Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 12
Prevalence and Associated Factors of Intestinal Helminths Among Kindergarten Children in Gondar Town, Northwest Ethiopia
Authors Ayele A , Tegegne Y, Derso A , Eshetu T , Zeleke AJ
Received 4 November 2020
Accepted for publication 27 January 2021
Published 5 February 2021 Volume 2021:12 Pages 35—41
DOI https://doi.org/10.2147/PHMT.S290265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Abiy Ayele,1 Yalewayker Tegegne,2 Adane Derso,2 Tegegne Eshetu,2 Ayalew Jejaw Zeleke2
1Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Medical Parasitology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Adane Derso
College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Tel +251930716000
Email [email protected]
Background: Intestinal parasitic infections (IPIs) are small organisms that infect the gastro-intestinal tract of human beings. Causes malnutrition, iron deficiency anemia, impairment of physical and mental development in children. The aim of this study was to determine the prevalence of intestinal helminthiasis and associated factors among Kindergarten (KG) children in Gondar Town, northwest Ethiopia.
Methods: Institution-based cross-sectional study was conducted on 390 Kindergarten children in Gondar town, Northwest, Ethiopia from March to May 2019. Study subjects were selected using systematic random sampling method. Data were gathered through direct interview by using a pretested questionnaire. Stool specimens were collected and examined using Kato Katz technique. Chi square was used to assess the association between variables and p-value less than 0.05 was taken as a statistical significance.
Results: The overall prevalence of intestinal helminthiasis was 16.7%, while those of participants infected by soil-transmitted helminths (STHs) and intestinal Schistosomiasis were 13.8% and 5.9%, respectively. Ascaris lumbricoides was the predominant STHs (9%) followed by Trichuris trichiura (2.3%) and hookworm (1.5%). Light infection was observed in almost all of the infected study participants. Intestinal helminthiasis was found to be significantly associated with age, KG level of children, maternal occupation, and educational status of parents.
Conclusion: A significant number of children were infected by intestinal parasites in the study area. A. lumbricoides and intestinal Schistosomiasis were the most predominant of the isolated parasites.
Keywords: soil-transmitted helminths, intestinal schistosomiasis, preschool children
Introduction
Intestinal parasitic infections (IPIs) are small organisms that infect the gastro-intestinal tract of human beings. They are more prevalent among children compared with the general population. About 12% of the global disease burden caused by intestinal parasites is observed among children aged 4 to 6 years in developing countries.1 Preschool children in Kindergartens are one of the groups of the people at high risk for IPIs.2
Currently, the big three soil-transmitted helminths (A. lumbricoides, T. trichiura and hookworm) are the leading cause of intestinal parasitosis. They are linked with significant figures of morbidity and mortality in the world.3,4 However, the incidence and prevalence of such infections may vary within and across countries due to several factors.5 According to the 2017 WHO report, the estimated number of people known to be infected globally with A. lumbricoides, T. trichuria, and hookworm was 820,440, 460 million, respectively.6 All of these parasites most commonly infect children and adolescents.7,8
The adult stage of STHs colonizes the intestine, whereas S. mansoni resides in the mesenteric blood vessels. They all produce thousands of eggs to the external environment every day. In places of poor sanitation, the eggs/larvae contaminate the soil and parasitize humans when ingested with foods or penetrate the skin. The worms do not multiply in the human host, and their intensity increases only as a result of re-infections.6
Poor hygiene, low immune status, overcrowding, close contact with soil and to each other, lack of latrine, and low provision of water in schools are some of the factors that put school-age children at high risk for intestinal parasitic infection.9
The prevention and control of STH and S. mansoni could be based on avoiding the contamination of food or water with fecal material.10 Health promotion and education aimed at improving personal hygiene and encouraging hand washing with soap and water, proper latrine utilization and food handling are also effective control activities for the reduction of person-to-person transmissions.11 Moreover, community-based mass deworming program, particularly among preschool children through annual or bi-annual distribution of a single dose of a broad spectrum benzimidazole drugs is the current advocated and practiced control strategy.12
Children are sadly reported to be at an increased risk for severe infections and the morbidity associated with intestinal parasitic infections.13 The adverse effects of intestinal parasites among preschool children are diverse and alarming. They have detrimental impacts on the survival, appetite, growth and physical fitness, school attendance and cognitive performance of children.14 For this reason, regular parasitological surveillance may have a crucial role in reducing a wide range of health consequences by these infections.
Though previous studies conducted in Ethiopia focusing on distribution of different intestinal parasites on various study groups such as school children and other study groups in hospitals, refugees, and the community, the prevalence of STH and intestinal schistosomiasis was not well addressed indifferent parts of Ethiopia including our study area. Therefore, the aim of this study was to determine the prevalence and associated factors of soil-transmitted helminths and S. mansoni infection among Kindergarten children in Gondar town, North West Ethiopia.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from University of Gondar School of Biomedical and Laboratory Science (Ref.No.SBMLS/1021/11) ethical committee and study was conducted in accordance with the Declaration of Helsinki. The kindergarten teachers and parents were informed about the purpose and procedures of the study. Written informed consent was obtained from the parents or legal guardian. All positive participants were treated according to the guidelines of the National Helminths Control Program of Ethiopia by referring to them to the nearby health center.
Materials and Methods
Study Setting
The study was conducted among kindergarten children in Gondar town of Central Gondar zone of the Amhara region in Northwestern Ethiopia. Gondar town is located 738km Northwest of Addis Ababa (the capital city of Ethiopia). Gondar is north of Lake Tana and southwest of the Simien Mountain. It has latitude of 12°36′N 37°28′E with an elevation of 2133 meters above sea level. Based on the 2007 census result, the town has a projected total population of 323,900.15
Study Design, Period, and Sampling Technique
Institution-based cross-sectional study was conducted from March to May 2019. The sample size was determined by using single population proportion formula. By considering prevalence of IPIs from previous study (p=15.5%).16 95% confidence interval (Z=1.96) and 5% marginal error (d=0.05) and design effect of 2. Then the sample size was calculated as n = [Z - a/2] 2 P (1-p)/d2, where: n = sample size, P = proportion problem in the study area, Z -a/2 = CI of 95%, d = Marginal error to be tolerated. Sample size was calculated to be 402.
The study was conducted in both private and governmental schools. There were about 75 (30 private and 45 governmental) Kindergarten schools in the Town. During the study period, the total number of children attending the Kindergartens in the town was 20,000. Through simple random sampling method, three governmental and five private schools were selected proportional to the total number of students. The students were stratified according to their Kindergarten (KG) grade level, Children whose age 4, 5 and 6 years were allocated in KG grade level 1, 2 and 3, respectively. Then, quota sampling was used to each grade based on the number of students. Then the number of the study participants in each grade was determined by using systematic random sampling technique using their class rosters as sampling frame.
Inclusion and Exclusion Criteria
All children who had no history of anti-helminthic drugs in the last 1 month before screening were included in the study. While, those school children who were not voluntary to give stool samples were excluded in the study.
Data Collection and Processing
Questionnaire Survey
Socio-demographic characteristics and associated factors were collected by face-to-face interview method using pre-tested structured questionnaire. English version of the questionnaire was translated to a local language “Amharic” version (see Supplementary Materials). Comparisons were made on the consistency of the two versions. After onsite training given for data collectors. A pretest was conducted among five percent of the total sample size that was selected randomly from all sections on which cross-sectional study was conducted. The interview included information such as age, marital status of mother, mother and father educational status, mother and father occupation.
Parasitological Analysis
A labeled, clean, leak proof empty container with unique identification (ID) numbers was distributed to the children and 3 gram of stool samples was collected. The samples were transported and processed at University of Gondar, School of Biomedical and Laboratory Science Medical Parasitology Laboratory Room. Each stool sample was examined by using Kato Katz technique. Hook worm ova detection was performed by examination of the Kato Katz slide within one hour of stool collection and its preparation. Specially, for the identification of S. mansoni and other helminths ova the prepared slides was left for 24 hours for better clearing and easy visualization of eggs. Infection intensity of the STHs and S. mansoni was estimated by multiplying the total number of eggs counted by 24, which gives as the eggs per gram (epg) of stool. Besides, the species-specific classes of infection intensity with S. mansoni and STH were classified as light, moderate and heavy as per the threshold set by WHO as Intensity of S. mansoni was classified into: light infection (1–99epg), moderate (100–399 epg) and heavy (greater than 400epg). Likewise, the classification for A. lumbricoidesis: light infection (1–4999epg), moderate (5000–49999epg) and heavy (greater than 50,000epg). Intensity of T. trichiura: light infection (1–999epg), moderate (1000–9999epg) and heavy (greater than 10,000epg). Classification of hookworm is: light infection (1–1999epg), moderate (2000–3999 epg) and heavy (greater than 4000epg).17
Data Management and Analysis
The obtained data was edited and analyzed by statistical package for social science (SPSS) software version 20.18 Descriptive statistics like frequency, mean and percentage was calculated to describe the study population characteristics. A chi square was used to assess the association between variables. The strength of associations was measured by 95% confidence interval and p value less than 0.05 was taken as a statistical significance. Compiled results were presented in the form of text, tables or graphs.
Results
Socio-Demographic Characteristics
A total of 390 Kindergarten school children aged 4 to 6 years were included in the study. The male to female ratio was 1:1. The majority were 5 years old. The other socio-demographic characteristics of the study participants are presented (Table 1).
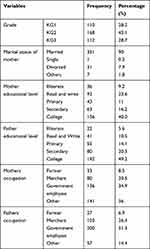 |
Table 1 Socio-Demographic Characteristics of Kindergarten Children in Gondar Town, Northwest Ethiopia, 2019 |
Prevalence of Intestinal Helminths Infections
The overall prevalence of intestinal helminthiasis was 16.7%. The prevalence of STHs was found to be 13.8%. A. lumbricoides, the most common STHs isolated was 9.0%, followed by T. trichiura (2.3%), and hookworm (1.5%). Schistosoma mansoni and H.nana were the other types of intestinal helminths whose detection rate was 23 (5.9%) and 6 (1.5%), respectively. The distribution of intestinal helminths among the study participants is shown (Figure 1).
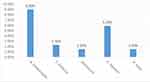 |
Figure 1 Prevalence of intestinal parasitic infection among kindergarten children in Gondar town, Northwest Ethiopia, 2019. |
Intensity of STHs and S. mansoni Infection
Almost all of the 35 Kindergarten children who were positive for A. lumbricoides had light infection, while only one 1 (2.8%) faced a moderate infection. The intensity of infection with T. trichiura, hookworm, and S. mansoni was light among all infected study participants (Table 2).
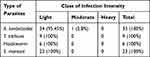 |
Table 2 Intensity of Infection in Kindergarten School Children with STH and S. mansoni Infection in Gondar Town, Northwest Ethiopia, 2019 |
Factors Associated with the Prevalence of Intestinal Helminths
In this study, the prevalence of intestinal helminthiasis was significantly associated with age, KG level, maternal education and occupation and paternal education (P. value <0.05), but not with sex of the participants (Table 3).
 |
Table 3 Factor Associated with the Prevalence of Intestinal Helminthiasis Among Kindergarten Children in Gondar Town, Northwest Ethiopia, 2019 |
Discussion
Studies that indicate the burden of STH and other intestinal parasites in different areas are crucial for identifying communities at high-risk for parasitic infections and for formulating suitable prevention and control measures. The results of the current study showed the existence of intestinal helminths infections among Kindergarten children in Gondar town, northwest Ethiopia.
In this study, the overall prevalence of intestinal helminthiasis among Kindergarten children was 16.7%. This result was lower than findings of similar studies conducted in other parts of Ethiopia, such as Mekele (18.8%),19 Jimma (46.6%),20 and Arbaminch (27.7%),21 and Yadot in Bale zone (21.9%).22 Similarly, the present finding was lower than prevalence study in Nigeria (30.0%).23 On the other hand, it is higher than a study conducted in Iran (7.1%).24 The differences in the prevalence of IPIs among different studies could be explained by variations in geography, households socio-economic status, water supply, environmental sanitation, study periods, implementation of prevention and control measures and also it might be laboratory methods used.
The present study showed that A. lumbricoides and S. mansoni were found to be the most dominant intestinal parasites with 9% and 5.9%, respectively. This predominance was consistent with a previous study conducted in a nearby village in Chuait, Dembia district in which A. lumbricoides and S. mansoni were the most frequently detected with parasites.16 The reason behind the high prevalence of A. lumbricoides both in the current and previous studies could be related to the hard nature of the egg to resist adverse environmental conditions and this can contribute to the easy transmission of this parasitic infection. The current study also presented that a significant number of pre-school children were positive for S. mansoni infection. This could be unusual report since most of us believe that they usually spend their time at home a part from kindergarten school. However, many people in the town are using several rivers for taking bathes and washing of their clothes.25 In connection to this, children frequently go to the river with their parents. The children are playing using water from the river in order to spend their time until their parents finish their work. As a result, they may get infected by cercarial form of the parasite from the water surface.
The prevalence of hookworm and H. nana infections were low in this study and are in agreement with findings of studies conducted in Tigray the northern part of the country,19 and Jimma in southern Ethiopia.20 In the current study, the 2.3% prevalence of T. trichiura was lower than the report 38.9% conducted from Jimma.20 The variation may be related to differences in study areas, that might be related with temperature difference and characteristics of the soil which aids for maturation and easily transmission of infective stage of the parasites, crowdedness and provision of water in the school, number of households, socioeconomic status, and study periods.
The intensity of intestinal helminthiasis in the current study was generally light. Only one child with A. lumbricoides experienced a moderate infection. This is in agreement with the result of a study conducted in Chuait, Dembia district.16 All hookworm, T. trichiura, and S. mansoni positive study participants were grouped under the light infection category. However, no heavy infection was detected in any of the study participants. That might be linked with the age (≤ 6 years) of the participants. In other words, children whose age less than six are less likely to be exposed to the soil or other risky behaviors to catch intestinal parasites.26
This study also investigated and determined several factors associated with intestinal helminthiasis. Accordingly, age, Kindergarten (KG) level, mothers’ education and occupation, and fathers’ education were significantly associated with intestinal parasitosis. The prevalence of intestinal helminthiasis among the Kindergarten children was significantly higher among the age of 6 followed by 5 years. This finding is supported by that of a study conducted in Mekelle.19 This is possibly as a result of more exposure of the children to the parasites as their age increased. In the study, sex, and parental marital status did not show any significant association with the prevalence of intestinal helminthic infections. The rate of infection was also higher among children whose mothers’ were illiterate than any other categories. This shows the role of education in preventing IPIs. Besides, personal hygiene and awareness about the transmission routes of intestinal parasites require literacy.21,27,28
Limitations of the Study
The infection intensity of the common intestinal helminthiasis was determined by the examination of a single stool sample of each participant. That might have affected the prevalence and the accuracy of the egg count of the parasites. Thus, the findings should be interpreted with that limitation in mind.
Conclusion
The present study showed that a significant number of Kindergarten children were infected by intestinal parasites. A. lumbricoides and Schistosomiasis were the most predominant of the isolated parasites. Age, KG level, and parental education were significantly associated with the prevalence of intestinal helminthiasis among Kindergarten children. Therefore, it is advisable to design and implement strategies that can control the burden of such infections among Kindergarten children.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We would like to thank University of Gondar. We are grateful to all study participants, parents, schoolteachers, and data collectors.
Author Contributions
All authors made significant contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of this research work.
Funding
This study was funded by University of Gondar.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Awasthi S, Bundy DA, Savioli L. Helminthic infections. BMJ. 2003;327(7412):431–433. doi:10.1136/bmj.327.7412.431
2. Ahmadiara E, Hajimohammadi B. Notification, an important neglected essential education for children in kindergartens and primary schools (Education about parasitic infections in kindergartens). Int J Pediatr. 2017;5(9):5723–5724.
3. Albonico M, Crompton D, Savioli L. Control strategies for human intestinal nematode infections. Adv Parasitol. 1999;42:277–341.
4. Ngui R, Ishak S, Chuen CS, Mahmud R, Lim YA. Prevalence and risk factors of intestinal parasitism in rural and remote West Malaysia. PLoS Negl Trop Dis. 2011;5(3):e974. doi:10.1371/journal.pntd.0000974
5. Astiazarán-García H, Espinosa-Cantellano M, Castañón G, Chávez-Munguía B, Martínez-Palomo A. Giardia lamblia: effect of infection with symptomatic and asymptomatic isolates on the growth of gerbils (Meriones unguiculatus). Exp Parasitol. 2000;95(2):128–135. doi:10.1006/expr.2000.4514
6. Organization WH. Integrating neglected tropical diseases into global health and development: fourth WHO report on neglected tropical diseases. World Health Organization; 2017.
7. Organization WH. Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. World Health Organization; 2017.
8. Organization WH. Prevention and control of intestinal parasitic infections: report of a WHO Expert Committee [meeting held in Geneva from 3 to 7 March 1986]; 1987.
9. Jarabo M, Garcia-Moran N, Garcia-Moran J. Prevalence of intestinal parasites in a student population. Enferm Infecc Microbiol Clin. 1995;13(8):464.
10. Mduluza T, Chisango TJ, Nhidza AF, Marume A. Global control efforts of schistosomiasis and soil-transmitted helminthiasis. Human Helminthiasis. IntechOpen; 2017.
11. Improving health and learning through better water, sanitation and hygiene in schools. An information package for school staff. Copenhagen: WHO Regional Office for Europe; 2019 Licence: CC BY-NC-SA 3.0 IGO.
12. Mary G. Effects of public health intervention on intestinal parasitic infection among school-going children in Murang, a county, Kenya; 2016.
13. Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC Infect Dis. 2017;17(1):362. doi:10.1186/s12879-017-2466-x
14. Connolly K, Kvalsvig J. Infection, nutrition and cognitive performance in children. Parasitology. 1993;107(S1):S187–S200. doi:10.1017/S0031182000075612
15. Commission FDRoEPC. Summary and statistical report of the 2007 population and housing census. Addis Ababa, Ethiopia; 2008.
16. Alemu A, Tegegne Y, Damte D, Melku M. Schistosoma mansoni and soil-transmitted helminths among preschool-aged children in Chuahit, Dembia district, Northwest Ethiopia: prevalence, intensity of infection and associated risk factors. BMC Public Health. 2016;16(1):422. doi:10.1186/s12889-016-2864-9
17. Schistosomiasis WECotCo, Organization WH. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Who; 2002.
18. Spss I. IBM SPSS Statistics for Windows, Version 20.0. New York: IBM Corp; 2011:440.
19. Alemu M, Bedemo H, Bugssa G, Bayissa S, Tedla K. Epidemiology of intestinal parasite infections among kindergarten children in Mekelle Town, Northern Ethiopia. Int J Pharm Sci Res. 2015;6(11):1392–1396.
20. Dana D, Mekonnen Z, Emana D, et al. Prevalence and intensity of soil-transmitted helminth infections among pre-school age children in 12 kindergartens in Jimma Town, southwest Ethiopia. Trans R Soc Trop Med Hyg. 2015;109(3):225–227. doi:10.1093/trstmh/tru178
21. Haftu D, Deyessa N, Agedew E. Prevalence and determinant factors of intestinal parasites among school children in Arba Minch town, Southern Ethiopia. Am J Health Res. 2014;2(5):244–274. doi:10.11648/j.ajhr.20140205.15
22. Tulu B, Taye S, Amsalu E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of South Eastern Ethiopia: a cross-sectional study. BMC Res Notes. 2014;7(1):848. doi:10.1186/1756-0500-7-848
23. Ihemanma C. Prevalence of intestinal parasites and associated risk factors among school children (5–16 years) in Ihite-Ude, Ofeme community in Umuahia North L.G.A, Abia State. Int J Res Rev. 2015;2(12):732–738.
24. Afrakhteh N, Marhaba Z, Mahdavi SA, et al. Prevalence of Enterobius vermicularis amongst kindergartens and preschool children in Mazandaran Province, North of Iran. J Parasit Dis. 2016;40(4):1332–1336. doi:10.1007/s12639-015-0683-z
25. Alemu A, Atnafu A, Addis Z, et al. Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis. 2011;11(1):189. doi:10.1186/1471-2334-11-189
26. Lander RL, Lander AG, Houghton L, et al. Factors influencing growth and intestinal parasitic infections in preschoolers attending philanthropic daycare centers in Salvador, Northeast Region of Brazil. Cad Saude Publica. 2012;28:2177–2188. doi:10.1590/S0102-311X2012001100017
27. Ayalew A, Debebe T, Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar, Ethiopia. J Parasitol Vector Biol. 2011;3(5):75–81.
28. Okyay P, Ertug S, Gultekin B, Onen O, Beser E. Intestinal parasites prevalence and related factors in school children, a western city sample-Turkey. BMC Public Health. 2004;4(1):1–6. doi:10.1186/1471-2458-4-64
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
