Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Prescribing Pathways to Triple Therapy: A Retrospective Observational Study of Adults with Chronic Obstructive Pulmonary Disease in the UK
Authors Quint JK , Venerus A , O'Leary C, Myland M, Holmgren U , Varghese P, Cabrera C
Received 21 August 2020
Accepted for publication 16 November 2020
Published 8 December 2020 Volume 2020:15 Pages 3261—3271
DOI https://doi.org/10.2147/COPD.S278101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Jennifer K Quint,1 Alessandra Venerus,2 Caroline O’Leary,2 Melissa Myland,2 Ulf Holmgren,3 Precil Varghese,4 Claudia Cabrera3,5
1Imperial College London, London, UK; 2IQVIA, London, UK; 3Real World Science and Digital, BioPharmaceuticals Medical, AstraZeneca, Gothenburg, Sweden; 4Global Medical Affairs, AstraZeneca, Gaithersburg, MD, USA; 5Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden
Correspondence: Claudia Cabrera
BioPharmaceuticals Medical, AstraZeneca, Pepparedsleden 1, SE-431 83 Mölndal, Gothenburg, Sweden
Tel +46 (0)31 776 2890
Email [email protected]
Purpose: Treatment guidance for chronic obstructive pulmonary disease (COPD) recommends inhaled corticosteroid (ICS)+long-acting muscarinic antagonist+long-acting β2-agonist (LABA) triple therapy for patients who experience recurrent exacerbations, persistent breathlessness, or exercise limitation on dual therapy. However, information is limited on pathways to triple therapy in the UK.
Patients and Methods: A retrospective cohort study was conducted using de-identified patient-level data from UK primary care electronic medical records from January 1, 2005 to May 1, 2016. Data were included from patients who had their first triple therapy regimen (index date) recorded during the study period and a minimum of 12 months’ pre-index data. Treatment pathways to triple therapy were recorded, and the proportion of patients on triple therapy before their COPD diagnosis was determined. Adherence to triple therapy was estimated using the proportion of days covered (PDC).
Results: After applying eligibility criteria, 82,300 patients were included, with a mean age at COPD diagnosis of 64.7 years. The major treatment pathway (27.9%) was the first initiation of ICS+LABA prior to triple therapy. Following COPD diagnosis, the median time to triple therapy was approximately 3.5 years. The estimated mean adherence to triple therapy was 81.8% PDC. Multivariate analysis showed that the following groups were more likely to have received previous therapy prior to triple therapy: females (versus males), patients with asthma (versus those without asthma), severe COPD (versus those with non-severe COPD), or fewer exacerbations (versus those with more exacerbations).
Conclusion: Treatment pathways to triple therapy in the UK are diverse, highlighting the need to better understand factors involved in clinical decision-making.
Keywords: chronic obstructive pulmonary disease, patient pathways, real-world, retrospective study, treatment initiation, triple therapy
Introduction
It is estimated that 1.2 million people in the UK are living with a diagnosis of chronic obstructive pulmonary disease (COPD), with 115,000 being diagnosed each year1 and many more remaining undiagnosed.2 COPD represents a considerable economic burden, with COPD exacerbations accounting for the greatest proportion of the total COPD burden on the healthcare system.3 Over £800 million of the annual National Health Service (NHS) budget relates to COPD healthcare.4
COPD diagnosis relies on clinical judgment of medical history and spirometry;3 symptoms may overlap with those of asthma and can vary between patients.3,5 Patients with COPD report low health-related quality of life5 and, as the severity of disease increases, so do more frequent and severe COPD exacerbations, which may lead to hospitalization.6 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) provides a classification scheme for COPD. This scheme was originally based on the degree of airflow obstruction using post-bronchodilator forced expiratory volume in 1 second spirometry results.7 However, in 2011 the GOLD classification scheme was updated to also include symptom burden and exacerbation history.3,8
Inhaled corticosteroid (ICS), long-acting muscarinic antagonist (LAMA), and long-acting β2-agonist (LABA) maintenance therapies, and combinations of these, are used to treat COPD.2,3 When exacerbations persist, or patients experience persistent breathlessness or exercise limitation, despite dual therapy, then ICS+LAMA+LABA triple therapy may be recommended in some patients, although this depends on blood eosinophil count, especially according to the GOLD recommendations.2,3 Additionally, GOLD 2020 suggests that the beneficial effects of triple therapy are most likely to be seen in patients who are severely symptomatic, have moderate-to-very severe airflow obstruction, and a history of frequent and/or severe COPD exacerbations.3 Given concerns about the long-term benefits and risks associated with ICS, it may be appropriate to remove ICS from triple therapy regimens for some patients, particularly those without asthma and without frequent exacerbations (termed “stepping down”).9 Furthermore, GOLD 2020 recommends that step-down should be considered for pneumonia, inappropriate original indication, or lack of response to ICS, and that if step-down is considered after stability is achieved, it should be undertaken cautiously.3
A previous study in the UK, which used the Clinical Practice Research Datalink (CPRD) (2008–2009) to examine treatment progression, found that nearly 50% of patients on monotherapy (LAMA or LABA) had additions to their therapy during the 24 months after COPD diagnosis, while approximately 25% of patients on triple therapy stepped down within this period.10
Little is known about how treatment patterns in the UK align with guidance, although some evidence suggests poor concordance between real-world prescribing and GOLD recommendations.11–13 For example, an earlier analysis of prescribing patterns in the UK found that triple therapy was initiated as the first therapy following diagnosis in 10% of patients with COPD.13 Following the publication of a multi-country, retrospective database study examining prescribing pathways to triple therapy,14 our study aimed to explore the current treatment pathways to triple therapy specifically in the UK.
Patients and Methods
Study Design and Patients
This was a retrospective cohort study using primary care electronic medical records of patients with COPD who received triple therapy. Data sources included IQVIA Medical Research Data (IMRD; incorporating The Health Improvement Network [THIN], a Cegedim database)15 -enhanced CPRD16 linked with Hospital Episode Statistics (HES).17 THIN and CPRD both capture data from the same theoretical source population (NHS UK general practitioner [GP] practices), allowing patients to be pooled and overlapping patients removed. CPRD alone was linked to HES to evaluate exacerbations.18
In May 2017, IMRD included active records from 2.9 million patients and CPRD included active records from 5.5 million patients, representing approximately 5% and 8% of the total UK population, respectively. Secondary care information was derived from CPRD linked to HES, which contains hospital data for England; linked CPRD-HES data were available up to March 2016.
The study period was January 1, 2005 to May 1, 2016, with the index date defined as the first instance of triple therapy during this period (defined as a prescription from each class of ICS, LABA, and LAMA with at least 14 days’ overlap according to recorded or calculated prescription duration). Triple therapy started the day the three drugs overlapped and ended when at least one ran out (or where there was a gap of at least 90 days between prescriptions of the same drug class).
A valid COPD diagnosis and at least 12 months’ data prior to the index date were required. COPD diagnosis was algorithmically defined as evidence of smoking (current or ex-smoker) at any point and at least one COPD diagnosis code on or after a patient’s 40th birthday to avoid common misclassifications of COPD.18 Patients were followed to the earliest of death, transfer out of practice, or end of study.
An Independent Scientific Advisory Committee approved the use of CPRD data (16_298R). A Scientific Review Committee approved the use of IMRD data (16THIN097).
Study Objectives
In order to explore the pathways to triple therapy, the primary objectives of the study were to: quantify the proportion of patients on triple therapy prior to, at and after COPD diagnosis; assess GOLD (2011) categories; understand time from diagnosis to the first prescription of triple therapy; and determine the treatment pathways to triple therapy, with respect to inhaled maintenance therapies.
The secondary objectives were to: identify factors influencing patient treatment pathways; calculate time to step-down from triple therapy; and identify treatment pathways following triple therapy.
Exploratory objectives were to: calculate the rate of adherence to triple therapy; investigate the association between adherence and exacerbations and healthcare resource utilization (HCRU); and assess changes to therapy following an annual review in patients with an exacerbation in the previous year.
Analysis
Baseline Characteristics
Descriptive statistics were summarized for categorical and continuous variables. For patients in the CPRD-HES linked dataset, GOLD 2011 categories were calculated directly to assess disease severity; a validated model was used to estimate GOLD categories in unlinked patients (please see Supplementary Information for details). GOLD 2011 was used as the majority of the data were collected prior to the GOLD 2015 update; also, limited revisions were made to GOLD 2015.19
Time (months) between diagnosis and triple therapy initiation was explored. Mean (standard deviation [SD]) and median (95% confidence interval [CI]) time to first instance of triple therapy after initial diagnosis was estimated with 25th and 75th percentiles.
All analyses were carried out using SAS version 9.4.
Pathways to Triple Therapy
Patient-level treatment pathways were created using a data-driven approach, with proportions of patients in each pathway reported. The pathways analysis focused on the three main COPD therapy drug classes – ICS, LABA, and LAMA – during the period from COPD diagnosis until the first record of triple therapy. The primary logistic model looked at factors influencing pathways to triple therapy, therefore the dependent variable was “pathway to triple therapy”, which was a categorical variable. Patients whose first instance of triple therapy was before their COPD diagnosis were not included in this analysis. Short-acting β2-agonist (SABA) and short-acting muscarinic antagonist use was recorded, but presented separately from the treatment pathways. Pathways containing ≥1% of patients are reported individually, with pathways containing <1% grouped into the “other” pathway category.
Factors that were considered as influencing pathways to triple therapy included: rate of exacerbations prior to triple therapy; GOLD 2011 classification (severe [C/D] or non-severe [A/B]) in the 12 months prior to, or at, index date; and post-COPD diagnosis, presence of asthma, gender, and age at COPD diagnosis.
Initially, bivariate analysis was performed to explore the association between each influencing factor and the treatment pathway, using crude multinomial logistic regression models. A multivariate model was then estimated, including all covariates.
Adherence to Triple Therapy
To estimate adherence to therapy, the proportion of days covered (PDC) was calculated for each patient based on prescriptions issued. Patients were defined as adherent if PDC was at least 80%. Adherence was calculated separately for the three drug classes and then combined to obtain a composite indicator, calculated as follows:
(1)
where i represents each of the three drug classes, LABA, LAMA, and ICS.
Multivariate negative binomial models adjusting for non-adherence to first triple therapy, asthma presence, GOLD group (calculated or estimated) pre-triple therapy, age, and sex were used to evaluate the association between non-adherence and exacerbations, and between non-adherence and HCRU.
The HCRU yearly rate was calculated considering COPD-related visits (recorded in primary or secondary care) from triple therapy initiation to 12 months post-initiation. Parameter (adjusted rate ratio) estimates associated with the covariates are presented with values, 95% CIs, and p values, calculated using Chi-square tests.
Changes to Therapy Following an Exacerbation
Changes to therapy (at a drug class level) were explored after annual review visits for patients experiencing one or more exacerbations in the previous 12 months. The UK’s National Institute for Health and Care Excellence recommends at least annual reviews for COPD patients, including a clinical assessment, to determine the effects of each drug treatment and any need for referral to specialist therapy services.2 We assessed the proportion of patients who: remained on the same treatment; changed treatment (step-down/up, change of drug class); and discontinued treatment. The last combination therapy started before the annual review was used as the reference and this was compared with treatments started on or after the review.
Stepping Down from Triple Therapy
The median duration of triple therapy (95% CI) and the 25th and 75th percentiles were estimated using Kaplan–Meier survival analysis and are reported as cumulative proportions alongside the survival curve. Pathways of stepping down following triple therapy were explored; in particular, for all patients who were not censored (censored patients being those who were still receiving triple therapy at the end of the study period), the first combination after triple therapy is reported using proportions (95% CIs). The numbers and percentages of patients who discontinued and patients who were censored are also reported.
Results
Baseline Characteristics
After applying inclusion/exclusion criteria, 82,300 patients were identified, with a mean age at COPD diagnosis of 64.7 years (SD: 10.6), of which 53.3% were males (Table 1). Cardiovascular disease was the most prevalent comorbidity (66.9%), with depression/anxiety also having a high prevalence (37.4%); most patients (77.2%) were non-asthmatic.
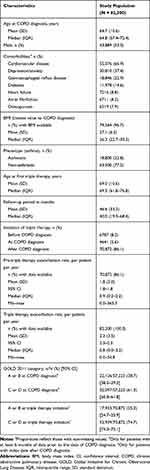 |
Table 1 Baseline Characteristics for the Study Population |
The mean age at initiation of triple therapy was 69.0 years (SD: 10.6), more than 4 years greater than the mean age at diagnosis (64.7 years). The average follow-up was almost 4 years (mean: 46.6 months, SD: 33.2) (Table 1).
The mean pre-triple therapy COPD exacerbation rate was 1.8 per patient per year (SD: 5.0), which slightly increased during triple therapy (2.3 per patient per year, SD: 3.5) (Table 1). Overall, 86.1% of patients initiated triple therapy after their COPD diagnosis compared with 8.2% before and 5.6% at diagnosis. At diagnosis, 61.3% (95% CI: 60.8–61.8) of patients were in GOLD 2011 category C or D (more severe). In comparison, at triple therapy initiation, GOLD category C/D increased to 74.7% (95% CI: 74.3–75.1) of patients.
Treatment Pathways to Triple Therapy
Triple therapy was initiated at a median of 42.5 months (interquartile range [IQR] 13.9–87.4) post-diagnosis (Table 2). There were a variety of treatment pathways leading to triple therapy (Table 3); those with <1% of patients were grouped in the “other” pathway category and represented 23.0% of patients in this analysis. The most frequently recorded pathways prior to initiation of triple therapy were ICS+LABA (27.9%), LAMA (13.1%), and “no therapy prior to triple therapy” (12.1%); together, these three pathways accounted for more than half (53.1%) of the patients in this analysis.
 |
Table 2 Time to, and Adherence to, Triple Therapy |
 |
Table 3 Most Frequently Recorded Treatment Pathways Prior to Triple Therapy |
Factors Influencing Pathways to Triple Therapy
Multivariate analysis (with “no therapy prior to triple therapy” as the reference category) showed that the following groups were more likely to have received previous therapy prior to initiating triple therapy: asthmatic patients (versus non-asthmatic patients); females (versus males); patients with severe COPD (GOLD 2011 C/D versus A/B); or patients with fewer exacerbations (versus those with a higher rate of exacerbations) (Table 4). Older patients were slightly less likely than younger patients to have received prior treatment before initiating triple therapy; however, this was not deemed to be clinically significant since the odds ratios for the age parameter were close to 1.
 |
Table 4 Factors Influencing the Pathway to Triple Therapy (Multivariate Analysis) [N = 70,872]a,b |
Patient Adherence
Estimated adherence was high at a mean of 81.8% PDC (SD: 15.5) (Table 2). When analyzing the association between adherence and exacerbations, adherent patients (defined as ≥80% PDC) had a significantly increased rate of exacerbations (means ratio: 1.05 [95% CI: 1.03–1.07], p <0.001) in adjusted analyses (Table 5). Those with GOLD C/D and asthmatic patients had an increased rate of exacerbations (means ratio: 1.86 [95% CI: 1.82–1.91], p <0.001; and 1.33 [95% CI: 1.30–1.36], p <0.001, respectively). Male patients tended to have a lower exacerbation rate than females (means ratio: 0.86 [95% CI: 0.85–0.88], p <0.001), while age did not impact the exacerbation rate.
 |
Table 5 Association Between Non-Adherence, Triple Therapy Exacerbation Rate and Healthcare Resource Utilization (Adjusted Analyses) |
In the analysis of association between adherence and HCRU, adherent patients tended to have increased HCRU (means ratio: 1.15 [95% CI: 1.14–1.16], p <0.001) in adjusted analyses (Table 5). Patients at higher risk of exacerbation (GOLD C/D) had a statistically significantly greater HCRU (means ratio: 1.21 [95% CI: 1.20–1.22], p <0.001); this positive association was also found for patients with asthma compared with those without (means ratio: 1.05 [95% CI: 1.04–1.05], p <0.001). Male patients had slightly lower HCRU rates than female patients (means ratio: 0.97 [95% CI: 0.97–0.98], p <0.001) and age did not have a clinically significant impact.
Changes in Therapy Following Exacerbations
Overall, 79.1% of patients recorded at least one annual review in the 12 months following an exacerbation, with a mean of 2.9 annual reviews per patient (Table 6). Most reviews (71.5%) did not lead to a change in the patient’s treatment regimen, while 23.6% resulted in a step-up of therapy. Treatment changes at a drug class level (without step-up or step-down of therapy) (2.3%), step-down (1.6%), and discontinuation (1.0%) combined constituted less than 5% of outcomes following review.
 |
Table 6 Changes in Therapy After Exacerbations |
Stepping Down Treatment
The median duration of triple therapy was 18.2 months (IQR: 3.5–86.9). The proportion of patients discontinuing treatment or stepping down from triple therapy was 59.1%. When excluding censored patients (n = 33,684 [40.9%]; those still on triple therapy at the end of the study), the median duration of therapy, ie time to step-down, decreased substantially to 5.2 months (IQR: 1.2–15.2). The most frequently recorded therapies following step-down were ICS+LABA+SABA (18.0%), LAMA+SABA (13.0%), and LAMA (11.3%) (Table 7).
 |
Table 7 Time to Step-Down from Initiation of Triple Therapy, and Most Frequent Treatments Following Step-Down from Triple Therapy |
Discussion
Following the publication of a multi-country, retrospective database study that provided insights into global prescribing pathways to triple therapy,14 this study focused solely on the prescribing pathways in UK clinical practice. The median time from diagnosis to initiation of triple therapy was approximately 3.5 years (42.5 months), which is comparable to findings of an earlier UK primary care database study by Brusselle et al, which found more than 50% of patients to have started triple therapy within 3 years.13 Treatment pathways to triple therapy were complex and varied, resulting in less common pathways being grouped in the “other” pathway category, which accounted for almost one-quarter of patients in the current study. The most frequently recorded treatment pathway to triple therapy was ICS+LABA → triple therapy (27.9%), which echoes the findings of Brusselle et al, who found that 24.8% of UK patients followed the same pathway.13
Interestingly, both studies also showed that a significant number of patients – around 1 in 10 (12.1% in our study and 9.7% in the study by Brusselle et al)13 – went straight to triple therapy without previous therapy for COPD. This is despite GOLD recommendations that dual therapy regimens should be given before progression to triple therapy.3 These findings may suggest, therefore, that many GPs do not adhere to recommended treatment strategies in response to patient symptoms. It has been reported previously that there is significant variability in adherence to GOLD guidelines in general practice,20 and it is not unreasonable to expect that it takes time for guidelines to be implemented, particularly in high-volume disease areas such as COPD. Indeed, real-world data show that in the year following release of the GOLD 2011 guidelines, only 65% of patients received treatment in line with recommended strategies; and treatment decisions were instead based on clinical experience.21 An alternative explanation may be that patients are receiving a late diagnosis of COPD, when the burden of symptoms and/or exacerbations is high. It should be noted that prior to initiation of triple therapy in the current study, 74.7% of patients were categorized as GOLD stage C or D, representing severe disease and possibly warranting a more intensive treatment regimen.
Pathways to triple therapy were most strongly affected by asthma phenotype and GOLD category, with the exacerbation rate also significantly impacting the majority of treatment pathways. Asthmatic patients were more likely than non-asthmatic patients to have received previous therapy prior to triple therapy, as would perhaps be expected, given the complexity of their comorbid conditions. Patients with severe COPD were also more likely to have received prior treatment, compared with those with less-than-severe disease, likely reflecting their disease history, the refractory nature of earlier treatment approaches, and the need for triple therapy in these individuals. The observation that patients with higher (12-month, pre-triple therapy) exacerbation rates tended to initiate on triple therapy rather than other therapeutic approaches is also to be expected, as it suggests that GPs start these patients on triple therapy in an attempt to minimize immediate symptoms and risk of the disease before considering step-down therapy. This is supported by double-blind, randomized controlled trials that have shown benefits of triple therapy versus LAMA/LABA and ICS/LABA combination therapies in reducing exacerbation rates.22–25 Additionally, all-cause mortality data from the ETHOS and IMPACT studies have shown a beneficial effect for triple therapy compared with LAMAs/LABAs.22,25
Adherence levels in our study (as defined by PDC) were high, with adherent patients experiencing significantly increased exacerbation rates versus non-adherent patients. This is to be expected, as patients are more likely to adhere to medication if they are experiencing exacerbations that are not under control. It follows that adherent patients exhibited increased HCRU, likely reflecting the increased number of prescriptions these patients received and the associated number of GP visits.
For patients experiencing at least one exacerbation in the 12 months prior to their annual review, most reviews did not result in any change to their treatment regimen. This could suggest a clinical reluctance to alter treatment plans after a single exacerbation. Alternatively, it may be that standard rescue packs, which all patients can receive following an exacerbation,2 adequately controlled exacerbations, masking the need for increased maintenance therapy.
The median duration of triple therapy was shown to be 18.2 months; previous observational research has not tended to examine the duration of triple therapy.11–13
After step-down from triple therapy, most patients were recorded as being treated with ICS+LABA+SABA; this deviates from recommendations to remove ICS in some patients at low risk of experiencing an exacerbation, or in those with a lack of response or side effects, eg pneumonia.3 Exploration of the reasons for step-down was beyond the scope of the current study, but it is apparent that further understanding of factors leading to such clinical decision-making is needed. Patients remaining on ICS could represent those severely affected or still experiencing exacerbations, as removal of ICS will not be appropriate for some patients.
The results of our study can be used to further understand adherence to recommendations and optimization of patient treatment patterns. A key strength of this study is the large population for analysis derived from the primary care setting, and that it provides a holistic view of treatment patterns over a considerable period of time, with results likely applicable to many populations of patients with COPD.
Limitations include that, firstly, as with other retrospective database studies,26 treatment use was based on prescriptions issued rather than treatment dispensed or taken by the patient. However, we assumed that medications were taken by the patient, with adherence estimated based on PDC, a commonly recognized study measure. Secondly, an algorithm for GOLD grouping was used in the absence of complete data (no HES linkage available) to estimate COPD severity; therefore, there is some possibility of misclassification due to a certain level of inaccuracy inherent in this approach. Finally, the use of LAMA+LABA combinations has increased slowly over the past two decades, with results from a UK database study showing that the proportion of patients maintained on LAMA+LABA (in free or fixed combination) increased from 1% in 2001 to 5% in 2016.27 It is likely that this proportion has continued to grow with the increased availability of fixed-dose combinations; therefore, an updated analysis including data from the last 4 years would possibly reveal changes in the pathways to triple therapy.
Conclusion
ICS+LABA, LAMA, and no prior therapy were the most common pathways to triple therapy. There was a relatively high frequency of prescribing ICS-containing regimens prior to triple therapy, particularly as the majority of patients were not asthmatic. This study also highlights the low (1.0%) prescribing frequency of dual bronchodilator therapy prior to triple therapy, despite GOLD recommendations.3 There is a need to better understand the factors driving clinical decision-making in the pharmacological treatment of patients with COPD, specifically the factors affecting decisions to initiate triple therapy.
Estimated treatment adherence was recorded as high; higher adherence was associated with increased exacerbation rates during triple therapy and HCRU, as expected. A large proportion of patients stepped down treatment, and a better understanding of factors guiding these decisions is needed. Our results can be used to further understand pathways to triple therapy and optimization of treatment patterns.
Abbreviations
BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; DF, degrees of freedom; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; GPP3, Good Publication Practice; HCRU, healthcare resource utilization; HES, Hospital Episode Statistics; ICS, inhaled corticosteroid; IMRD, IQVIA Medical Research Data; IQR, interquartile range; ISAC, Independent Scientific Advisory Committee; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; NHS, National Health Service; OR, odds ratio; PDC, proportion of days covered; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation; THIN, The Health Improvement Network.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
Access to CPRD data was approved (16_298R) for this study by an Independent Scientific Advisory Committee (ISAC). ISAC is a non-statutory expert advisory body established in 2006 by the Secretary of State for Health to provide scientific advice on research requests to access data provided by CPRD. The THIN data collection was approved by the NHS South-East Multi-centre Research Ethics Committee in 2003. Under the terms of this ethics approval, studies using pre-collected, pseudonymized data must undergo scientific review to help ensure appropriate analysis and interpretation of the data. The independent Scientific Review Committee, which was set up in July 2009, provided approval for the use of the THIN data for the current study (16THIN097).
Consent for Publication
Not applicable.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Nicholas Hudson, IQVIA, London, UK, who also assisted with interpretation of results, and Lindsey O’Mahony, PhD, on behalf of CMC Connect, McCann Health Medical Communications, funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines.28 IQVIA Medical Research Data incorporates data from The Health Improvement Network (THIN). THIN is a registered trademark of Cegedim SA in the UK and other countries. Reference made to the THIN database is intended to be descriptive of the data asset licensed by IQVIA. This work used de-identified data provided by patients as a part of their routine primary care.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
IQVIA received funding from AstraZeneca to conduct this study. The funder of the study was involved in data interpretation and writing of the report.
Disclosure
Jennifer K Quint’s research group has received funding from AstraZeneca, Bayer, Boehringer Ingelheim, British Lung Foundation, GlaxoSmithKline, Insmed, Medical Research Council, The Health Foundation, and Wellcome Trust for other projects, none of which relates to this work. Jennifer K Quint’s research group has received funds from IQVIA relating to this work. Jennifer K Quint has received funds from AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Teva for advisory board participation or travel. Caroline O’Leary, Alessandra Venerus, and Melissa Myland are employees of IQVIA, which received consulting fees from AstraZeneca for conducting the study. Precil Varghese and Ulf Holmgren are employees of AstraZeneca. Claudia Cabrera is an employee of AstraZeneca and has an adjunct research position with the Department of Medical Epidemiology and Biostatistics at the Karolinska Institute. The authors report no other conflicts of interest in this work.
References
1. British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics. Available from: https://statistics.blf.org.uk/copd.
2. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. NICE guideline [NG115]; 2018. Available from: https://www.nice.org.uk/guidance/ng115.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report); 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
4. National Institute for Health and Care Excellence. Guideline scope: chronic obstructive pulmonary disease in over 16s: diagnosis and management (update); 2017. Available from: https://www.nice.org.uk/guidance/ng115/documents/draft-scope.
5. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279–285. doi:10.3109/02770903.2011.555576
6. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138.
7. Rodriguez-Roisin R, Rabe KF, Vestbo J, Vogelmeier C, Agusti A. Global initiative for Chronic Obstructive Lung Disease (GOLD) 20th anniversary: a brief history of time. Eur Respir J. 2017;50(1):1700671. doi:10.1183/13993003.00671-2017
8. Global Initiative for Chronic Obstructive Lung Disease. 2011 Report: global Strategy for the Diagnosis, Management and Prevention of COPD; 2011. Available from: https://goldcopd.org/archived-reports/.
9. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535–2548. doi:10.2147/COPD.S93321
10. Wurst KE, Punekar YS, Shukla A, Hartl D. Treatment evolution after COPD diagnosis in the UK primary care setting. PLoS One. 2014;9(9):e105296. doi:10.1371/journal.pone.0105296
11. Hurst JR, Dilleen M, Morris K, Hills S, Emir B, Jones R. Factors influencing treatment escalation from long-acting muscarinic antagonist monotherapy to triple therapy in patients with COPD: a retrospective THIN-database analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:781–792. doi:10.2147/COPD.S153655
12. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–905. doi:10.2147/COPD.S62750
13. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217.
14. Quint JK, O’Leary C, Venerus A, et al. Prescribing pathways to triple therapy: a multi-country, retrospective observational study of adult patients with chronic obstructive pulmonary disease. Pulm Ther. 2020. doi:10.1007/s41030-020-00132-7
15. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255.
16. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098
17. NHS Digital. Hospital episode statistics. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics.
18. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi:10.1371/journal.pone.0151357
19. Global Initiative for Chronic Obstructive Lung Disease. 2015 report: global strategy for the diagnosis, management and prevention of COPD; 2015. Available from: https://goldcopd.org/archived-reports/.
20. Sehl J, O’Doherty J, O’Connor R, O’Sullivan B, O’Regan A. Adherence to COPD management guidelines in general practice? A review of the literature. Ir J Med Sci. 2018;187(2):403–407. doi:10.1007/s11845-017-1651-7
21. Bargiel A, Obojski A. Impact of the GOLD 2011 guidelines on treatment regimen in COPD patients. Eur Respir J. 2015;46(suppl.59):PA3939.
22. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
23. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X
24. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758.
25. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
26. Ford ES, Mannino DM, Giles WH, et al. Prescription practices for chronic obstructive pulmonary disease: findings from the national ambulatory medical care survey 1999–2010. COPD. 2014;11:247–255.
27. Bloom CI, Elkin SL, Quint JK. Changes in COPD inhaler prescriptions in the United Kingdom, 2000 to 2016. Int J Chron Obstruct Pulmon Dis. 2019;14:279–287. doi:10.2147/COPD.S190086
28. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. doi:10.7326/M15-0288
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
