Back to Journals » Infection and Drug Resistance » Volume 10
Prevalence and antibiotic resistance of coagulase-negative Staphylococci isolated from poultry farms in three regions of Ghana
Authors Boamah VE, Agyare C , Odoi H, Adu F, Gbedema SY , Dalsgaard A
Received 7 March 2017
Accepted for publication 27 April 2017
Published 13 June 2017 Volume 2017:10 Pages 175—183
DOI https://doi.org/10.2147/IDR.S136349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Vivian Etsiapa Boamah,1 Christian Agyare,1 Hayford Odoi,1 Francis Adu,1 Stephen Yao Gbedema,1 Anders Dalsgaard2
1Microbiology Section, Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; 2Section of Food Safety and Zoonoses, Department of Veterinary and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Frederisksberg, Denmark
Abstract: The use of antibiotics in animal production has been associated with the development and spread of antibiotic-resistant organisms including commensals. Coagulase-negative Staphylococcus (CoNS) species, which were until recently considered non-pathogenic, have been associated with opportunistic infections and high resistance to several antibiotics. This study sought to determine the prevalence, identity, and phenotypic resistance of coagulase-negative Staphylococcus spp. isolated from some selected poultry farms and farm workers in the Ashanti, Brong Ahafo, and Greater Accra regions of Ghana. Poultry litter samples and oral swabs of poultry farm workers were collected, from which bacterial species were isolated, identified, and analyzed. Various selective media were used for the presumptive identification of the different species. Confirmation of bacterial identity was done using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry. Antibiotic susceptibility testing of the isolates was performed using the Kirby-Bauer disk diffusion method. Zones of growth inhibition were interpreted based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. Two hundred and fifty-six coagulase-negative Staphylococcus spp., comprising S. sciuri (42.97%), S. lentus (35.94%), S. gallinarum (6.64%), S. xylosus (4.30%), S. haemolyticus (3.91%), S. saprophyticus (1.95%), and S. cohnii (0.39%) were confirmed by MALDI-TOF. CoNS were isolated from samples from the Brong Ahafo (48.83%), Ashanti (33.59%), and Greater Accra (17.78%) regions. Isolates from poultry litter constituted 55.47%, and farm workers 44.53%. All the isolates were susceptible to amoxicillin/clavulanic acid and amikacin. The isolates exhibited high resistance toward tetracycline (57.03%), doxycycline (43.75%), and oxacillin (43.36%). Multi-drug resistance (MDR) was observed in 19.14% of the isolates. MDR was higher in isolates obtained from poultry farm workers (61.22%) than isolates from poultry litter (38.78%). The above findings call for stricter monitoring of antibiotic usage in both animal production and in humans.
Keywords: poultry farms, antibiotic resistance, antibiotics, antimicrobials
Introduction
The bacteria genus Staphylococcus is a Gram-positive, facultative anaerobe, which is round in shape and appears in clusters.1 Staphylococcus spp. are also known to be non-motile, non-sporing, glucose fermenting, and catalase producing.2 Staphylococcus aureus is the most commonly known member of the family, and the most commonly known coagulase-positive strain.1 Members of the Staphylococcus genus that do not produce coagulase are known as coagulase-negative Staphylococci (CoNS). Until recently, CoNS were considered as the non-pathogenic members of the genus and thus were not of much interest to the research community. However, due to their implication in infections in both humans and animals, research interest in CoNS has increased over the past decade.3–5 In addition, CoNS have, over the last decade, developed resistance to multiple antibiotics,4,6,7 making their study worthwhile, especially, since they are known commensals and could be prevalent in most environments.5,8 The economic burden of Staphylococcal infections in animal husbandry includes decreased weight gain, drop in egg production (with respect to poultry), mortality, condemnation at slaughter, and lameness, among others.9,10
In Ghana, a developing country, with a growing population of about 25 million people and a land area of 385,500 square kilometers, agriculture provides employment and serves as the major source of livelihood for over 55% of the population.11 Several individuals engage in livestock production as a means of protein (meat and eggs) supplementation in the home, and also as a source of income.12,13 Commercial poultry production in Ghana is highly concentrated in the Ashanti, Brong Ahafo, and Greater Accra regions and these three regions contribute to almost 70% of commercial poultry production in the country.14,15
However, commercial poultry production in Ghana is challenged with occurrences of bacterial, viral, and parasitic infections.16 This compels poultry farmers to employ several antimicrobial agents, including essential antibiotics, on their farms.13,16 Studies have also shown that the farmers in the said regions employ these antibiotics even in the management of viral infections.13 The misuse of antibiotics in animal production has been closely associated with the development and spread of antibiotic-resistant organisms, including non-pathogenic species and commensals.8,17
Notwithstanding the fact that research interest in CoNS has increased in recent years, there is very little data on their prevalence and resistance profiles in Ghana. Information on CoNS is currently unavailable, especially in areas where misuse of antibiotics could lead to selection pressure, development of resistance, and spread of resistant strains. Hence, the aim of this study was to determine the prevalence and antibiograms of coagulase-negative Staphylococcus spp. isolated from poultry bedding materials (poultry litter) and poultry farm workers in the Ashanti, Brong Ahafo, and Greater Accra regions of Ghana.
Materials and methods
Ethical clearance and approval
Ethical clearance for the study was obtained from the Committee on Human Research Publications and Ethics (CHRPE), Kwame Nkrumah University of Science and Technology (KNUST), Kumasi. In addition, written consent was obtained from all participants.
Selection of farms
A total of 400 poultry farms were randomly selected from the three regions as previously described by Boamah et al.13 Sixty-one percent of the farms were located in the Ashanti region, 28.5% in the Brong Ahafo region, and 10.5% in the Greater Accra region. Eighty-two (20.5%) of the farms were small scale, 254 (63.5%) medium, and 64 (16%) large, as categorized by the Food and Agriculture Organisation (FAO).12
Sampling from poultry bedding and poultry workers
One gram of poultry bedding material (litter) was aseptically collected from three different points in a pen, in sterile sample containers. In a situation where a farm had less than five pens, samples were collected from at least three different pens, and from farms with more than five pens, samples were collected from at least five different pens. Oral swabs from farm workers who had worked on the farm for at least 1 month, and those directly involved in the day-to-day management of birds in the farm were also taken. The samples were appropriately labeled and transported to the laboratory in a cold box.
Isolation of CoNS species
Five and ten milliliters of trypticase soy broth (TSB) (Thermo Fisher Scientific, Waltham, MA, USA) were added to the samples from humans and poultry litter respectively, and then incubated at 37°C for 24 h. CoNS were isolated from the media by methods described by Reynolds18 and Kateete et al.,19 with slight modifications. Five hundred microliters suspension of TSB was aseptically transferred into 10 mL Mueller-Hinton broth (Thermo Fisher Scientific), supplemented with 6.5% NaCl, and incubated at 37°C for 24 h. After incubation, 10 µL was spread on 20 mL mannitol salt agar (MSA) (Thermo Fisher Scientific), and incubated at 37°C for 24 h. Well-isolated colonies on MSA medium were aseptically streaked onto 20 mL plates of Staphylococcus-aureus-select (SaSelect), (Bio-Rad Laboratories Inc., Hercules, CA, USA) and incubated at 37°C for 24 h. Bluish, purple-like, and whitish colonies grown on SaSelect were further streaked on 20 mL nutrient agar (Thermo Fisher Scientific) enriched with 5% sheep blood and then incubated at 37°C for 24 h. Identity of non-hemolytic colonies was then confirmed by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry.
Confirmation of isolates by MALDI-TOF mass spectrometry
Twenty-four hour cultures of non-hemolytic colonies on blood agar plates were used. Isolated colonies were uniformly spread on the MALDI-TOF slide to form a thin film and then covered with 1 µL of ready-to-use α-cyano-4-hydroxycinnamic acid and allowed to air-dry. Escherichia coli 25922 was used as the control strain. The MALDI-TOF slide was loaded into the VITEK MS (BioMérieux Corporate, Paris, France) for identification. The identity of the isolate was determined using Saramis® program (BioMérieux Corporate). Isolates with identities >99% were confirmed as CoNS. S. aureus 25923, E. coli ATCC 25922 (American Type Culture Collection, Manassas, VA, USA), and all other strains of CoNS were stored at -80°C until needed.
Antibiotic susceptibility testing
Antibiotic sensitivity of the Staphylococcus spp. was determined using the disk diffusion method described by EUCAST20 and Bauer et al.21 Twenty-four hour colonies of Staphylococci growing on blood agar plates were used for the susceptibility testing. To ensure a good representation of the isolates in the culture, approximately five to seven well-separated colonies were picked and suspended in 5 mL sterile distilled water and vortexed at high speed until the suspension was uniform. The turbidity of the suspension was determined using a nephelometer already calibrated to 0.5 McFarland. The turbidity of the suspensions were then adjusted appropriately to 0.5 McFarland with the addition of more colonies or sterile distilled water.
A sterile cotton swab was soaked in the inoculum and rotated twice against the inner side of the test tube to remove excess liquid. The swab was used to streak the entire surface of 20 mL MHA plate whilst rotating the plate at an angle of 60° with repeated streaking (three times in total). With the aid of a disk dispenser, antibiotic disks (benzyl penicillin 1 unit; ampicillin 10 µg; oxacillin 1 µg; cefoxitin 30 µg; trimethoprim/sulfamethoxazole 25 µg; ciprofloxacin 5 µg; norfloxacin 10 µg; gentamicin 10 µg; erythromycin 15 µg; tetracycline 30 µg; doxycycline 30 µg; chloramphenicol 30 µg) purchased from Thermo Fisher Scientific, were placed on the surface of the MHA. The plates were incubated at 37°C for 20 h. Zones of growth inhibition (mm) were measured and recorded. Susceptibility of the isolates to different antibiotics was determined and classified according to EUCAST.20
Results
Distribution of different Staphylococcus spp. in the selected farms
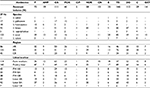
Of the 368 presumptive Staphylococcal colonies isolated using selective media, 256 (69.57%) were confirmed by MALDI-TOF mass spectrometry as coagulase-negative Staphylococcus spp. One hundred and fourteen (44.53%) were from farm workers whilst isolates from the poultry bedding material constituted 55.47%. Seven different CoNS species were identified and these include S. sciuri (42.97%), S. lentus (35.94%), S. gallinarum (6.64%), S. xylosus (4.30%), S. haemolyticus (3.91%), S. saprophyticus (1.95%), and S. cohnii (0.39%) (Table 1).
Antibiotic sensitivity profiles of isolated CoNS
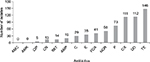
All the isolates (256) were susceptible to amoxicillin/clavulanic acid and amikacin. Fifty-seven percent (57.03%) of the isolates were resistant to tetracycline, 43.75% to doxycycline, 43.36% to oxacillin, and 28.52% to penicillin. The isolates showed low resistance to ampicillin (7.42%), trimethoprim/sulfamethoxazole (5.47%), gentamycin (5.00%), and ciprofloxacin (1.95%) (Figure 1).
Distribution of resistant coagulase-negative Staphylococcus spp.
Each of the seven different coagulase-negative Staphylococcus spp. exhibited some level of resistance toward penicillin, oxacillin, cefoxitin, and tetracycline. All the different species were most susceptible toward ciprofloxacin. Isolates from all the three regions showed varying levels of resistance to the different antibiotics, with the exception of isolates from the Ashanti region which were totally susceptible to ciprofloxacin. Both sets of isolates from farm workers and poultry litter exhibited various levels of resistance to the antibiotics. However, isolates from farm workers’ samples in the Brong Ahafo and Ashanti regions and isolates from litter samples in the Ashanti regions did not exhibit any resistance toward ciprofloxacin. Also, none of the isolates obtained from litter samples in the Greater Accra region was resistant to chloramphenicol (Table 2).
Multi-drug resistant CoNS
Forty-nine (19.14%) of the isolates exhibited multi-drug resistance (MDR) (simultaneous resistance to three or more antibiotics) (Table 3).22 Thirty isolates (61.22%) from farm workers were MDR isolates whilst 19 (38.78%) MDR isolates were from poultry litter. Twenty-three (46.94%) of the MDR strains were from samples from the Ashanti region, 15 (30.61%) from the Brong Ahafo region, and 11 (22.45%) from the Greater Accra region (Table 4). Among the Staphylococcus spp., 27 (55.1%) of the MDR isolates were S. sciuri, 9 (18.37%) S. lentus, 5 (10.20%) S. gallinarum, 3 (6.12%) S. haemolyticus, 2 (4.08%) isolates each were S. saprophyticus and S. xylosus, and 1 (2.04%) S. cohnii. Farm workers contributed 30 (61.22%) of the MDR Staphylococcal isolates whilst poultry litter had 19 (38.78%) of the MDR isolates (Figure 2).
  | Figure 2 Multi-drug resistant patterns of coagulase-negative Staphylococci isolated from poultry farms in the selected regions. |
Discussion
The increasing rates of antibiotic resistance and MDR among pathogenic, non-pathogenic, commensals, and opportunistic bacteria call for increased report on the distribution (prevalence) of these organisms and their antibiotic profiles.23 Many Staphylococcus spp. are part of the normal bacterial flora in humans and animals.18 Several of these commensal and non-pathogenic Staphylococci have been implicated in infections.5 Many of such species have also been reported as multi-drug resistant, which has resulted in increased cost of treating infections and increased disease burden in both humans and animal husbandry.3,23
Seven different coagulase-negative Staphylococcus spp. including S. sciuri, S. lentus, S. gallinarum, S. xylosus, S. haemolyticus, S. saprophyticus, and S. cohnii were identified in both farm workers and poultry litter samples. The presence of different Staphylococcus spp. among poultry litter and farm workers has been reported by Simjee et al.24 and Vadari et al.25 The latter identified 19 different Staphylococcus spp. from poultry litter using molecular techniques (16s rDNA). The species included all the species identified in this study, with the exception of S. gallinarum. The low number of species identified in this study compared to that of Vadari et al.25 could be due to the identification method used. Molecular techniques detect the presence of both viable and non-viable organisms, whilst only viable organisms are identified using morphological and biochemical means.
In the report by Simjee et al.,24 38% of the coagulase-negative Staphylococcus spp. were S. sciuri, whereas S. lentus and S. xylosus constituted 21% and 14%, respectively. The prevalence of S. sciuri among the farms in this study was 44%, whilst S. lentus and S. xylosus were 37% and 4%, respectively. Staphylococcus simulans was not found in this current study but was detected by Simjee et al.24 On the other hand, Staphylococcus spp. including S. cohnii, S. saprophyticus, and S. gallinarum were not identified and reported by Simjee et al.24 The differences in the Staphylococcus spp. identified could be due to the different bacterial flora within the study areas.26
There have been reports of increasing resistance of Staphylococcus spp. to several essential antibiotics over the past 30 years26–28 in different countries including Ghana.29 In this study, coagulase-negative Staphylococcus spp. equally showed a high level of resistance mostly to tetracycline (57%), doxycycline (44%), and penicillin (28%). However, the resistance observed toward penicillin was less than the number Lerbech et al.29 reported, with 98% CoNS isolates from humans being resistant to penicillin. The high resistance of CoNS to penicillin reported by Lerbech et al.29 could be due to the fact that all the isolates were obtained from patients, and such isolates have been found to be highly resistant to beta-lactams.30 Resistance of the isolates to tetracycline (57%) was almost similar to the 63% reported by Lerbech et al.29
All the isolates from this study were susceptible to amoxicillin/clavulanic acid and amikacin. This may be due to the fact that these two antibiotics are not part of the antimicrobial agents routinely employed in poultry production in Ghana,31 hence, amoxicillin/clavulanic acid and amikacin preparations remain reserved antibiotics used for the treatment of Staphylococcus spp. infections.32
Forty-nine (19.14%) of the CoNS isolated were multi-drug resistant with the highest resistance to tetracycline (59.18%), doxycycline (59.18%), and erythromycin (57.14%). Suleiman et al.33 reported 65% MDR Staphylococci isolates in a similar study in Nigeria. The high level of MDR Staphylococci could be due to the misuse of antibiotics in the poultry industry in many developing countries, including Ghana13 and Nigeria.34 Coagulase-negative Staphylococci are also known to form biofilms and this reduces the effect of antimicrobial agents against them.26,27
The majority (61%) of the MDR CoNS isolates were isolated from humans including farm workers, owners, and managers, whereas the bedding material had 39% of the MDR isolates. These findings could be due to the fact that farm workers are more exposed to MDR Staphylococcus strains because of the direct contact or interactions between humans and animals. These findings confirm the reports by Lerbech et al.,29 Feglo et al.,35 Obeng-Nkrumah et al.,36 and Newman et al.,37 which showed a high level of antibiotic resistance among humans in Ghana.
Conclusion
Coagulase-negative Staphylococci (256 isolates) from seven different species were identified and they exhibited varying levels of resistance to the selected antibiotics, with 49 multi-drug resistant isolates, and over 60% of these MDR Staphylococcus strains were from human samples. These call for increased surveillance measures and monitoring of antibiotic use in both animal husbandry and in humans in Ghana.
Acknowledgments
The authors are grateful to L’Oreal/African Network of Scientific and Technological Institutions (ANSTI)/United Nations Educational, Scientific and Cultural Organization (UNESCO)/Fellowship for Women in Science (FWIS) and the Danish International Development Agency (DANIDA)/Building Stronger Universities (BSU) for grants and fellowships to VEB.
Disclosure
The authors report no conflicts of interest in this work.
References
Barrow GI, Feltham RK, editors. Cowan and Steel’s Manual for the Identification of Medical Bacteria. 3rd ed. Cambridge University Press; 2009. | ||
Harley JP, Prescott LM. Laboratoy Exercises in Microbiology. 5th ed. New York: The McGraw-Hill Companies, New York; 2002. | ||
Boerlin P, Kuhnert P, Hussy D, Schaellibaum M. Methods for identification of Staphylococcus aureus isolates in cases of bovine mastitis. J Clin Microbiol. 2003;41(2):767–771. | ||
Koksal F, Yasar H, Samasti M. Antibiotic resistance patterns of coagulase-negative staphylococcus strains isolated from blood cultures of septicemic patients in Turkey. Microbiol Res. 2009;164(4):404–410. | ||
May L, Klein EY, Rothman RE, Laxminarayan R. Trends in antibiotic resistance in coagulase-negative staphylococci in the United States, 1999 to 2012. Antimicrob Agents Chemother. 2014;58(3):1404–1409. | ||
Ma XX, Wang EH, Liu Y, Luo EJ. Antibiotic susceptibility of coagulase-negative staphylococci (CoNS): emergence of teicoplaninnon-susceptible CoNS strains with inducible resistance to vancomycin. J Med Microbiol. 2011;60(Pt 11):1661–1668. | ||
Qu Y, Daley AJ, Istivan TS, Garland SM, Deighton MA. Antibiotic susceptibility of coagulase-negative staphylococci isolated from very low birth weight babies: comprehensive comparisons of bacteria at different stages of biofilm formation. Ann Clin Microbiol Antimicrob. 2010;9:16. | ||
Carlet J, Jarlier V, Harbarth S, Voss A, Goossens H, Pittet D; Participants of the 3rd World Healthcare-Associated Infections Forum. Ready for a world without antibiotics? The Pensières Antibiotic Resistance Call to Action. Antimicrob Resist Infect Control. 2012;1(1):11. | ||
Andreasen CB. Overview of Staphylococcosis in Poultry. Merck Vet Man. 2013. | ||
Giguère S, Prescott JF, Dowling PM. Antimicrobial Therapy in Veterinary Medicine. 5th ed. Wiley Blackwell; 2013. | ||
Ghana Statistical Service. 2010 Population and Housing Census; 2012. Available from: http://www.statsghana.gov.gh/docfiles/2010phc/Census2010_Summary_report_of_final_results.pdf. Accessed April 29, 2015. | ||
Food and Agriculture Organization of the United Nations; FAO Animal Production and Health Division. Poultry Sector Ghana; FAO Animal Production and Health; Livestock Country Reviews; 2014. Available from: http://www.fao.org/docrep/019/i3663e/i3663e.pdf. Accesssed May 20, 2015. | ||
Boamah VE, Agyare C, Odoi H, Dalsgaard A. Antibiotic practices and factors influencing the use of antibiotics in selected poultry farms in Ghana. J Antimicrob Agents. 2016;2(2):120. | ||
Sumberg J, Awo M, Fiankor DD, Kwadzo GT-M, Thompson J. Ghana’s Poultry Sector: Limited Data, Conflicting Narratives, Competing Visions; 2013. Available from: https://steps-centre.org/wp-content/uploads/Ghana-Poultry-online.pdf. Accessed June 6, 2015. | ||
Food and Agriculture Organization of the United Nations; FAO Animal Production and Health Division. Poultry sector country review; Ghana; 2008. Available from: ftp://ftp.fao.org/docrep/fao/011/ai354e/ai354e00.pdf. Accessed April 29, 2015. | ||
Annan-Prah A, Agbemafle E, Asare PT, Akorli SY. Antibiotic use, abuse and their public health implication: the contributory role of management flaws in the poultry industry in two agro-ecological zones in Ghana. Journal of Veterinary Advances. 2012;2(4):199–208. | ||
Cogliani C, Goossens H, Greko C. Restricting antimicrobial use in food animals: lessons from Europe. Microbe. 2011;6(6):274–279. | ||
Reynolds J, editor. Genus Staphylococcus: Identification of Species. New York, NY: VCH Publisher, Inc; 2011. | ||
Kateete DP, Kimani CN, Katabazi FA, et al. Identification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase test. Ann Clin Microbiol Antimicrob. 2010;9:23. | ||
EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Available from: www.eucast.org. Accessed May 5, 2017. | ||
Bauer A, Kirby W, Sherris J, Turk M. Antibiotic susceptibility testing by a standard single disc diffusion method. Am J Clin Pathol. 1966;45(4):493–496. | ||
Saana SB, Adu F, Agyare C, Gbedema SY, Boamah VE, George DF. Antibiotic resistance patterns of strains of Staphylococcus aureus isolated from patients in three hospitals in Kumasi, Ghana. African Journal of Bacteriology Research. 2013;5(3):35–40. | ||
World Health Organization (WHO). Antimicrobial resistance: global report on surveillance 2014; 2014. Available from: http://www.who.int/drugresistance/documents/surveillancereport/en/. Accessed April 29, 2016 . | ||
Simjee S, McDermott P, White DG, et al. Antimicrobial susceptibility and distribution of antimicrobial-resistance genes among enterococcus and coagulase-negative Staphylococcus isolates recovered from poultry litter. Avian Dis. 2007;51(4):884–892. | ||
Vadari Y, Mason BP, Doerner KC. Isolation from poultry litter and characterization [corrected] of Staphylococcus spp. capable of growth in high phosphate conditions [corrected]. Lett Appl Microbiol. 2006;43(1):64–70. | ||
John JF, Harvin AM. History and evolution of antibiotic resistance in coagulase-negative staphylococci: Susceptibility profiles of new anti-staphylococcal agents. Ther Clin Risk Manag. 2007;3(6):1143–1152. | ||
Hamilton-Miller JM, Iliffe A. Antimicrobial Resistance in coagulase-negative staphylococci. J Med Microbiol. 1985;19(2):217–226. | ||
Yurdakul NE, Erginkaya Z, Ünal E. Antibiotic resistance of enterococci, coagulase negative staphylococci and staphylococcus aureus isolated from chicken meat. Czech Journal of Food Sciences. 2013;31(1):14–19. | ||
Lerbech AM, Opintan JA, Bekoe SO, et al. Antibiotic exposure in a low-income country: screening urine samples for presence of antibiotics and antibiotic resistance in coagulase negative staphylococcal contaminants. PLoS One. 2014;9(12):e113055. | ||
Sharma V, Jindal N, Devi P. Prevalence of methicillin resistant coagulase negative staphylococci in a tertiary care hospital. Iran J Microbiol. 2010;2(4):185–188. | ||
Turkson PK. Use of drugs and antibiotics in poultry production in Ghana. Ghana Journal of Agricultural Science. 2008;41(1). | ||
Ministry of Health (GNDP) Ghana. Standard Treatment Guidelines. 6th ed. Yamens Press Ltd Accra; 2010. | ||
Suleiman A, Zaria LT, Grema HA, Ahmadu P. Antimicrobial resistant coagulase positive Staphylococcus aureus from chickens in Maiduguri, Nigeria. Sokoto Journal of Veterinary Sciences. 2013;11(1):51–55. | ||
Mainda G, Bessell PB, Muma JB, et al. Prevalence and patterns of antimicrobial resistance among Escherichia coli isolated from Zambian dairy cattle across different production systems. Sci Rep. 2015;5:12439. | ||
Feglo P, Adu-Sarkodie Y, Ayisi L, et al. Emergence of a novel extended-spectrum-β-Lactamase (ESBL)-producing, fluoroquinolone-resistant clone of extraintestinal pathogenic Escherichia coli in Kumasi, Ghana. J Clin Microbiol. 2013;51(2):728–730. | ||
Obeng-Nkrumah N, Twum-Danso K, Krogfelt KA, Newman MJ. High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistance. Am J Trop Med Hyg. 2013;89(5):960–964. | ||
Newman MJ, Frimpong E, Donkor ES, Opintan JA, Asamoah-Adu A. Resistance to antimicrobial drugs in Ghana. Infect Drug Resist. 2011;4:215–220. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.