Back to Journals » OncoTargets and Therapy » Volume 13
Pretreatment Albumin-to-Fibrinogen Ratio Independently Predicts Chemotherapy Response and Prognosis in Patients with Locally Advanced Rectal Cancer Undergoing Total Mesorectal Excision After Neoadjuvant Chemoradiotherapy
Authors Li H, Wang H, Shao S, Gu Y, Yao J, Huang J
Received 22 October 2020
Accepted for publication 11 December 2020
Published 23 December 2020 Volume 2020:13 Pages 13121—13130
DOI https://doi.org/10.2147/OTT.S288265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Hongzhi Li,1 Honggang Wang,2 Shanshan Shao,1 Yawen Gu,1 Juan Yao,1 Junxing Huang1
1Department of Oncology, Taizhou People’s Hospital, Taizhou, Jiangsu, People’s Republic of China; 2Department of General Surgery, Taizhou People’s Hospital, Taizhou, Jiangsu, People’s Republic of China
Correspondence: Junxing Huang
Department of Oncology, Taizhou People’s Hospital, No. 210 Yingchun Road, Taizhou City, 225300 Jiangsu Province, People’s Republic of China
Email [email protected]
Background: Neoadjuvant chemoradiotherapy (nCRT) followed by surgery of total mesorectal excision (TME) is currently accepted as the standard treatment for locally advanced rectal cancer (LARC). This study aimed to investigate the potential prognostic factors, including the albumin-to-fibrinogen ratio (AFR) for LARC patients.
Methods: We retrospectively recruited LARC patients (cT3-4 and/or cN1-2) who underwent nCRT followed by TME between January 2011 and January 2015. The cut-off value of pretreatment AFR for overall survival (OS) was determined by the receiver operating characteristic (ROC) curve. The potential predictive factors for prognosis in the LARC patients were assessed by the univariate and multivariate Cox’s proportional hazard regression and Kaplan–Meier curve analyses.
Results: AFR was a significant predictor for OS with a cut-off value of 8.65 and an AUC of 0.882 (P< 0.001). The pretreatment AFR level was the only independent risk factor for pathologic response to nCRT (HR: 2.44, 95% CI: 1.43– 4.17, P=0.003), 5-year OS (HR: 3.31, 95% CI: 1.51– 6.77, P=0.005) and disease-free survival (DFS) (HR: 2.73, 95% CI: 1.34– 5.47, P=0.007) in LARC patients. A low pretreatment AFR level was significantly associated with a poor 5-year OS and DFS by the Log rank test (P=0.003 and 0.006, respectively).
Conclusion: Pretreatment AFR level was an independent prognostic factor in LARC patients undergoing TME after nCRT.
Keywords: locally advanced rectal cancer, total mesorectal excision, neoadjuvant chemoradiotherapy, prognosis, albumin-to-fibrinogen ratio
Introduction
Colorectal cancer (CRC), is the third most commonly diagnosed cancer and the second leading cause of cancer-related death in both sexes combined worldwide.1 According to GLOBOCAN reports, there were over 1.8 million new CRC cases and 881,000 CRC-related deaths in 2018, accounting for almost 10% of cancer cases and deaths.1 Rectal cancer (RC) approximately accounts for 30% of CRC and has a worse clinical prognosis.2 To improve tumor resectability, preserve anal sphincter, and localize tumor for locally advanced rectal cancer (LARC) patients, neoadjuvant chemoradiotherapy (nCRT) followed by surgery of total mesorectal excision (TME) is currently accepted as the standard treatment.3,4 The prognosis and survival rates of the LARC patients with nCRT have significantly improved.5 However, the clinical efficacy differs significantly in different patients due to personal heterogeneity. Thus, to investigate the effective predictors and design individualized treatment for LARC patients is of great clinical meaning.
Accumulating evidence has indicated that the host factors, including the status of nutrition, inflammation, and immune system, are significantly associated with the prognosis in patients with malignancies as well as tumor characteristics.6,7 Albumin (Alb), and fibrinogen (Fib) are two widely used inflammatory markers and they are both accepted as potential prognostic factors in various malignancies, eg, lung cancer,8 gastric cancer,9 and colon cancer.10 Alb-to-Fib ratio (AFR), which is calculated based on Alb and Fib concentrations, has also been reported as a prognostic parameter in various malignancies.11,12 However, whether AFR could serve as a predictor for treatment response and survival in LARC patients undergoing nCRT remains unclear. This study aimed to investigate potential prognostic factors (including AFR) for LARC patients.
Patients and Methods
Patients
This single-center retrospective study was approved by the Medical Institutional Ethics Committee of our hospital (approval number: KY200901103). We retrospectively recruited LARC patients (cT3-4 and/or cN1-2) who underwent nCRT followed by TME at the Department of Oncology and General Surgery, Taizhou Peoples’ Hospital between January 2009 and January 2016. All enrolled patients were required to offer the signed informed consent for nCRT and operations. The inclusion criteria were as follows: (a) aged between 18 and 75 years; (b) histologically diagnosed with LARC within 12 cm from the anal verge; (c) with the imaging results of abdominal and pelvic computed tomography (CT) or PET-CT, pelvic magnetic resonance imaging (MRI); and (d) undergoing TME after nCRT. The exclusion criteria were as follows: (a) with distant metastasis; (b) combined with hepatic, kidney, or hematological disorders; (c) with autoimmune diseases, acute infections, or other malignancies; (d) with molecular targeted drugs therapy; (e) who did not finish the nCRT due to adverse reactions; and (f) with incomplete data or lost to follow-up. This study was conducted in accordance with the Declaration of Helsinki.
Treatment and Follow-Up
Before the start of nCRT, all enrolled patients received the pathological diagnosis by rectal biopsy. The nCRT protocols, surgical procedures, and follow-ups were performed according to the latest Chinese Guidelines for the diagnosis and treatment of colorectal cancer (2009, 2015). In brief, preoperative radiotherapy was administered to the area of the pelvic region (radiation dose: 45.0–50.4 Gy, 1.8–2.0 Gy per time, 5 times per week, for 25–28 times). During the radiotherapy, chemotherapy was concomitantly performed. FOLFOX or XELOX was the general chemotherapy regimen with a duration of 2–3 months and 6–12 weeks after the completion of nCRT, TME was performed as the standard surgical procedure. Based on the guidelines, the postoperative 5-year follow-up was performed every 3 months within the first 2 years, and every 6 months in the following 3 years.
Pathologic Response to nCRT
As described by previous reports by Mandard et al13, the pathologic response to nCRT was evaluated using five tumor regression grades (TRG1–5). In brief, TRG1 (complete regression): absence of residual cancer and fibrosis; TRG2: presence of rare residual cancer cells scattered through the fibrosis; TRG3: increased number of residual cancer cells, but fibrosis predominated; TRG4: residual cancer outgrowing fibrosis; and TRG5: absence of regressive changes. A good pathologic response to nCRT was defined as TRG1-3, while a poor pathologic response was defined as TRG4-5.
Prognosis Evaluation
All the enrolled patients were followed-up until death or over a period of 5 years. Locoregional recurrence was defined as the tumor recurrence of lymphatic vessels, anastomosis, or adjacent organs. Distant metastasis was defined as the tumor spread outside the pelvic cavity. Disease-free survival (DFS) was calculated from the day of initial nCRT beginning to the disease progression (distant metastasis, or locoregional recurrence), death or the 5-year follow-up. Overall survival (OS) was calculated from the day of initial nCRT beginning to death or the 5-year follow up.
Data Collection
The demographics and clinicopathological characteristics of the enrolled patients were collected, including the age, gender, body mass index (BMI), pretreatment comorbidities (hypertension, diabetes), habits of smoking and drinking, distance from anal verge, clinical T and N stage before nCRT, lymph vascular invasion, perineural invasion, pathologic differentiation, and postoperative TNM stage. The TNM stage was verified based on the CRC TNM Staging System by the American Joint Committee on Cancer/International Union Against Cancer (7th edition). The treatment-related parameters including radiotherapy dose, operation type, operation time and estimated blood loss were also recorded. The tumor mutational status including KRAS, BRAF, and mismatch repair (MMR) were also recorded. As described in previous reports, if one or more of the four proteins (MLH1, PMS2, MSH2, and MSH6) in the tumor epithelial cell nuclei was defective, the patients were identified as defective MMR (dMMR). If all the proteins were positive, then the patients were identified as proficient MMR (pMMR).
Laboratory Tests
Fasting blood samples of all the enrolled patients were obtained on 1 day before nCRT initiation. Blood cell and biochemical analyses including hemoglobin (Hb), white blood cell (WBC), hematocrit (Hct), C-reactive protein (CRP), creatinine, urea, Alb, and Fib were detected in the laboratory of our hospital using the blood samples obtained from them. AFR was calculated as Alb (g/L) divided by Fib (g/L).
Statistical Analysis
The statistical analysis was performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Inc., CA, USA). Categorical and quantitative data were expressed as number (n) with percentage (%) and mean levels with standard deviation (SD) as appropriate. Chi-square test, Student’s t-test, and Mann–Whitney U-test were used for data analysis as appropriate. The receiver operating characteristic (ROC) curve was plotted to identify the cut-off value of the pretreatment AFR for OS. The univariate and multivariate Cox’s proportional hazard regression analyses were performed to investigate the risk factors for pathologic response to nCRT, 5-year OS and DFS. The Kaplan–Meier curve analyses were performed to identify the association between the pretreatment AFR and 5-year OS and DFS. A two-sided P value <0.05 was accepted as statistically different.
Results
Patient Characteristics
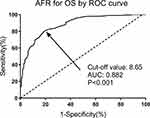
The flowchart of this study is shown in Figure 1. According to the inclusion criteria, 378 patients were initially enrolled and 58 were excluded on the basis of the exclusion criteria (8 with distant metastasis, 10 combined with hepatic, kidney, or hematological disorders, 10 with autoimmune diseases, infections, or other malignancies, 5 with molecular-targeted drugs therapy, and 20 with incomplete data or lost to follow-up). As a result, 320 LARC patients who underwent TME after nCRT were included in this retrospective study. To identify the cut-off value of pretreatment AFR for OS, ROC curve analysis was conducted. As shown in Figure 2, AFR was a significant predictor for OS with a cut-off value of 8.65, an AUC of 0.882, a sensitivity of 80.68%, a specificity of 80.53% (95% CI: 0.844–0.920, P<0.001). Based on the cut-off value, the enrolled patients were categorized into two groups, the high AFR group (≥8.65, n=131) and the low AFR (<8.65, n=189) group. The detailed demographics and clinicopathological characteristics are summarized in Table 1. The median age was 54 years and male patients occupied 70.3% (225/320). The differences in clinicopathological characteristics were compared between the low and high AFR groups (Table 1). The patients in low the AFR group had a higher rate of elderly (≥65 years) patients (P=0.022), a higher Charlson Comorbidity Index (P=0.008), comorbidity of hypertension (P=0.038), and positive lymph vascular invasion (P=0.032) than those in the high AFR group. Moreover, the low AFR group was associated with a poorer (or mucinous) pathologic differentiation (P=0.021), a higher pathological TNM stage (P=0.021), and a poorer pathologic response to nCRT (TRG4-5). No statistical differences were observed with respect to gender, BMI, diabetes, habits of smoking and drinking, distance from anal verge, cT and cN stage before nCRT, perineural invasion, radiotherapy dose, operation type, operation time and estimated blood loss between the patients with high and low AFR (P>0.05). Table 2 lists the laboratory tests associated with AFR in the LARC patients. Patients in the low AFR group had a higher rate of elevated CRP level (>0.8mg/L) in comparison with those in the high AFR group (P=0.008).
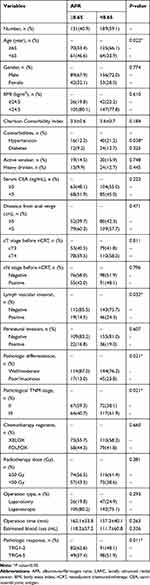 |
Table 1 Clinicopathological Variables Associated with AFR in LARC Patients |
 |
Table 2 Laboratory Tests Associated with AFR in LARC Patients |
Risk Factors for Pathologic Response to nCRT, 5-Year OS and DFS
To investigate potential prognostic factors for LARC patients, we choose pathologic response to nCRT, 5-year OS and DFS as the observational endpoints. As illustrated in Table 3, lymph vascular invasion (HR: 2.15, 95% CI: 1.03–4.37, P=0.019) and pretreatment AFR (<8.65 vs ≥8.65) (HR: 2.44, 95% CI: 1.43–4.17, P=0.003) were two independent risk factors for TRG4-5 (poor pathological response) by univariate and multivariate Cox regression analyses. Moreover, lymph vascular invasion (HR: 0.46, 95% CI: 0.33–0.61, P=0.023) and pretreatment AFR (HR: 0.39, 95% CI: 0.21–0.62, P=0.009) were also risk factors for TRG1 (complete response, see Table 4). In addition, age (<65 vs ≥65) (HR: 2.42, 95% CI: 1.10–5.32, P=0.029), pathologic differentiation (well/moderate vs poor/mucinous) (HR: 2.83, 95% CI: 1.44–7.33, P=0.018) and pretreatment AFR (≥8.65 vs <8.65) (HR: 3.31, 95% CI: 1.51–6.77, P=0.005) were the three independent predictors for 5-year OS in LARC patients undergoing TME after nCRT (see Table 5). Furthermore, pretreatment AFR (≥8.65 vs <8.65) (HR: 2.73, 95% CI: 1.34–5.47, P=0.007) and CEA (<5 vs ≥5) (HR: 1.73, 95% CI: 1.12–2.66, P=0.034) were two significant factors for 5-year DFS, which is shown in Table 6.
 |
Table 3 Risk Factors Associated with Poor Pathological Response (TRG4-5) in LARC Patients Undergoing nCRT by Univariate and Multivariate Cox Proportional Hazards Analyses |
 |
Table 4 Risk Factors Associated with Complete Response (TRG1) in LARC Patients Undergoing nCRT by Univariate and Multivariate Cox Proportional Hazards Analyses |
 |
Table 5 Risk Factors Associated with 5-Year OS in LARC Patients by Univariate and Multivariate Cox Proportional Hazards Analyses |
 |
Table 6 Risk Factors Associated with 5-Year DFS in LARC Patients by Univariate and Multivariate Cox Proportional Hazards Analyses |
Pretreatment AFR Associated with 5-Year OS and DFS
To further identify the association between pretreatment AFR and survival in LARC patients, Kaplan–Meier curve analyses were performed. As shown in Figure 3A and B, the results indicated that a low pretreatment AFR level was significantly associated with a poor 5-year OS and DFS by Log rank test (P=0.003 and 0.006, respectively).
Tumor Mutational Status, AFR and Oncologic Outcomes
We also evaluated the status of tumor mutational status, AFR and oncologic outcomes in 248 patients with complete mutational data. As shown in Table 7, no significant association between tumor mutational status (KRAS, BRAF, and MMR) and AFR was observed (P>0.05). In addition, the mutational status was also not significantly associated with 5-year OS (see Figure 4A–C, P>0.05).
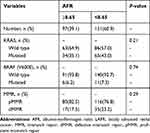 |
Table 7 KRAS, BRAF and MMR Status Associated with AFR in LARC Patients |
Discussion
Our findings firstly indicated that pretreatment AFR was an independent prognostic factor for the LARC patients undergoing TME after nCRT. In this study, three observational endpoints including pathologic response to nCRT, 5-year OS and DFS were set for prognosis evaluation. Our univariate and multivariate Cox proportional hazard regression analyses revealed different risk factors for different endpoints (eg, age, pathologic differentiation, and pretreatment AFR for 5-year OS). However, our results only supported the pretreatment AFR level as the only independent risk factor for pathologic response to nCRT, 5-year OS and DFS.
The specific mechanism for the prognostic role of AFR predicting cancer prognosis remains incomplete in terms of clarity. Fib, an important protein in the maintenance of hemostasis, is also widely reported to be an acute-phase protein that is involved in inflammatory responses.14 Moreover, the synthesis of Fib can be regulated by several inflammatory cytokines, including interleukin-1 (IL-1) and IL-6.15 There is increasing evidence that an elevated Fib level can serve as a strong predictor for unfavorable outcomes in some types of cancers, such as epithelial ovarian cancer16 and pancreatic cancer.17 The frequently observed elevated Fib level in patients with multiple cancers is related to unfavorable prognoses.18,19 In cancer patients, elevated Fib expression due to the abnormally activated coagulation system can possess anti-cancer properties combined with sodium selenite.20 In addition, Fib can regulate the growth of tumor cells by binding to various growth factors,21 and enhance cell invasion, migration, and metastasis via epithelial–mesenchymal transition.22 Furthermore, Fib can also participate in tumor progression by involving in angiogenesis.23
Alb, a well-established nutritional biomarker, is also an acute-phase protein in response to systemic inflammation.14 The synthesis of Alb in hepatocytes can be significantly affected by proinflammatory cytokines released by inflammatory cells or tumor tissues, eg IL-4, and IL-6, resulting in decreased Alb expression.24 A previous study of 431 CRC patients identified that serum Alb expression was a reliable prognostic factor for overall survival.25 A low serum Alb level usually heralds the status of malnutrition, which indicates the weakness of the immune system in patients.26 In addition, decreased serum Alb correlates to an enhanced inflammatory response to cancers and increased release of various cancer-related cytokines involved in tumor development.27
AFR, which combines these two biomarkers, has attracted a lot of attention in recent decades. Recently, accumulating evidence has verified the prognostic value of AFR in various diseases, including peritonitis-induced sepsis,28 non-small-cell lung cancer after platinum-based chemotherapy,29 and advanced epithelial ovarian cancer.30 In addition, some other biomarkers reflecting systematic inflammatory status, such as CRP, and neutrophil-to-lymphocyte ratio (NLR), have also shown prognostic values in malignancies.31 It has been well established that inflammation can promote carcinogenesis and increase the risk of cancer development, including CRC.32 Moreover, regular non-steroidal anti-inflammatory drugs are associated with a decreased risk of CRC.33 The complicated and close correlation between inflammation and tumors might be a possible explanation for the prognostic role of AFR in LARC patients. A previous study by Shen et al revealed baseline NLR (≥2.8) as a prognostic factor for LARC patients undergoing nCRT.34 A clinical trial by Dudani et al reported that NLR and platelet-to-lymphocyte ratio (PLR) are two useful predictive and prognostic markers in LARC patients.35 However, some other studies did not indicate a significant association between NLR, PLR and outcomes in LARC patients.36,37 A recent meta-analysis by Jin et al indicated lymph node ratio (LNR) as an independent prognostic factor for RC patients after nCRT.38 A multi-institutional study on 965 LARC patients undergoing nCRT indicated elevated platelet count as a negative predictive and prognostic marker.39 To our knowledge, this study firstly highlighted AFR as an independent risk factor for both pathological response for nCRT and prognosis in LARC patients.
Due to the individual heterogeneity to treatment response, it is necessary for clinical practice to investigate novel predictors and generate personalized treatment strategies. The laboratory parameters added to clinicopathological characteristics (eg, TNM stage, pathologic differentiation) may have important roles in the personalized treatment determination. Among the clinicopathological and laboratory variables, AFR has some significant advantages, such as high sensitivity, wide availability, easy acquirement, and low economic cost. Pre-treatment evaluation of the AFR may have significant meanings in risk stratifications and prognosis prediction of LARC. More intensive care, frequent treatment efficacy evaluation and postoperative follow-up, and timely therapeutic strategies adjustment are suggested for patients with low AFR levels. We consider that pretreatment AFR evaluation may be beneficial for the therapeutic management and follow-up of LARC patients. However, we admit that this study has some great limitations. First, this is a single-center study with a relatively small sample size. Second, whether the modulation of pretreatment AFR level (eg Alb supplement, coagulation function improvement) can improve the prognosis of LARC patients remains unknown due to the retrospective nature of this study. Last, only pretreatment AFR was calculated and whether the AFR level at some other time points (eg after nCRT treatment, after the surgery) can also predict the prognosis in LARC patients is unclear.
Conclusions
This study indicated that pretreatment AFR level was an independent risk factor for pathologic response to nCRT, 5-year OS and DFS in LARC patients undergoing TME after nCRT.
Data Sharing Statement
Please contact the corresponding author (Junxing Huang, email: [email protected]) for data requests.
Ethics Approval and Consent to Participate
This study was approved by the Medical Institutional Ethics Committee of Taizhou People’s Hospital. All patients included were required to offer written informed consent.
Funding
This study was supported by the Jiangsu Provincial Medical Innovation Team (No. CXTDA2017042).
Disclosure
All the authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150(1):17–22. doi:10.1001/jamasurg.2014.1756
3. Rana N, Chakravarthy AB, Kachnic LA. Neoadjuvant treatment for locally advanced rectal cancer: new concepts in clinical trial design. Curr Treat Options Oncol. 2017;18(2):13. doi:10.1007/s11864-017-0454-4
4. Franke AJ, Parekh H, Starr JS, Tan SA, Iqbal A, George TJ. Total neoadjuvant therapy: a shifting paradigm in locally advanced rectal cancer management. Clin Colorectal Cancer. 2018;17(1):1–12. doi:10.1016/j.clcc.2017.06.008
5. Zorcolo L, Rosman AS, Restivo A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19(9):2822–2832. doi:10.1245/s10434-011-2209-y
6. Sanchez-Lara K, Turcott JG, Juarez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer. 2012;64(4):526–534. doi:10.1080/01635581.2012.668744
7. Hung HY, Chen JS, Yeh CY, et al. Effect of preoperative neutrophil-lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int J Colorectal Dis. 2011;26(8):1059–1065. doi:10.1007/s00384-011-1192-x
8. Jones JM, McGonigle NC, McAnespie M, Cran GW, Graham AN. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006;53(1):97–101. doi:10.1016/j.lungcan.2006.03.012
9. Son HJ, Park JW, Chang HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20(9):2908–2913. doi:10.1245/s10434-013-2968-8
10. Lien YC, Hsieh CC, Wu YC, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8(8):1041–1048. doi:10.1016/j.gassur.2004.09.033
11. Wang Y, Chen W, Hu C, et al. Albumin and fibrinogen combined prognostic grade predicts prognosis of patients with prostate cancer. J Cancer. 2017;8(19):3992–4001. doi:10.7150/jca.21061
12. Matsuda S, Takeuchi H, Kawakubo H, et al. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow prognostic score. Ann Surg Oncol. 2015;22(1):302–310. doi:10.1245/s10434-014-3857-5
13. Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686. doi:10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C
14. Tang MJ, Ding SB, Hu WY. Fibrinogen and albumin score changes during preoperative treatment can predict prognosis in patients with locally advanced rectal cancer. Gastroenterol Res Pract. 2019;2019:3514586. doi:10.1155/2019/3514586
15. Zhang Z, Fuller GM. Interleukin 1beta inhibits interleukin 6-mediated rat gamma fibrinogen gene expression. Blood. 2000;96(10):3466–3472. doi:10.1182/blood.V96.10.3466
16. Man YN, Wang YN, Hao J, et al. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer. 2015;25(1):24–32. doi:10.1097/IGC.0000000000000303
17. Qi Q, Geng Y, Sun M, Chen H, Wang P, Chen Z. Hyperfibrinogen is associated with the systemic inflammatory response and predicts poor prognosis in advanced pancreatic cancer. Pancreas. 2015;44(6):977–982. doi:10.1097/MPA.0000000000000353
18. Huang G, Jiang H, Lin Y, et al. Prognostic value of plasma fibrinogen in hepatocellular carcinoma: a meta-analysis. Cancer Manag Res. 2018;10:5027–5041. doi:10.2147/CMAR.S175780
19. Zhong H, Qian Y, Fang S, Wang Y, Tang Y, Gu W. Prognostic value of plasma fibrinogen in lung cancer patients: a meta-analysis. J Cancer. 2018;9(21):3904–3911. doi:10.7150/jca.26360
20. Kieliszek M, Lipinski B, Blazejak S. Application of sodium selenite in the prevention and treatment of cancers. Cells. 2017;6(4):39. doi:10.3390/cells6040039
21. Adams GN, Rosenfeldt L, Frederick M, et al. Colon cancer growth and dissemination relies upon thrombin, stromal PAR-1, and fibrinogen. Cancer Res. 2015;75(19):4235–4243. doi:10.1158/0008-5472.CAN-15-0964
22. Zhang F, Wang Y, Sun P, et al. Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(12):2413–2424. doi:10.1007/s00432-017-2493-4
23. Zhao C, Su Y, Zhang J, et al. Fibrinogen-derived fibrinostatin inhibits tumor growth through anti-angiogenesis. Cancer Sci. 2015;106(11):1596–1606. doi:10.1111/cas.12797
24. Brenner DA, Buck M, Feitelberg SP, Chojkier M. Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia. J Clin Invest. 1990;85(1):248–255. doi:10.1172/JCI114419
25. Heys SD, Walker LG, Deehan DJ, Eremin OE. Serum albumin: a prognostic indicator in patients with colorectal cancer. J R Coll Surg Edinb. 1998;43(3):163–168.
26. Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58(5):1836–1846. doi:10.1002/hep.26338
27. Seaton K. Albumin concentration controls cancer. J Natl Med Assoc. 2001;93(12):490–493.
28. Tai H, Zhu Z, Mei H, Sun W, Zhang W. Albumin-to-fibrinogen ratio independently predicts 28-day mortality in patients with peritonitis-induced sepsis. Mediators Inflamm. 2020;2020:7280708. doi:10.1155/2020/7280708
29. Ying J, Zhou D, Gu T, Huang J, Liu H. Pretreatment albumin/fibrinogen ratio as a promising predictor for the survival of advanced non small-cell lung cancer patients undergoing first-line platinum-based chemotherapy. BMC Cancer. 2019;19(1):288. doi:10.1186/s12885-019-5490-y
30. Yu W, Ye Z, Fang X, Jiang X, Jiang Y. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J Ovarian Res. 2019;12(1):88. doi:10.1186/s13048-019-0563-8
31. Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109(1):24–28. doi:10.1038/bjc.2013.330
32. Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10(6):639–645. doi:10.1016/j.cgh.2012.01.010
33. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–2087. doi:10.1016/S0140-6736(11)61049-0
34. Shen L, Zhang H, Liang L, et al. Baseline neutrophil-lymphocyte ratio (>/=2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat Oncol. 2014;9:295. doi:10.1186/s13014-014-0295-2
35. Dudani S, Marginean H, Tang PA, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer. 2019;19(1):664. doi:10.1186/s12885-019-5892-x
36. Shen J, Zhu Y, Wu W, et al. Prognostic role of neutrophil-to-lymphocyte ratio in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Med Sci Monit. 2017;23:315–324. doi:10.12659/MSM.902752
37. Portale G, Cavallin F, Valdegamberi A, Frigo F, Fiscon V. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio are not prognostic biomarkers in rectal cancer patients with curative resection. J Gastrointest Surg. 2018;22(9):1611–1618. doi:10.1007/s11605-018-3781-2
38. Jin C, Deng X, Li Y, He W, Yang X, Liu J. Lymph node ratio is an independent prognostic factor for rectal cancer after neoadjuvant therapy: a meta-analysis. J Evid Based Med. 2018;11(3):169–175. doi:10.1111/jebm.12289
39. Belluco C, Forlin M, Delrio P, et al. Elevated platelet count is a negative predictive and prognostic marker in locally advanced rectal cancer undergoing neoadjuvant chemoradiation: a retrospective multi-institutional study on 965 patients. BMC Cancer. 2018;18(1):1094. doi:10.1186/s12885-018-5022-1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.