease. We designed this multicenter study to better study the prevalence of CMV ocular disease in children with CMV viremia, identify risk factors, and determine if this information could be used to provide guidance on surveillance.
Methods
This retrospective study involved four major university hospital-based pediatric referral centers between 2005–2018: Lucile Packard Children’s Hospital (Stanford, CA), University of California San Francisco Benioff Children’s Hospital (San Francisco, CA), The Hospital for Sick Children (Toronto, Canada), and Boston Children’s Hospital (Boston, MA). This study was approved by the Institutional Review Boards at all participating institutions and was in compliance with the Health Insurance Portability and Accountability Act. This study adhered to the tenets of the Declaration of Helsinki. All solid organ transplants were voluntarily donated with written informed consent in accordance with the Declaration of Istanbul.
Study patients were less than 18 years of age with CMV viremia, which was defined as >135 CMV copies/mL by PCR. Both symptomatic and asymptomatic (or unable to communicate symptoms) populations were included in the analysis.
Data collection consisted of a detailed review of the patient’s medical history, including key dates and values related to CMV viremia. Historical details of the primary diagnosis and immunodeficiency were also extracted from the chart. The occurrence of an ophthalmic examination and reason for consultation were included, as was the presence of any eye findings. Ophthalmologic examination included measurement of best-corrected visual acuity, pupils, ocular adnexa, anterior segment, posterior segment, visual fields where permissible, extraocular motility and alignment, tonometry, and dilated funduscopic examination. Main outcome measures included the (1) occurrence of ophthalmologic examination, and (2) occurrence of CMV ocular disease, defined as retinitis, vasculitis, retinal hemorrhages, optic nerve atrophy, or anterior uveitis in the setting of CMV viremia without other identifiable causes (ie, no other infections or immunologic conditions diagnosed at the time). Intraocular fluids were not routinely sent off for confirmation of virus. Diagnoses of eye conditions were made by pediatric ophthalmologists, though management may have involved retina specialists at each site if there was involvement of the posterior segment.
For the statistical analysis, patients were categorized into seven groups based on their primary diagnosis: (1) history of cancer, (2) primary immunologic disorder, (3) history of organ transplant, (4) non-malignant blood disorder, (5) congenital CMV (viremic in first 3 weeks of life), (6) CMV-related organ disease, and (7) other diagnoses. This category of ‘other diagnoses’ included patients who were found to be CMV viremic as part of their work up for their inpatient admission (Table 1). Within each group, we calculated the number of cases of CMV ocular disease. Given the burden of their underlying conditions, children were often admitted multiple times within a short period of time. To take this into account, the highest viral load amongst all the admissions was used as the maximum viral load in the analysis. We also calculated rates of ophthalmologic examination and the reason for each consultation request (ie, whether the patient was symptomatic or a screening eye exam was needed as part of the patient’s care). Statistical significance for associations between diagnoses, history of SCT, steroid/steroid-sparing agent use, or CMV treatment and occurrence of CMV eye disease was evaluated using Fisher’s exact test in univariate analysis. Statistical significance of association of an eye exam with a particular diagnosis and association of death with a particular diagnosis was calculated using multivariate logistic regression. Statistical analysis was performed using Stata software (StataCorp LLL, College Station, TX). In all cases, p<0.05 was considered statistically significant.
 |
Table 1 Demographic Data |
Results
A total of 794 patients were identified across sites based on the inclusion criteria (site 1: 291, site 2: 153, site 3: 225, site 4: 125 patients). After initial review, 793 of the 794 charts were included for analysis (Figure 1).
 |
Figure 1 Study methodology: (1) patient selection criteria; (2) data collection; and (3) breakdown of analysis. Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia. |
Demographic data are presented in Table 1. Males and females were similarly represented in the study sample (male: 431, 54%; female 362, 46%). The most common risk factor was solid organ transplant (306, 39%), followed by cancer (212, 27%), primary immunologic disorder (91, 11%), non-malignant blood dyscrasia (52, 7%), congenital CMV infection (41, 5%), CMV-related organ disease (13, 2%), and other diagnoses (78, 10%). Multivariate logistic regression revealed a higher odds ratio of death associated with higher maximal viral load (OR=1.11; p=0.009) and history of SCT (OR=1.78; p<0.025) (Table 2).
 |
Table 2 Frequency of Clinical Findings and Odds Ratio of Death and Eye Exam by Multivariate Logistic Regression |
There were 296 patients (37% of the total group) who underwent ophthalmologic examination following CMV viremia. None of the sites had an institution-specific protocol for screening patients who were CMV viremic. There was a statistically significant difference in the occurrence of an eye exam based on age across sites (p<0.001). Patients had higher odds of undergoing an ophthalmologic evaluation based on maximum viral load (OR=1.25, p<0.001) (Table 2) though this finding may be confounded by varying consultation patterns amongst the different institutions. Twenty-three patients had ocular symptoms prompting evaluation while the rest were asymptomatic and had an eye exam for baseline screening. Though there was variation on when and which patients were screened with an eye exam amongst the various sites, in general, if persistently viremic, the various sites reported that children were re-evaluated with follow-up eye exams (varying frequency). All sites also reported using prophylactic anti-viral therapy for CMV.
Thirteen cases of CMV ocular disease were identified (Table 3). Children with congenital CMV infection accounted for three of the cases, five had severe combined immunodeficiency disorder (SCID) status post-stem cell transplantation, three had a hematologic malignancy, two of whom had undergone stem cell transplantation, one had a history of Evans syndrome status post-stem cell transplantation, and one had a history of bone marrow transplantation for treatment of medulloblastoma. Using Fisher’s exact test in univariate analysis, SCID was identified as the solitary independent risk factor for ocular involvement of CMV viremia in our sample (p<0.001). Nine of the 13 patients had a history of SCT (p=0.08). No patients with solid organ transplant had CMV ocular disease in this cohort. Only four of the 13 confirmed CMV cases were in symptomatic children. Ocular findings included anterior uveitis (N=1), nerve edema and pallor (N=1), retinitis/hemorrhage with subretinal lesions, retinal detachment, macular scarring, and fibrosis (N=11) (Figure 2).
 |
Table 3 Demographics of Patients with Confirmed CMV Ocular Disease |
Discussion
In our study population, the overall prevalence of CMV ocular disease in children who underwent an eye exam was 4%. Baumal et al’s27 study, which looked at the overall prevalence of CMV ocular disease in children with different risk factors including SCID, SCID status post-bone marrow transplantation, AIDS, and acquired immunodeficiency syndrome status post-bone marrow transplantation for leukemia, renal transplantation, and chemotherapy for leukemia found similar rates. When we break down the numbers by sub-group, we see CMV ocular disease develop in 4% (4/101) of children with a history of cancer, 8.6% (3/35) with congenital CMV, and a higher rate of 14% (6/42) in children with a primary immunologic disorder. Our rates of CMV ocular disease were higher in this study than previously reported for children with congenital CMV.15 Larochelle et al23 and Hiwarkar et al24 both found higher rates of CMV ocular disease in patients with CMV viremia and a history of SCT, at 38% and 23%, respectively. Our finding that the majority of the patients with CMV ocular disease were status post-stem cell transplantation is consistent with previous literature. Interestingly, Hiwarkar et al24 found that patients with a history of a primary immunodeficiency disorder and CMV viremia even prior to undergoing SCT were at the highest risk for developing CMV ocular disease. In our study population, there were no cases of CMV ocular disease in patients with a history of organ transplantation, CMV-related organ disease, non-malignant blood disorders, or in the ‘other diagnoses’ group. With the caveat that not all CMV serum positive patients underwent an eye exam in our study, our results agree with previous studies that have described CMV ocular disease as an uncommon complication post-solid organ transplantation with a prevalence in the order of 0.1–3%.21,22
None of the sites had an institution-specific protocol for screening patients who were CMV viremic. Although we pooled patients from four large pediatric referral centers, the low number of cases of CMV ocular disease limited our ability to identify statistically significant risk factors in this study other than for a history of SCID. There are some inferences that can be made from our findings that add onto prior studies’ suggestions for screening and surveillance, especially given most affected patients were status post-stem cell transplantation (Figure 3). For the low risk population, ie, Non-malignant blood dyscrasia, solid organ transplants, an eye evaluation may only be warranted in these groups if a child is symptomatic or too young or ill to communicate symptoms. However, an eye evaluation for this population may still be warranted for other reasons (ie, use of high-risk medications such as steroids). For those patients with persistent CMV viremia or with a high viral load, regardless of the underlying condition, an ophthalmology consultation is likely warranted at the discretion of the primary team. For children with a history of congenital CMV, SCT, and a primary immunodeficiency disorder like SCID, frequent screening exams are warranted as these groups appear to be at higher risk for developing CMV ocular disease. Ghekiere et al28 and Pass29 have previously recommended dilated fundus examination at birth, 6 months, then annually for the first 10–12 years of life, as well as a follow-up eye exam following cessation of any antiviral treatment to assess for recurrence of ocular disease in congenital CMV children. For children with a history of SCT, Larochelle et al23 have recommended a baseline eye evaluation for all children set to undergo SCT. If a child did not develop CMV viremia, a 3-month post-SCT examination followed by a 12-month post-SCT examination sufficed. If a child developed CMV viremia, a new baseline eye exam was recommended within 2 weeks of CMV viremia then every 6–8 weeks while the child is viremic. Along the same thread, Hiwarkar et al24 recommended baseline screening for high risk children, which in their group included children with significant pre-transplant CMV viremia, a history of a primary immunodeficiency disorder, severe graft-versus-host disease, and an acute mismatched graft. They then recommended monitoring with serial eye exams every few weeks until resolution of CMV viremia. While we cannot comment on the appropriate timing of ophthalmic screening exams based on the data from this study, the majority of our patients who did develop CMV ocular disease had a history of SCT and a primary immunodeficiency disorder. Given the severity of eye disease in our patients by the time of detection, we recommend a higher frequency of exams, every 2 weeks instead of 6–8 weeks, in the highest risk population. It is also worth noting that 9/13 children with suggestive CMV ocular disease were asymptomatic. The need for a more standardizing eye exam protocol is further emphasized by this vulnerable population as many will not or are unable to report symptoms. A lower threshold for a sedated eye exam is also strongly recommended in this population if the patient does not allow for an adequate bedside exam. As initial fundus findings in these high risk populations can be subtle, likely due the immunocompromised state of many of these patients, more than a fly-by posterior pole exam is needed to definitively rule out ocular disease. Collaboration with stem cell and oncology colleagues is critical in coordinating episodes of anesthesia for various procedures (ie, Bone marrow biopsy, lumbar punctures, MRI’s, etc).
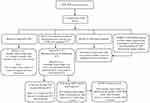 |
Figure 3 Risk factor-based screening recommendations. |
Limitations of this study include the inability to confirm that all eye findings were truly caused by CMV. Fluid from the eye was not sent off in identified patients in this study. Ocular manifestations, if not explained by the underlying condition, were presumed to be secondary to CMV given the variability of presentation of the disease depending on severity, chronicity, and immune status of the host. Though the final visual outcomes were not reported in this study, the most common presentation being retinitis is concerning as visual prognosis with CMV retinitis is most often devastating. Another potential limitation of this study was that only patients with a positive CMV serum PCR at the included study site were analyzed in the study. Patients who were viremic at another point in time previous to their admission or at another site (another hospital/lab) would have been missed in this study. One of the main limitations for a purely evidence-based screening protocol for CMV ocular disease is the low incidence of disease. Though we suspect stem cell transplantation to be a significant risk factor for CMV ocular disease, we were unable to find statistical significance given such low incidence of eye disease. Harnessing the numeric power of large national databases that are being developed around the world, such as the Intelligent Research in Sight (IRIS) registry within the United States, will be important in further elucidating the epidemiology of CMV ocular disease in the pediatric population. Such a registry would allow for more rapid adaptability of the screening guideline when the detection methods for disease further improves just as it occurred with availability of PCR.
Acknowledgments
We thank Dr. Lu Tian at Stanford University for his help with the statistical analysis for this study. Material has been presented at the American Academy of Ophthalmology Annual Meeting, 2020.
Funding
An unrestricted grant from Research to Prevent Blindness and the National Eye Institute P30-EY026877.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Demmler GJ; Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1991;13:315–329. doi:10.1093/clinids/13.2.315
2. Centers for Disease Control and Prevention (CDC). Cytomegalovirus (CMV) and congenital CMV infection; 2020. Available from: https://www.cdc.gov/cmv/index.html.
3. Andriesse GI, Weersink AJ, de Boer J. Visual impairment and deafness in young children: consider the diagnosis of congenital infection with cytomegalovirus even years after birth. Arch Ophthalmol. 2006;124:743. doi:10.1001/archopht.124.5.743
4. Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:99–126. doi:10.1128/CMR.00023-08
5. Coclite E, Di Natale C, Nigro G. Congenital and perinatal cytomegalovirus lung infection. J Matern Fetal Neonatal Med. 2013;26:1671–1675. doi:10.3109/14767058.2013.794207
6. Furutate S, Iwasaki S, Nishio SY, Moteki H, Usami S. Clinical profile of hearing loss in children with congenital cytomegalovirus (CMV) infection: CMV DNA diagnosis using preserved umbilical cord. Acta Oto Laryngol. 2011;131:976–982. doi:10.3109/00016489.2011.583268
7. Pass RF, Stagno S, Myers GJ, et al. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics. 1980;66(5):758–762. doi:10.1542/peds.66.5.758
8. Townsend CL, Forsgren M, Ahlfors K, et al. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56(9):1232–1239. doi:10.1093/cid/cit018
9. Oliver SE, Cloud GA, Sanchez PJ, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol. 2009;46S:S22–S26.
10. Kimberlin DW, Lin C-Y, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi:10.1016/S0022-3476(03)00192-6
11. Stagno S, Reynolds DW, Amos CS, et al. Auditory and visual defects resulting from symptomatic and subclinical congenital cytomegaloviral and toxoplasma infections. Pediatrics. 1977;59:669–678. doi:10.1542/peds.59.5.669
12. Stagno S, Pass RF, Dworsky ME, Alford CA. Congenital and perinatal cytomegalovirus infections. Semin Perinatol. 1983;7:31–42.
13. Istas AS, Demmler GJ, Dobbins JG, Stewart JA; National Congenital Cytomegalovirus Disease Registry Collaborating Group. Surveillance for cytomegalovirus disease: a report from the National Congenital Cytomegalovirus Disease Registry. Clin Infect Dis. 1995;20:665–670. doi:10.1093/clinids/20.3.665
14. Jones CA, Isaacs D. Predicting the outcome of symptomatic congenital cytomegalovirus infection. J Paediatr Child Health. 1995;31:70–71. doi:10.1111/j.1440-1754.1995.tb00749.x
15. Jin H, Demmler-Harrison GJ, Coats DK, et al. The congenital CMV longitudinal study group.long-term visual and ocular sequelae in patients with congenital cytomegalovirus infection. Ped Infect Dis J. 2017;36(9):877–882. doi:10.1097/INF.0000000000001599
16. Du LT, Coats DK, Kline MW, et al. Incidence of presumed cytomegalovirus retinitis in HIV-infected pediatric patients. J AAPOS. 1999;3:245–249. doi:10.1016/S1091-8531(99)70010-8
17. Studies of Ocular Complications of AIDS (SOCA) Research Group in Collaboration with the AIDS Clinic Clinical Trials Group (ACTG). Foscarnet-ganciclovir cytomegalovirus retinitis trial: 5. Clinical features of cytomegalovirus retinitis at diagnosis. Am J Ophthalmol. 1997;124:141–157. doi:10.1016/S0002-9394(14)70778-0
18. Baumal CR, Levin AV, Read SE. Cytomegalovirus retinitis in immunosuppressed children. AJO. 1999;127(5):550–558.
19. Frenkel LD, Gaur S, Tsolia M, Scudder R, Howell R, Kesarwala H. Cytomegalovirus infection in children with AIDS. Rev Infect Dis. 1990;12(suppl 7):S820–S825. doi:10.1093/clinids/12.Supplement_7.S820
20. De Smet MD, Butler KM, Rubin BI, et al. The ocular complications of HIV in the pediatric population. In: Dernouchamps JP, Verougstraete C, Caspers-Velu L, Tassignon MJ, editors. Recent Advances in Uveitis: Proceedings of the Third International Symposium on Uveitis. Amsterdam: Kugler Publications; 1992:315–319.
21. Egli A, Bergamin O, Mullhaupt B, Seebach JD, Mueller NJ, Hirsch HH. Cytomegalovirus-associated chorioretinitis after liver transplantation: case report and review of the literature. Transpl Infect Dis. 2008;10:27–43. doi:10.1111/j.1399-3062.2007.00285.x
22. Bradfield YS, Kushner BJ, Gangnon RE. Ocular complications after organ and bone marrow transplantation in children. J AAPOS. 2005;9(5):426–432. doi:10.1016/j.jaapos.2005.06.002
23. Larochelle MB, Phan R, Craddock J, et al. Cytomegalovirus retinitis in pediatric stem cell transplants: report of a recent cluster and the development of a screening protocol. Am J Ophthalmol. 2017;175:8–15. doi:10.1016/j.ajo.2016.09.039
24. Hiwarkar P, Gajdosova E, Qasim W. Frequent occurrence of cytomegalovirus retinitis during immune reconstitution warrants regular ophthalmic screening in high-risk pediatric allogeneic hematopoietic stem cell transplant recipients. Clin Infect Dis. 2014;58(12):1700–1706. doi:10.1093/cid/ciu201
25. Nelson CT, Istas AS, Wilkerson MK, Demmler GJ. PCR detection of cytomegalovirus DNA in serum as a diagnostic test for congenital cytomegalovirus infection. J Clin Microbiol. 1995;33(12):3317–3318. doi:10.1128/jcm.33.12.3317-3318.1995
26. Brytting M, Xu W, Wahren B, Sundqvist VA. Cytomegalovirus DNA detection in sera from patients with active cytomegalovirus infections. J Clin Microbiol. 1992;30(8):1937–1941. doi:10.1128/jcm.30.8.1937-1941.1992
27. Baumal CR, Levin AV, Kavalec CC, Petric M, Khan H, Read SE. Screening for cytomegalovirus retinitis in children. Arch Pediatr Adolesc Med. 1996;150(11):1186–1192. doi:10.1001/archpedi.1996.02170360076013
28. Ghekiere S, Allegaert K, Cossey V, et al. Ophthalmological findings in congenital cytomegalovirus infection: when to screen, when to treat? J Pediatr Ophthalmol Strabismus. 2012;49(5):274–282. doi:10.3928/01913913-20120710-03
29. Pass R. Congenital cytomegalovirus infection: screening and treatment. J Pediatr. 2010;157:179–180. doi:10.1016/j.jpeds.2010.04.045
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

