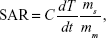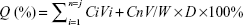Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Preparation of folic acid-conjugated, doxorubicin-loaded, magnetic bovine serum albumin nanospheres and their antitumor effects in vitro and in vivo
Authors Yang R , An Y, Miao F, Li M, Liu P, Tang Q
Received 4 May 2014
Accepted for publication 18 June 2014
Published 4 September 2014 Volume 2014:9(1) Pages 4231—4243
DOI https://doi.org/10.2147/IJN.S67210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Rui Yang,1 YanLi An,2 FengQin Miao,1 MengFei Li,1 PeiDang Liu,1 QiuSha Tang1
1School of Medicine, Southeast University, 2Jiangsu Key Laboratory of Molecular and Functional Imaging, Department of Radiology, Zhongda Hospital, Nanjing, Jiangsu Province, People’s Republic of China
Background: This study aimed to generate targeted folic acid-conjugated, doxorubicin-loaded, magnetic iron oxide bovine serum albumin nanospheres (FA-DOX-BSA MNPs) that lower the side effects and improve the therapeutic effect of antitumor drugs when combined with hyperthermia and targeting therapy. A new nanodrug using magnetic nanospheres for heating and addition of the folate receptor with cancer cell specificity was prepared. The characteristics of these nanospheres and their antitumor effects in nasopharyngeal carcinoma were explored.
Methods: FA-DOX-BSA MNPs comprising encapsulated magnetic iron oxide nanoparticles were prepared using a desolvation cross-linking method. Activated folic acid (N-hydroxysuccinimide ester of folic acid) was conjugated to the surface of albumin nanospheres via amino groups.
Results: Folic acid was successfully expressed on the surface of the nanospheres. Electron microscopy revealed that the FA-DOX-BSA MNPs were nearly spherical and uniform in size, with an average diameter of 180 nm. The nanomaterial could deliver doxorubicin at clinically relevant doses with an entrapment efficiency of 80%. An increasing temperature test revealed that incorporation of magnetic iron oxide into nanospheres could achieve a satisfactory heat treatment temperature at a significantly lower dose when placed in a high-frequency alternating magnetic field. FA-DOX-BSA MNPs showed greater inhibition of tumors than in the absence of folic acid in vitro and in vivo. Compared with chemotherapy alone, hyperthermia combined with chemotherapy was more effective against tumor cells.
Conclusion: Folic acid-conjugated bovine serum albumin nanospheres composed of mixed doxorubicin and magnetic iron oxide cores can enable controlled and targeted delivery of anticancer drugs and may offer a promising alternative to targeted doxorubicin therapy for nasopharyngeal carcinoma.
Keywords: doxorubicin, bovine serum albumin, folic acid, KB cells
Introduction
Nasopharyngeal carcinoma (NPC) is a common cancer and has a relatively high incidence in southern China, Southeast Asia, and North Africa.1 Annually, approximately 80,000 new cases of oral cancer are reported worldwide. Death will occur in more than 60% of these cases.2 NPC is an Epstein-Barr virus-related cancer, in which radiation and chemotherapy demonstrate antitumor effects but cause injury to normal tissue cells to varying degrees, leading to a poor prognosis and high mortality after treatment. Despite improvement in the clinical cure rate for early-stage NPC, multidrug resistance and side effects encountered in advanced or intracranially recurrent NPC remain issues to be addressed. Thus, identifying more aggressive treatment strategies for NPC would be beneficial.
Chemotherapy has long been used in the treatment of many cancers. Doxorubicin, an anthracycline antibiotic, is an effective chemotherapeutic agent in numerous types of tumor, including solid malignancies, acute leukemias, and malignant lymphoma.3 However, its use has been limited by concomitant dose-related and irreversible cardiotoxicity, as well as the emergence of drug resistance.4 Damage to nontargeted tissues often stymies cancer therapy by limiting the doxorubicin dosage.5 The underlying causes of drug resistance remain unknown, so it is important to establish delivery systems to minimize doxorubicin-induced toxicity while obtaining maximum anticancer efficacy. The framework used in drug nanocarrier systems comprises mainly two materials, ie, natural and synthetic polymers. Natural polymers, particularly albumin with its unique physical and chemical attributes, are versatile protein carriers for drug targeting and improving the pharmacokinetic profiles of peptide-based or protein-based drugs.6 The particle size of serum albumin is at the nanoscale to mesoscale level. Additionally, serum albumin is a polymer consisting of amino acids linked by peptide bonds, which facilitates the embedding and loading of drugs. Carriage of drugs by serum albumin improves their biodegradability and stability, and enables slow release and uptake by the tumor and inflamed tissue.7 Furthermore, its numerous lysine residues facilitate chemical coupling and modification of bovine serum albumin. These characteristics suggest that this protein can transport therapeutic drugs. Therefore, we prepared doxorubicin-loaded serum albumin nanoparticles to enhance the antitumor effects by using serum albumin as drug carrier.
Nanotechnology is attracting great attention worldwide in biomedicine. Targeted therapy based on drug nanocarrier systems enhances the treatment of tumors and enables the development of targeted drug delivery systems. Compared with nontargeted drug delivery systems, targeted systems not only increase therapeutic efficacy and reduce the side effects of antitumor drugs but also enhance targeting and tissue absorption. Receptor-ligand interactions represent the most important method of targeting antitumor drugs to living cells. The focus has been on identifying highly specific receptors in the membranes of tumor cells that demonstrate a significantly different drug uptake ability compared with nontarget tissues. The folic receptor, also known as folic acid-binding protein, is expressed at a low level in normal cells, but is overexpressed during cellular activation and proliferation, particularly in human tumors such as NPC, ovarian cancer, and gliomas. Consequently, the folic receptor is a promising target ligand for cancer treatment. Folic acid is essential for the maintenance of cell functions. Binding of folic acid to the folic receptor results in receptor-mediated endocytosis and internalization. The functionalized polylactide-co-glycolide-polyethylene glycol-folic acid nanoparticles containing anticancer drugs developed by Saxena et al showed a two-fold increase in cytotoxicity and considerably higher intracellular uptake of folic-targeted nanoparticles compared with free drug or nontargeted nanoparticles in MCF-7 human breast cancer cells.8 Further, Lee et al prepared folic acid/secondary lymphoid tissue chemokine (CCL21)/upconversion fluorescent nanoparticle conjugates that can target folic receptor-expressing tumor cells and successfully attract immune cells.9 Other ligands, such as antibodies and hormones, are transported to lysosomes and cleared.10,11 Together, the above data suggest that folic acid may be a good ligand for targeting of tumors.
Heat treatment is an ancient and promising method for treating tumors and was first used in 5,000 BC by the ancient Egyptians to treat breast cancer.12 Heating tissues to 41°C–45°C to achieve the therapeutic aim was known as green therapy. The combination of chemotherapy or radiotherapy with heat not only enables a decreased dose of chemotherapy or radiotherapy to be applied, thus reducing side effects, but also can improve therapeutic efficacy by enhancing the killing of tumor cells. Pelicci et al evaluated the antitumor effect of heat in combination with radiotherapy. The combination of increased temperature at the tumor site generated by laser treatment of intravenously injected gold nanoshells and ionizing radiation enhanced both the radiosensitivity of cancer stem cells and the tumor response. The results suggested that heating is a potent radiation sensitizer if provided appropriately.13 The drawbacks of heat treatment include the possibility of failure to deliver and monitor the temperature in deep-seated tumors or use of an inefficient traditional-type heater that has long been out of use. Magnetic-targeted heat treatment, initially proposed by Gilchrist et al has promoted heat treatment for tumors because it can precisely deliver heat to the target.14 The benefits of using magnetic nanomaterials for tumor heating are that they can enter tumor tissue either alone or as conjugates with small molecules, or be included in bulky cargos such as liposomes or proteins.15–17 The temperature of the tissue can be effectively controlled by an external magnetic field; therefore, no thermal damage to nontarget zones occurs. Magnetic nanoparticles, particularly magnetic fluids, are a new heating technology for the treatment of tumors and were first reported by Jordan et al in 1997.18 Magnetic fluids are nanostructured materials with magnetic properties. Iron oxide (Fe3O4) displays considerable magnetism, catalysis, and wave absorption properties, making it the most commonly used magnetic fluid for tumor hyperthermia.19,20 However, Fe3O4 has a short half-life and no specific tumor-targeting effect because it is readily taken up by reticuloendothelial cells and removed by macrophages. We have designed bovine serum albumin nanospheres containing folic acid-conjugated Fe3O4 to improve the pharmacokinetic profile and targeting effect, due to the long half-life of albumin in the body and binding of folic acid to the folic receptor.
In the present study, we used bovine serum albumin nanospheres containing Fe3O4 and doxorubicin conjugated to folic acid (FA-DOX-BSA MNPs). We compared the ability of various FA-DOX-BSA MNPs to inhibit tumors, including doxorubicin-loaded bovine serum albumin nanospheres (DOX-BSA NPs), magnetic bovine serum albumin nanospheres (BSA MNPs), doxorubicin-loaded magnetic bovine serum albumin nanospheres (DOX-BSA MNPs), and FA-DOX-BSA MNPs in vivo and in vitro, to investigate whether targeted delivery is effective for NPC therapy. The tumor-targeting efficacy of FA-DOX-BSA MNPs in vitro and in vivo has been confirmed by our laboratory.21
Materials and methods
Materials
Bovine serum albumin, 3-(4,5-dimethyl-2-thiazyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT), dimethyl sulfoxide, phosphate-buffered saline (pH 7.4), N-dicyclohexylcarbodiimide, dicyclohexylurea, N-hydroxysuccinimide (NHS), and doxorubicin hydrochloride were purchased from Sigma-Aldrich (St Louis, MO, USA). Ammonium ferric sulfate dodecahydrate, hydroxylamine hydrochloride, 1,10- phenanthroline, and sodium acetate were purchased from JiangLai Biological (Shanghai, People’s Republic of China). All solvents were of high-performance liquid chromatography grade. An Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was supplied by Beyotime Institute of Biotechnology (Shanghai, People’s Republic of China). Fe3O4 was prepared as described by Li et al.22 All reagents were of analytical grade.
Synthesis of targeting FA-DOX-BSA MNPs
DOX-BSA MNPs were firstly prepared using a desolvation cross-linking technique. Briefly, bovine serum albumin (0.25 g), Fe3O4 (0.05 g), and doxorubicin (0.01 mg) were dissolved in 20 mL of deionized water. The pH was adjusted to 8.0 after thorough mixing. Absolute alcohol (150 mL) was added to the solution, followed by stirring at room temperature for 1–2 hours until a precipitate appeared. Next, 0.25% glutaraldehyde (50 μL) solution was added to cross-link the amino groups in the nanoparticles. This process was performed during stirring for 12 hours. Finally, the mixture was centrifuged at 1,500 rpm for 5 minutes to remove nonencapsulated Fe3O4, and then at 21,000 rpm for 30 minutes to remove nonencapsulated doxorubicin, followed by washing three times with deionized water. The solution was redispersed in 3.0 mL of purified water.
The NHS ester of folic acid (folic-NHS) was prepared as follows. Folic acid (0.5 g) was dissolved in dimethyl sulfoxide (10 mL), to which was added triethylamine (0.25 mL). N-dicyclohexylcarbodiimide (4.8 g) and NHS (2.6 g) were added to the folic acid-dimethyl sulfoxide solution while stirring. The reaction was continued at room temperature overnight. Dicyclohexyl urea, which is the byproduct of the synthesis of folic-NHS, was removed by filtering. After these steps, we obtained folic-NHS.
FA-DOX-BSA MNPs was prepared by conjugating DOX-BSA MNPs with folic acid via amino groups. NaCO3/NaHCO3 buffer (pH 11.5) was added to a solution of DOX-BSA MNPs (3 mL) to adjust the pH to 10.0. The dimethyl sulfoxide solution of folic-NHS (1 mL) was added to the solution, followed by stirring at room temperature for 2 hours. The reactants were placed in a dialysis bag (3,500 Da) to remove the dimethyl sulfoxide. A Sephadex G-50 column was used to separate the FA-DOX-BSA MNPs from unreacted folic acid and other byproducts. The eluent containing FA-DOX-BSA MNPs was centrifuged at 21,000 rpm for 30 minutes and the pellets were redispersed in 4.0 mL of purified water.
Characterization of targeted FA-DOX-BSA MNPs
Assessment of morphology by transmission electron microscopy
The FA-DOX-BSA MNP sample was redispersed in an ultrasonication bath for 15 minutes and then dripped onto a copper net with carbon support film to prepare for visualization using a transmission electron microscope (Hitachi, Tokyo, Japan).
Dynamic light scattering analysis
The particle size and size distribution of the FA-DOX-BSA MNPs were assessed by dynamic light scattering (Brookhaven Instruments Co, Holtsville, NY, USA). To assess the stability of the nanoparticles, the hydrodynamic size of the nanoparticles in phosphate-buffered saline or Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum was analyzed by dynamic light scattering within 24 hours. Particle size was measured for 18 days with storage at 4°C and ambient humidity.
Iron content, specific absorption rate, and increasing temperature tests
Iron content was measured using 1,10-phenanthroline spectrophotometry based on the principle that 1,10-phenanthroline generates stable color complexes in aqueous solution at pH 2–9. The steps were as follows. First, 5 μL of FA-DOX-BSA MNP solution was brought up to 1 mL with 1 M HCl and blended in an ultrasonication bath. Second, 10% hydroxylamine hydrochloride (1 mL), 0.1% phenanthroline solution (2 mL), acetic acid, and sodium acetate buffer (pH 5, 5 mL) were added, and the solution was brought up to 50 mL with ultrapure water. A standard solution was prepared by dissolving an accurately measured quantity of FeCl3·6H2O according to the second step. The different optical density (OD) values were detected by ultraviolet spectrophotometry (DU 800, Beckman Coulter, Brea, CA, USA) at 510 nm. A standard curve was constructed using the iron content of the standard solution (mg) as the abscissa and the OD value as the y coordinate. The regression equation of the iron content and the OD value was also derivative. The iron concentration in the sample solution was determined from the standard regression equations. The sample was diluted proportionally in ultrapure water to the appropriate concentrations. Next, the solution was placed in a flat-bottomed test tube on an SPG-06A high-frequency induction heater from Shenzhen, People’s Republic of China (f =200 kHz; I =20 A) for 60 minutes. The distance between the bottom of the tube and center of the heating coil of the alternating magnetic field was 0.5 cm. The temperature was tested using a digital thermometer at 5-minute intervals. The specific absorption rate (SAR) values for Fe3O4 and FA-DOX/BSA MNPs were calculated using the following formula:
|
where C is the specific heat capacity of the suspension, dT/dt is the initial slope of temperature versus time graph, ms is the mass of the suspension, and mm is the mass of the magnetic material in the suspension.
Drug encapsulation, loading, and cumulative drug release
Drug encapsulation efficiency was measured using an ultraviolet-visible near-infrared spectrophotometer (UV-3600; Shimadzu, Tokyo, Japan). First, 1 mL of FA-DOX-BSA MNPs was dispersed into 9 mL of 0.5% pepsin aqueous solution and digested for 3 hours at 37°C. The supernatant was collected after centrifuging for 30 minutes at 21,000 rpm and detected at 480 nm using ultraviolet spectrophotometry. Drug encapsulation efficiency was calculated as follows: (actual amount of drug encapsulated in MNPs)/(initial amount of drug used in the fabrication of MNPs) ×100%. Drug loading was calculated as follows: (actual amount of drug encapsulated in MNPs)/(amount of MNPs) ×100%. Doxorubicin release from the nanoparticles in phosphate-buffered saline with three different pH values (pH 5.4, 7.4, and 9.0) at 37°C was evaluated using the dynamic dialysis method. A sample (15 mL) was placed in a dialysis bag (molecular weight cutoff 3,500 Da), which was then immersed in a 50 mL centrifuge tube containing 30 mL of phosphate-buffered saline. The tube was placed in a horizontal shaking incubator (Shanghai, People’s Republic of China) at 37°C and 110 rpm. A 5 mL sample was removed from the centrifuge tube and replaced with 5 mL of fresh buffer at regular time intervals. Doxorubicin release from the nanoparticles in phosphate-buffered saline (pH 7.4) at 43°C was also evaluated using the same methods. The doxorubicin concentration in each sample was quantified by ultraviolet spectrophotometry at 480 nm. The cumulative release rate, Q (%), was calculated as follows:
|
where Ci and Cn are the drug concentration in released mediators, Vi is the volume of mediator removed, V is the total amount of leaching mediator, and W and D are the weight of the microspheres and drug concentration in the microspheres, respectively.
Surface functional groups
An ultraviolet-visible near-infrared spectrophotometer was used to determine whether folic acid was conjugated to the DOX-BSA MNPs.
Toxicity assessment
As we know, folic acid is essential for the maintenance of cell functions, bovine serum albumin has good biodegradability, and both are nontoxic to normal cells. Our laboratory has been studying the applications of Fe3O4 for a long time. It shows good biocompatibility, which has been proven by Chen et al.23 So the folic acid conjugated MNPs also have good biodegradability.
Antitumor effects of FA-DOX-BSA MNPs in vitro
Cell culture
NPC KB cells were obtained from the Shanghai Institute of Cell Biology (Shanghai, People’s Republic of China) and cultured in folic acid-free RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Shanghai, People’s Republic of China), 100 U/mL penicillin, and 100 mg/mL streptomycin. Cells were maintained at 37°C with 5% CO2 in a humidified incubator.
MTT
The antitumor effect of FA-DOX-BSA MNPs on KB cells was measured by MTT assay. KB cells were seeded in 96-well tissue culture plates at a density of 5×104 per well and cultured in folic acid-free RPMI 1640 medium for 24 hours. Subsequently, the cells were divided into the following groups: (1) a negative control group (folic acid-free RPMI 1640 medium containing 10% fetal calf serum); (2) a DOX-BSA NP group; (3) a DOX-BSA MNP group with no heating; (4) a FA-DOX-BSA MNP group with no heating; (5) a BSA MNP group with heating; (6) a DOX-BSA MNP group with heating; (7) a FA-DOX-BSA MNP group with heating; (8) a negative control group with heating (folic acid-free RPMI 1640 medium containing 10% fetal calf serum); and (9) a BSA MNP group with no heating. Groups 5, 6, 7, and 8 were placed on the heating coil of a high-frequency induction heater (f =200 kHz; I =20 A) for 60 minutes. After incubation for 48 hours, MTT solution (20 μL) was added to all groups, followed by incubation at 37°C for 4 hours. Next, dimethyl sulfoxide (200 μL) was added, and the solution was mixed until the precipitate dissolved. The 96-well plate was read at 590 nm using a spectrophotometer (Bio-Tek, Winooski, VT, USA). The cell growth inhibition rate (IR) was calculated using the following formula: IR = (1− the experimental group OD/the negative control group OD) ×100%.
Apoptosis assay
The apoptosis rate was determined by flow cytometry. The KB cells were divided into nine groups and treated as described above. The cells were then collected into tubes and washed with phosphate-buffered saline. Annexin V-FITC (5 μL) and propidium iodide (5 μL) were added to the tubes, followed by incubation at 25°C for 10 minutes in the dark. The effect of FA-DOX-BSA MNPs on the apoptosis rate was evaluated using flow cytometry (FACSCalibur; Becton Dickinson, Mountain View, CA, USA). All experiments were performed in triplicate.
Antitumor effect of FA-DOX-BSA MNPs in vivo
BALB/C nude mice (male, aged 5 weeks) were purchased from the Shanghai Institute of Biological Sciences, Chinese Academy of Sciences (Shanghai, People’s Republic of China). Xenograft tumors were induced by subcutaneous injection of KB cells under the right leg. After 2 weeks, the mice were divided into five groups of six mice each as follows: a negative control group (folic acid-free RPMI medium containing 10% fetal calf serum); a DOX-BSA NP group; a BSA MNP group with heating; a FA-DOX-BSA MNP group with no heating; and a FA-DOX-BSA MNP group with heating. The mice had been injected intraperitoneally with sodium pentobarbital (50 mg/kg) beforehand. Next, all solutions were directly injected into the tumors at the 3, 6, 9, and 12 o’clock positions, at a volume equal to half of the tumor volume. After 24 hours, all of the groups were placed on a high-frequency induction heater (f =200 kHz; I =20 A) for 60 minutes every other day. The maximal temperature of the rectal tissue did not exceed 40°C, and the maximal temperature of the tumor core was maintained at 50°C. The tumor volume was calculated as follows: (long diameter × short diameter2)/2. The rate of inhibition of tumor volume was calculated as (1- volume of experimental group/volume of control groups) ×100%. Six days later, all of the mice were euthanized, and then weighed and sectioned with hematoxylin-eosin staining. The rate of inhibition of tumor weight was calculated as follows: (1− weight of experimental group/weight of control groups) ×100%.
Statistical analysis
Values are shown as the mean ± standard deviation. The data were analyzed with Statistical Package for the Social Sciences version 16.0 software (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered to be statistically significant.
Results and discussion
Characterization of targeted FA-DOX-BSA MNPs
Assessment of morphology by transmission electron microscopy
DOX-BSA MNPs was prepared using a desolvation cross-linking method with ethanol as the desolvating agent and 0.25% glutaraldehyde as the cross-linking agent. During the desolvation process, the pH was adjusted to 8.0 after bovine serum albumin, Fe3O4, and doxorubicin were dissolved in deionized water because pH is important for maintenance of the structure and characteristics of albumin, which has the ability to undergo a major reversible conformational isomerization with changes in pH. Foster classified the pH-dependent forms of albumin as N, B, F, E, and A.24,25 When pH ≥8, the structure of albumin is F and the albumin molecule expands by dissociation of the C-terminal half. Albumin at F has an increased viscosity and decreased dissolvability,26 which aids its formation into nanospheres. Transmission electron microscopy (Figure 1) showed FA-DOX-BSA MNPs to be approximately round in shape, and this has also been demonstrated by Ak et al27 and Shen et al.28 The particles were uniform in size. Moreover, an Fe3O4 area in the particle center could be seen in all particles. Its hydrodynamic size was about 20 nm.
Dynamic light scattering analysis
Nanoparticle size and size distribution as measured by dynamic light scattering is shown in Figure 2A. The size of FA-DOX-BSA MNPs was 180±2.2 nm. To assess the stability of the nanoparticles, the hydrodynamic size of the FA-DOX-BSA MNPs was analyzed by dynamic light scattering. The hydrodynamic sizes did not change significantly over 24 hours in phosphate-buffered saline or RPMI 1640 medium supplemented with 10% fetal bovine serum (Figure 2B). In addition, the nanoparticles showed limited (<10%) variation in hydrodynamic size after 3 weeks of storage in phosphate-buffered saline at 4°C, indicating excellent stability in aqueous medium.
Iron content, SAR and increasing-temperature tests
When the iron concentration was 0–100 μg/mL, the regression equation was Y =0.00417X +0.01099, r2=0.9997, where X and Y are the OD values and iron concentration, respectively. The iron content was 2.2 mg/mL. The SAR value for FA-DOX-BSA MNPs was 60±2.1 w/g. This result is consistent with that obtained for Fe3O4 alone (59.7±3.2 w/g). When the alternating magnetic field intensity was fixed, a perfect correlation between the heating ability of the FA-DOX-BSA MNPs and iron content was obtained, ie, the greater the iron concentration, the greater the increase in temperature. The heating curves for the various iron concentrations were similar: during the first 30 minutes, the temperature of the sample increased rapidly, and then only slightly between 30 and 35 minutes. After 35 minutes, the temperature remained constant (Figure 3). The 0.375 mg/mL solution could reach a temperature of 43°C (effective treatment temperature is 41°C–44°C) and the temperature could remain stable. Therefore, 0.375 mg/mL was selected for follow-on magnetic fluid hyperthermia in our experiment.
Drug encapsulation efficiency, drug loading efficiency and drug release
We used bovine serum albumin nanospheres containing Fe3O4 and doxorubicin encapsulated by a direct drug-loading process. For direct drug-loading, bovine serum albumin solution was prepared and doxorubicin and Fe3O4 were mixed into it; ethanol was then added dropwise in a continuous manner. Drug encapsulation efficiency and drug loading efficiency of nanoparticles are crucial for their clinical application. In our study, we found a favorable drug encapsulation efficiency, drug loading efficiency, and drug release time. Drug loading efficiency was ~4% and drug encapsulation efficiency was ~80%. Release of doxorubicin from the samples was determined by ultraviolet spectrophotometry, and the cumulative release at various time points in three pH solutions (pH 5.4, 7.4, 9.0) was calculated (Figure 4). Cumulative release of FA-DOX-BSA MNPs at 72 hours in pH 7.4 solutions was less than 6%. However, it had significantly increased in a weak acid medium, with a cumulative release rate of 78.3%. The magnetic nanoparticles were very stable at neutral and alkaline pH. No significant difference in cumulative release rate was observed in the presence of heating. The pH values in tumor tissues and lysosome were slightly acidic. With the magnetic nanoparticles inside the cancer cells, doxorubicin would release from it. However, when the magnetic nanoparticles in normal tissues, the doxorubicin would not release. This result confirmed that bovine serum albumin could carry anticancer drugs, which would be stable in blood during in vivo circulation and be released quickly after being selectively taken up by tumor tissue. Better release properties help chemotherapy drugs to have a stronger inhibitory and killing effect on cancer cells, which may improve patient acceptance and compliance.
Surface functional groups
Ultraviolet-visible near-infrared spectrophotometry was used to confirm the binding of folic acid to bovine serum albumin. The ultraviolet spectra for FA-NHS, DOX-BSA MNPs, and FA-DOX-BSA MNPs are shown in Figure 5. An absorption maximum for FA-NHS of 250–300 nm was detected by ultraviolet-visible near-infrared spectrophotometry. The absorption peak of FA-DOX-BSA MNPs at 286 nm clearly indicated successful contact between folic acid and DOX-BSA MNPs.
Antitumor effects of FA-DOX-BSA MNPs in vitro
MTT
For this study, KB cells were heated to a defined temperature (43°C) on the heating coil of a high-frequency induction heater (f =200 kHz; I =20 A) for 60 minutes. The antitumor effect on KB cells was determined by measuring the cell IR by MTT assay after 48 hours of drug treatment. As depicted in Table 1, the cell IRs for the DOX-BSA NP and DOX-BSA MNP groups were 22.50% and 25.20%, respectively. After using FA-DOX-BSA MNPs containing the same drug concentration, the cell IR increased to 43.15%. Thus, FA-DOX-BSA MNPs was effective in terms of inhibiting KB cell growth (P<0.05). Additionally, placement of the BSA MNP, DOX-BSA MNP, and FA-DOX-BSA MNP groups in an alternating magnetic field (f =200 kHz; I =20 A) for one hour of heating resulted in an increase in cell IRs to 47.84%, 74.06%, and 97.79%, respectively, indicating that heating itself inhibits KB cell growth. Cell viability in the negative control group, the negative control group with heating, and the BSA MNP group with no heating was not affected. These results suggest that FA-DOX-BSA MNPs have enhanced antitumor efficacy. When combined with hyperthermia, FA-DOX-BSA MNPs were more effective in terms of inhibiting cell proliferation than the other groups.
Apoptosis assay
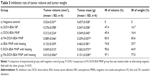
The number of apoptotic KB cells after 48 hours of incubation is shown in Figure 6. Apoptosis and necrosis can be found in all treatment groups, and the apoptosis take up a big proportion. It showed that the main function of our therapy was to induce apoptosis of KB cells in vitro. The function of hyperthermia at temperatures ranging from 42°C to 45°C is to cause inhibition of cellular enzymes and cell damage largely through the process of apoptosis.29,30 Using our therapy, the magnetic nanoparticle hyperthermia groups induced apoptosis even in the absence of doxorubicin. Induction of apoptosis after treatment with doxorubicin magnetic nanoparticles combined with hyperthermia was considerably greater than in the doxorubicin nanoparticle and magnetic nanoparticle hyperthermia groups (P<0.05). This means that hyperthermia had a positive impact on the effect of chemotherapy. Additionally, FA-DOX MNPs combined with hyperthermia induced apoptosis more rapidly than did nontargeted magnetic nanoparticles at an identical doxorubicin concentration (P<0.05). Therefore, a combined therapy mode could achieve a higher rate of apoptosis in NPC KB cells than chemotherapy alone. The percentages of apoptotic cells after treatment with DOX-BSA NPs, DOX-BSA MNPs, FA-DOX-BSA MNPs, BSA MNPs with heating, DOX-BSA MNPs with heating, FA-DOX-BSA MNPs with heating, the negative control with heating, and BSA MNPs with no heating were 23.68%±3.01%, 26.24%±2.94%, 43.87%±3.92%, 48.33%±2.01%, 77.32%±6.67%, 96.24%±2.81%, 0.84%±2.1% and 1.01%±0.25%, respectively (Table 2). These findings are consistent with the MTT data shown in Table 1.
Antitumor effect of FA-DOX-BSA MNPs in vivo
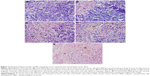
We determined the effect of FA-DOX-BSA MNPs on NPC tumor growth by calculating the changes in tumor volume and weight and by hematoxylin-eosin staining. During this process, the temperature inside a tumor can reach 41°C–44°C. Hemorrhage, necrosis, and eschar were evident throughout treatment, and hyperthermia-treated and thermochemotherapy-treated gray–white tumor tissue showed a dark brown color at the end of the treatment cycle. No such finding was evident in the control and chemotherapy only groups. Tumor volume and weight IRs are shown in Table 3. Consistent with our previous in vitro findings, FA-DOX-BSA MNPs combined with hyperthermia yielded significantly higher inhibition rates than the other groups (P<0.05). Specifically, the folic acid-targeted and nonheated groups exhibited decreased tumor volume and weight, respectively, from 0% with each pretreatment to 83.0% and 60.6%, respectively, at 1 week post-treatment. By contrast, the heat-treated, folic acid-targeted groups showed inhibition rates of 97.1% and 77.32%, respectively, over the same period. Hematoxylin-eosin staining revealed that tumor cells proliferated actively in the control group, with a low degree of differentiation (Figure 7). However, in the thermal therapy groups, some tumor tissues showed obvious necrosis and hemorrhage, particularly in the folic acid-targeted groups, where the tumor structures were completely destroyed and the tumor cells were irregularly shaped. The most intense inflammatory reaction and infiltration by inflammatory cells were also found in the thermal therapy groups. Brown iron was deposited in the tumors in the heated groups. These findings suggested that folic acid-targeted hyperthermia combined with chemotherapy could kill tumor cells. Taken together, these data demonstrate that administration of FA-DOX-BSA MNP is an effective method for tumor chemotherapy. Heating combined with chemotherapy had the most significant effect on tumors.
FA-DOX-BSA MNPs combined with hyperthermia therapy had a significantly greater effect on average tumor volume and weight than either non-FA-DOX-BSA MNPs or chemotherapy alone. In contrast, histopathological evaluation indicated that tumor structures in the FA-DOX-BSA MNP group were destroyed more completely than those in the non-FA-DOX-BSA MNP group. It is likely that the following mechanisms are involved:
- Hyperthermia as an effective treatment modality to augment chemotherapy-based anticancer treatments. Hyperthermia in combination chemotherapy can not only increase the drug concentration in tumor cells but also reduce the toxic effects to normal tissue and prevent the development of drug resistance. Clinical tests have shown that hyperthermia combined with chemotherapy has increased efficacy against tumors such as high-grade soft tissue sarcomas.31 In our experiment, when combined with hyperthermia therapy, FA-DOX-BSA MNPs had a greater effect on cell viability and inhibited NPC cells significantly (a four-fold increase in cytotoxicity versus DOX-BSA MNPs).
- Fe3O4 has a short half-life and no specific tumor-targeting effect, which limits its application in tumor therapy. In our study, we incorporated Fe3O4 into BSA NPs to avoid them being taken up by reticuloendothelial cells and removed by macrophages. BSA NPs with a smaller size (50–300 nm) compared with microparticles are easily prepared and could be widely accepted in the pharmaceutical industry.
- Tumor cells have a unique ability to absorb magnetic nanoparticles (8–400-fold more than normal cells) and distribute them in daughter cells uniformly, making them highly susceptible to magnetic fluid hyperthermia and avoiding damage to normal tissues.32
- FA-DOX-BSA MNPs showed a two-fold increase in cytotoxicity compared with nontargeted nanoparticles.
Active targeting of folic acid is a promising approach via which a drug delivery system can reach and penetrate into tumor cells with overexpression of folate receptors on their surface, and then release its encapsulated therapeutic agent in a controlled and sustained manner. FA-BSA MNPs had less in vitro toxicity and higher antitumor activity. Inhibition of in vivo tumor growth demonstrated that these carriers have excellent antitumor activity and induce a high rate of apoptosis in cancer cells.
Conclusion
Several recent studies have investigated the synthesis of folic acid-targeted albumin nanoparticles to avoid the side effects of antitumor drugs and improve tumor-targeted drug delivery to improve the treatment of tumors. However, FA-DOX-BSA MNPs targeting NPC are rarely used. In this study, we successfully synthesized FA-DOX-BSA MNPs as nanocarriers, and achieved thermotherapy, chemotherapy, and targeted therapeutic effects against NPC. However, further studies must be performed to optimize this therapy for clinical use and determine whether it can be used to treat other tumors.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program of China (973 Program; 2013 CB933904, 2011CB933500) and the National Natural Science Foundation of China (81271635, 81071881, 81301270, 81101139).
Disclosure
The authors report no conflicts of interest in this work.
References
Glastonbury CM, Salzman KL. Pitfalls in the staging of cancer of NPC. Neuroimaging Clin N Am. 2013;23:9–25. | ||
Han BL, Xu XY, Zhang CZ, et al. Systematic review on Epstein-Barr virus (EBV) DNA in diagnosis of NPC in Asian populations. Asian Pac J Cancer Prev. 2012;13:2577–2581. | ||
Agudelo D, Bourassa P, Bruneau J, Bérubé G, Asselin E, Tajmir-Riahi HA. Probing the binding sites of antibiotic drugs DOX and N-(trifluoroacetyl) DOX with human and BSAs. PLoS One. 2012; 7:e43814. | ||
Hanušová V, Boušová I, Skálová L. Possibilities to increase the effectiveness of DOX in cancer cells killing. Drug Metab Rev. 2011;43: 540–557. | ||
Carvalho C, Santos RX, Cardoso S, et al. DOX: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. | ||
Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–183. | ||
Zhao D, Zhao X, Zu Y, et al. Preparation, characterization, and in vitro targeted delivery of folic-decorated paclitaxel-loaded BSA nanoparticles. Int J Nanomedicine. 2010;5:669–677. | ||
Saxena V, Naguib Y, Hussain MD. Folic receptor targeted 17-allylamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric nanoparticles for breast cancer. Colloids Surf B Biointerfaces. 2012;94: 274–280. | ||
Lee KY, Seow E, Zhang Y, Lim YC. Targeting CCL21-folic acid-upconversion nanoparticles conjugates to folic receptor-α expressing tumor cells in an endothelial-tumor cell bilayer model. Biomaterials. 2013;34:4860–4871. | ||
Rijnboutt S, Jansen G, Posthuma G, Hynes JB, Schornagel JH, Strous GJ. Endocytosis of GPI-linked membrane folate receptor-alpha. J Cell Biol. 1996;132:35–47. | ||
Birn H, Selhub J, Christensen EI. Internalization and intracellular transport of folate-binding protein in rat kidney proximal tubule. Am J Physiol. 1993;264:C302–C310. | ||
Hall EJ, Cox JD. Physical and biological basis of radiation therapy. In: Moss WT, Brand WN, Battifora H, editors. Radiation Oncology: Rationale, Technique, Results. St Louis, MO, USA: CV Mosby Co; 2003. | ||
Pelicci PG, Dalton P, Orecchia R. Heating cancer stem cells to reduce tumor relapse. Breast Cancer Res. 2011;13:305. | ||
Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg. 1957;146:596–606. | ||
Wang L, Zhang J, An Y, et al. A study on the thermochemotherapy effect of nanosized As2O3/MZF thermosensitive magnetoliposomes on experimental hepatoma in vitro and in vivo. Nanotechnology. 2011; 22:315102. | ||
Hayashi K, Nakamura M, Sakamoto W, et al. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics. 2013;3:366–376. | ||
Kumar M, Singh G, Arora V, et al. Cellular interaction of folate acid conjugated superparamagnetic iron oxide nanoparticles and its use as contrast agent for targeted magnetic imaging of tumor cells. Int J Nanomedicine. 2012;7:3503–3516. | ||
Jordan A, Scholz R, Wust P, et al. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia. 1997; 13:587–605. | ||
Li XH, Rong PF, Jin HK, Wang W, Tang JT. Magnetic fluid hyperthermia induced by radiofrequency capacitive field for the treatment of transplanted subcutaneous tumors in rats. Exp Ther Med. 2012;3: 279–284. | ||
Béalle G, Di Corato R, Kolosnjaj-Tabi J, et al. Ultra magnetic liposomes for MR imaging, targeting, and hyperthermia. Langmuir. 2012;28: 11834–11842. | ||
Tang QS, An YL, Yang R. In vitro and in vivo evaluation of tumor-targeting efficacy of folate-conjugated magnetic albumin nanospheres by MRI. Chinese Journal of Magnetic Resonance. 2013;30:499–506. | ||
Li Y, Liu J, Zhong Y, et al. Biocompatibility of Fe3O4@ Au composite magnetic nanoparticles in vitro and in vivo. Int J Nanomedicine. 2011;6: 2805–2819. | ||
Chen D, Tang Q, Li X, et al. Biocompatibility of magnetic Fe3O4 nanoparticles and their cytotoxic effect on MCF-7 cells. Int J Nanomedicine. 2012;7:4973–4982. | ||
Foster JF. In: Rosenoer VM, Oratzand M, Rothschild MA, editors. Albumin Structure, Function and Uses. Oxford: Pergamon Press; 1977:53–84. | ||
Foster JF. In: Putnam FW, editor. The Plasma Proteins. 1st ed. Vol. 1. New York: Academic Press; 1960:179–239. | ||
Budavari S, O’Neil MJ, Smith A, Heckleman PE, Kinneary JF, editors. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ, USA: Merck; 1989. | ||
Ak G, Yɪlmaz H, Sanlɪer SH. Preparation of magnetically responsive albumin nanospheres and in vitro drug release studies. Artif Cells Nanomed Biotechnol. 2014;42:18–26. | ||
Shen Z, Li Y, Kohama K, et al. Improved drug targeting of cancer cells by utilizing actively targetable folic acid-conjugated albumin nanospheres. Pharmacol Res. 2011;63:51–58. | ||
Overgaard J, Suit HD. Time-temperature relationship in hyperthermic treatment of malignant and normal tissue in vivo. Cancer Res. 1979;39: 3248–3253. | ||
Tao Y, Guo Y, Liu W, et al. AKT inhibitor suppresses hyperthermia-induced Ndrg2 phosphorylation in gastric cancer cells. Braz J Med Biol Res. 2013;46:394–404. | ||
Franckena M, De Wit R, Ansink AC, et al. Weekly systemic cisplatin plus locoregional hyperthermia: an effective treatment for patients with recurrent cervical carcinoma in a previously irradiated area. Int J Hyperthermia. 2007;23:443–450. | ||
Jordan A, Wust P, Scholz R, et al. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int J Hyperthermia. 1996;12:705–722. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.