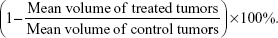Back to Journals » International Journal of Nanomedicine » Volume 12
Preparation, cytotoxicity, and in vivo antitumor efficacy of 111In-labeled modular nanotransporters
Authors Slastnikova TA , Rosenkranz AA , Morozova NB , Vorontsova MS, Petriev VM, Lupanova TN , Ulasov AV , Zalutsky MR , Yakubovskaya RI, Sobolev AS
Received 21 October 2016
Accepted for publication 22 November 2016
Published 10 January 2017 Volume 2017:12 Pages 395—410
DOI https://doi.org/10.2147/IJN.S125359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Video abstract presented by Slastnikova et al.
Views: 199
Tatiana A Slastnikova,1,* Andrey A Rosenkranz,1,2,* Natalia B Morozova,3 Maria S Vorontsova,3 Vasiliy M Petriev,4,5 Tatiana N Lupanova,1 Alexey V Ulasov,1 Michael R Zalutsky,6 Raisa I Yakubovskaya,3 Alexander S Sobolev1,2
1Laboratory of Molecular Genetics of Intracellular Transport, Institute of Gene Biology, Russian Academy of Sciences, 2Department of Biophysics, Biological Faculty, Lomonosov Moscow State University, 3Department of Anticancer Therapy Modifiers and Protectors, Moscow Hertsen Research Institute of Oncology, Russian Ministry of Health Care, Moscow, 4National Medical Research Radiological Center, Russian Ministry of Health Care, Obninsk, Moscow Region, 5Department of Nuclear Medicine, National Research Nuclear University MEPhI (Moscow Engineering Physics Institute), Moscow, Russia; 6Department of Radiology, Duke University Medical Center, Durham, NC, USA
*These authors contributed equally to this work
Purpose: Modular nanotransporters (MNTs) are a polyfunctional platform designed to achieve receptor-specific delivery of short-range therapeutics into the cell nucleus by receptor-mediated endocytosis, endosome escape, and targeted nuclear transport. This study evaluated the potential utility of the MNT platform in tandem with Auger electron emitting 111In for cancer therapy.
Methods: Three MNTs developed to target either melanocortin receptor-1 (MC1R), folate receptor (FR), or epidermal growth factor receptor (EGFR) that are overexpressed on cancer cells were modified with p-SCN-Bn-NOTA and then labeled with 111In in high specific activity. Cytotoxicity of the 111In-labeled MNTs was evaluated on cancer cell lines bearing the appropriate receptor target (FR: HeLa, SK-OV-3; EGFR: A431, U87MG.wtEGFR; and MC1R: B16-F1). In vivo micro-single-photon emission computed tomography/computed tomography imaging and antitumor efficacy studies were performed with intratumoral injection of MC1R-targeted 111In-labeled MNT in B16-F1 melanoma tumor-bearing mice.
Results: The three NOTA-MNT conjugates were labeled with a specific activity of 2.7 GBq/mg with nearly 100% yield, allowing use without subsequent purification. The cytotoxicity of 111In delivered by these MNTs was greatly enhanced on receptor-expressing cancer cells compared with 111In nontargeted control. In mice with B16-F1 tumors, prolonged retention of 111In by serial imaging and significant tumor growth delay (82% growth inhibition) were found.
Conclusion: The specific in vitro cytotoxicity, prolonged tumor retention, and therapeutic efficacy of MC1R-targeted 111In-NOTA–MNT suggest that this Auger electron emitting conjugate warrants further evaluation as a locally delivered radiotherapeutic, such as for ocular melanoma brachytherapy. Moreover, the high cytotoxicity observed with FR- and EGFR-targeted 111In-NOTA–MNT suggests further applications of the MNT delivery strategy should be explored.
Keywords: nuclear delivery, cancer, melanoma, radionuclide therapy, Auger electrons
Introduction
Targeted subcellular delivery systems have attracted growing attention in development of anticancer drugs.1–3 It is especially concerned with the nuclear targeting system4 because the majority of anticancer agents act inside the cell nucleus. However, their real in vivo efficacy is little studied in most cases and for many perspective active principles, it depends on both the efficacy of the delivery system and the attachment technique of these active principles to the vehicle. The high cytotoxicity of Auger electrons when localized in close proximity to cellular DNA is an attractive feature for molecularly targeted radiotherapy.5 Moreover, their circumscribed tissue range (generally <0.1 μm) should also be advantageous in those settings where minimizing collateral damage to the normal tissues adjacent to the tumor is critical, such as in the treatment of central nervous system malignancies.6 Although radiopharmaceuticals labeled with Auger electron emitters have been considered to be promising as anticancer therapeutic agents for several decades and a lot of research has been done in the field, translation from attractive concept to clinical reality has been difficult.7 This can mainly be attributed to the flip side of the coin of their subcellular range of action, namely, that methods must be devised to localize the Auger electron emitter specifically to cancer cells within a tumor and in close proximity to the cell nucleus or preferably within the cell nucleus in order to maximize therapeutic effectiveness. A wide variety of approaches have been investigated for achieving this difficult task, including antibodies,8 functionalized antibodies,9 small molecules,10 block copolymer micelles,11 and peptides,12 with varying degrees of success.
We have been working on an alternative strategy for achieving molecularly directed selective delivery of short-range therapeutics such as Auger electron emitters to malignant cell populations, based on an engineered polypeptide platform.13 These modular nanotransporters (MNTs) are artificial multifunctional molecules designed to facilitate receptor-specific transport from the cell surface into its nucleus through inclusion of polypeptide domains for accomplishing receptor binding and internalization as well as endosomal escape and nuclear translocation. Moreover, MNTs possess interchangeable component domains, permitting relatively facile exchange of receptor recognition domains, which ultimately might be adapted for different diseases or might help improve homogeneity of drug delivery by targeting several tumor-specific molecules for personalized medicine. MNTs reactive with a variety of tumor-associated receptors have shown promise for delivering short range of action therapeutics including photosensitizers,13,14 the α-emitter 211At,15 and the Auger electron emitters 125I16 and 67Ga,17 as they were demonstrated to colocalize in the nuclei of target cancer cells in vitro (up to 60% of internalized found in nuclei)13,14,16,17 and in vivo,14 as well as enhance significantly cytotoxicity of all the above-mentioned cargoes in vitro13,15–18 and the therapeutic potential of different photosensitizers in vivo.14 Moreover, manifold advantage of full-size MNT over MNTs lacking any of the functional domains for delivery of cytotoxic drugs has been demonstrated previously.18 Recent efforts in Auger electron-targeted radiotherapy have been primarily focused on applications involving 111In8,9,11,12 based, in part, on its ready availability, commercial and clinically compatible 2.8-day half-life, and the wealth of experience in patients with 111In-labeled compounds as imaging agents. With regard to its low-energy electron spectrum, 111In emits 14.7 Auger and Coster–Kronig electrons per decay;19 moving its site of decay from the cell surface to the nucleus would increase the radiation dose delivered to the cell nucleus by more than tenfold for typical cell geometries.20 Based on its excellent properties for imaging and Auger electron radiotherapy, 111In is an ideal candidate for developing theranostics for use in settings where sparing normal tissue is of paramount importance, such as in the treatment of glioblastoma infiltrating normal brain or in the ocular brachytherapy and prostate brachytherapy.
The objective of the current study was first to adapt 111In polypeptide labeling methodologies for use with MNTs, which have isoelectric points in the optimum pH range for indium chelation, which complicates this process, and then to evaluate the resultant 111In-labeled MNT as targeted radiotherapeutics in cell culture and animal cancer models.
Materials and methods
Cell lines
The mouse melanoma B16-F1 and human epidermoid carcinoma A431 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Human cervical carcinoma HeLa cells and human ovarian carcinoma SK-OV-3 cells were kindly provided by Prof E Sverdlov and Prof S Deev, respectively, both from the Institute of Bioorganic Chemistry, RAS. The use of HeLa and SK-OV-3 cell lines was approved by Moscow State University Commission on Bioethics. Human glioma U87 cells transfected to express epidermal growth factor receptor (EGFR; U87MG.wtEGFR) were kindly provided by Dr Darell Bigner, Duke University Medical Center. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; B16-F1, A431), Zink Option Improved MEM (U87MG.wtEGFR), or in-house folate-deficient media (HeLa, SK-OV-3) supplemented with both 10% calf fetal serum and gentamicin (50 mg/L) at 37°C in a 5% CO2 atmosphere.
Modular nanotransporters
Three different MNT molecules were used in these experiments (Figure 1): 1) melanocortin-1 receptor targeted tDTox-HMP-NLS-MSH (MNT-MSH), Mw 70,430 Da, consisting of α-melanocyte-stimulation hormone (MSH) as the ligand module, the diphtheria toxin translocation domain (tDTox) as an endosomolytic module, the optimized nuclear localization sequence of SV-40 large T-antigen (NLS), and hemoglobin-like protein (HMP) as a carrier module;14 2) epidermal growth factor (EGF) receptor targeted tDTox-HMP-NLS-EGF (MNT-EGF),13,14 Mw 76,306 Da; and 3) folate receptor (FR)-targeted MNT, possessing folic acid (FA; Sigma, St Louis, MO, USA) covalently attached to the polypeptide tDTox-HMP-NLS (Mw 70,125 Da) by N-hydroxysuccinimide and ethylene dicyclohexyl carbodiimide, as it was described for albumin.21 All MNTs were synthesized and purified as described earlier.13,14,18 Briefly, each MNT was expressed in an Escherichia coli suspension (OD600 0.6–1.0) by the addition of isopropyl-β-D-1-thiogalactopyranoside to a final concentration of 200 μM at 18°C and shaking at 170–180 rpm for 2 h. Bacterial cells were pelleted by centrifugation at 10,000 rpm with a JA-10 rotor at 4°C for 30 min. Then, the pellets were frozen for subsequent lysis in ice-cold 50 mM sodium phosphate, 300 mM NaCl, pH 8.0, 1 mM phenylmethylsulfonyl fluoride, 5 mg/mL lysozyme, and 0.5% Triton X-100. The obtained lysate was clarified by centrifugation at 18,000 rpm with a JA-20 rotor at 4°C for 30 min. Then, the supernatant was loaded onto an Ni-NTA agarose (Qiagen NV, Venlo, the Netherlands) column, washed with 50 mM sodium phosphate, 300 mM NaCl, 20 mM imidazole, pH 8.0, 0.5% Triton X-100, and 1% glycerol, followed by 50 mM sodium phosphate, 300 mM NaCl, 20 mM imidazole, pH 8.0. MNTs were eluted with 50 mM sodium phosphate, 300 mM NaCl, 700 mM imidazole, pH 8.0, and dialyzed against 10 mM sodium phosphate, 150 mM NaCl, pH 7.4.
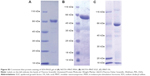
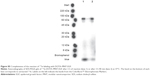
The FR-targeted MNT was synthesized by chemical attachment of FA to ligand-free MNT according to the protocol described previously for FA attachment to albumin.21 Briefly, FA (Sigma) was dissolved in dimethyl sulfoxide to a concentration of 0.1 M and incubated with an 1.2-fold molar excess of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide for 1 h at room temperature (RT), followed by the addition of N-hydroxysuccinimide in 1.2-fold molar excess. After 4 h of incubation at RT, a 15-fold molar excess of the “activated” FA was added to the ligand-free MNT solution and incubated for 12 h at RT. The unconjugated FA as well as the reaction by-products were removed by five cycles of ultrafiltration with an Amicon Ultra Centrifugal Filter Unit (Ultracel-30K; Merck Millipore Ltd, Tullagreen, Ireland), yielding purified MNT-FA. FA:MNT ratio in the obtained MNT-FA was estimated spectrophotometrically. Purity and integrity of MNT were determined by Laemmli sodium dodecyl sulfate (SDS)-PAGE using Mini-Protean TGX Any kD gels (Bio-Rad Laboratories Inc., Hercules, CA, USA) with subsequent Coomassie Blue staining. The obtained images were analyzed using ImageQuant TL 5.0 software (Bio-Rad Laboratories Inc.). All three MNTs were visualized by atomic force microscopy (Figure S1).
Conjugation of p-SCN-Bn-NOTA or p-SCN-Bn-DOTA to MNT
To permit subsequent 111In labeling, the chelator S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA; Macrocyclics, Dallas, TX, USA; −log KIn3+ NOTA =26.2)22 or S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (p-SCN-Bn-DOTA; Macrocyclics; −log KIn3+ DOTA =23.9)22 was conjugated to MNT according to a recently published protocol,17 using 10-fold chelator molar excess over MNT. All buffers for chelator conjugation and labeling procedures were first passed through a Chelex-100 resin (200–400 mesh, Bio-Rad Laboratories Inc.) to minimize adventitious heavy metal ion contamination. Briefly, 1–16 mg of each MNT was incubated with a 10-fold molar excess of the chelator in 1× pH 8.6 conjugation buffer17 for 24 h at RT with a final concentration of MNT ≥1.5 mg/mL. The chelator–MNT conjugate was concentrated and separated from the excess chelator by five cycles of ultrafiltration with an Amicon Ultracel-30K unit. During this process, the conjugation buffer was gradually replaced with 10 mM HEPES, 15 mM NaCl, pH 7.4. The chelator–MNT molar ratio was determined by MALDI-MS performed on a MALDI TOF/TOF Mass Spectrometer (Shimadzu, Kyoto, Japan) using a nitrogen laser at 337 nm with 500 shots per probe. Purity and integrity of chelator–MNT were determined by Laemmli SDS-PAGE using Mini-Protean TGX Any kD gels (Bio-Rad Laboratories Inc.) with subsequent Coomassie Blue staining. The obtained images were analyzed using ImageQuant TL 5.0 software (Bio-Rad Laboratories Inc.).
Labeling of chelator–MNT conjugates with 111In
We searched for optimal conditions for labeling chelator–MNT with 111In that would be compatible with the isoelectric points of these MNTs (5.5–5.6, calculated) and would also provide high radiochemical yield and specific activity. Variables included reaction mixture pH, SDS and citrate concentrations, temperature, and time. Moreover, this was done with 111InCl3 preparations having either relatively low (standard diagnostic agent; 0.22 GBq/mL) or high (custom-made; 2.3±0.2 GBq/mL) volumetric activity concentrations. The optimized protocol for MNT labeling that follows was used for subsequent experiments. In a typical procedure, 0.12 mg of chelator–MNT in 10 mM HEPES, 15 mM NaCl, pH 7.5, was mixed with 29 μL of 1 M HEPES, pH 7.5, 21 μL of 0.1 M citrate, pH 6.7, 8 μL of 1% SDS, and 42 μL of 0.25 M HCl (Ultrapure Grade; EMD Millipore, Billerica, MA, USA). Then, 102 μL (322 MBq) of 111InCl3 (Federal Center of Nuclear Medicine Projects Design and Development, Moscow, Russia) in 0.048 M HCl was added. The pH of the final reaction mixture was 4.5. The reaction mixture was incubated at 37°C for 1 h and then the reaction was stopped by addition of 3 μL of 0.05 M ethylenediaminetetraacetic acid (EDTA), pH 8.0, followed by gentle mixing and incubation for 10 min at 37°C. Finally, the pH was adjusted to neutral with 24 μL of 1 M NaOH. The specific activity obtained for the three 111In-NOTA–MNT types using this protocol was 2.7 GBq/mg. As a control for subsequent in vitro and in vivo studies, 111In was treated according to the same procedures except that the chelator–MNT was omitted. Radiochemical yields and 111In–chelator–MNT integrity were analyzed by Laemmli SDS-PAGE using Mini-Protean TGX Any kD gels (Bio-Rad Laboratories Inc.) with subsequent detection of radioactivity on a Storm 865 PhosphorImager (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The obtained images were analyzed using ImageQuant TL 5.0 software (Bio-Rad Laboratories Inc.). We have chosen Laemmli SDS-PAGE instead of the usually used instant thin layer chromatography to evaluate the labeling yields, since this method not only provides the data about the percentage of unreacted 111In that migrates immediately before bromophenol blue on the gel, but also demonstrates the integrity of protein molecule after labeling.
Cytotoxicity studies
B16-F1 (melanocortin receptor-1 [MC1R]-positive),23 HeLa or SK-OV-3 (both FR-positive),24,25 and A431 or U87MG.wtEGFR (both EGFR-positive)15 cells were seeded in 24-well plates (7×103 cells/well for B16-F1 and 2.5×104 cells/well for other cell lines). Two days later, the medium was refreshed and dilutions of 111In-NOTA–MNT (0.03–9.5 MBq/mL) or 111In-EDTA as a control (0.3–9.5 MBq/mL) were added. Cells were incubated for 48 h in a humidified atmosphere at 37°C in 5% CO2. Afterward, the medium containing unbound radioactivity was removed and the cells were washed, trypsinized, harvested, and resuspended in 1 mL of fresh medium. The cells were seeded for a colony-forming assay in 25 cm2 flasks (2,000 cells per flask for HeLa, SK-OV-3, A431, and U87MG.wtEGFR and 500 cells per flask for B16-F1) in DMEM or DMEM/F12 medium supplemented with 10% calf fetal serum. Colonies were stained 6–11 days later with 0.3% Crystal Violet and counted.
Animal studies
These experiments were performed on 8–9-week-old female C57BL/6J mice (Andreevka, Moscow Region, Russia). The animals were maintained under specific pathogen-free conditions with access to mouse chow and water ad libitum. The experimental protocol was approved by the Institute Commission for Animals and was performed in accordance with the National Institutes of Health guide for the care and use of laboratory animals. B16-F1 mouse melanoma tumors were established in mice by subcutaneous injection of 106 cells suspended in 100 μL DMEM medium into the flank region as described previously.14
Single-photon emission computed tomography/computed tomography (SPECT/CT) imaging
For SPECT/CT imaging, the animals were anesthetized with 0.8%–1.8% isoflurane in air. The mice (n=4; mean tumor volume: 88±19 mm3) received intratumoral bolus injections of 7.3±1.1 MBq of 111In-NOTA–MNT-MSH in 45 μL. Whole body imaging was performed on an U-SPECT-II/CT (MILabs, Utrecht, the Netherlands) scanner, beginning immediately after injection and continuing for 2.5 h (13×10-min frames) using a 1 mm diameter pinhole collimator, with subsequent immediate whole-animal CT acquisition. Additional SPECT/CT imaging was performed up to 8 days (5 frames ×10 min). The images were reconstructed using U-SPECT-Rec2.34b software obtained from the manufacturer, followed by co-registration of SPECT images to corresponding CT images. Quantitative analysis of images after three-dimensional reconstruction was performed using PMOD 3.4 software (PMOD Technologies Ltd., Zürich, Switzerland).
Therapy studies
For therapy studies, B16-F1 mouse melanoma tumor-bearing mice (n=5) were i.t. injected with 2.6 MBq/1.4 μg, 5.2 MBq/2.9 μg, or 10.4 MBq/5.7 μg of either 111In-NOTA–MNT-MSH, or 111In-EDTA or unlabeled NOTA–MNT-MSH, or saline in a volume 40 μL. The efficacy of the treatment was monitored using the tumor growth inhibition index, defined as:
|
Statistical analysis
The data were analyzed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Data on the plots represent the mean values, with bars indicating the standard error of mean of 3–6 repetitive values. The significance of the difference was evaluated using the one-way analysis of variance, the Kruskal–Wallis, or F-test.
Results
Modular nanotransporters
MNT-MSH and MNT-EGF are artificial modular polypeptide nanostructures with the functional properties that have been described earlier which are targeted to MC1R and EGFR.13,16,18,26 In this study, we also used the same structure lacking any ligand module to produce MNT with FR targeting. FR-targeted MNT was obtained by covalent attachment of FA to MNT molecule, resulting in FA:MNT molar ratio of 1.6:1 in the final product. The ability of MNT-FA to bind target FR receptors specifically by competition studies with free FA was demonstrated on two different cancer cell lines expressing FR (Figure S2).
Labeling of chelator–MNT conjugates with 111In
MNTs were successfully conjugated with bifunctional p-SCN-Bn-NOTA or p-SCN-Bn-DOTA chelators, yielding chelator:MNT ratio of 2.5±0.2. SDS-PAGE of chelator–MNTs is presented in Figure S3.
Due to the hydrolysis of 111In at neutral pH as well as the increased hydrophobicity of these MNTs at slightly acidic pH, attempts to label the NOTA–MNT or DOTA–MNT conjugates with 111In either in the acetate buffer routinely used for 111In labeling of proteins27 or using a protocol that was successful for 67Ga labeling of NOTA–MNT17 were futile. Based on the studies by Brom et al28 and Morfin and Toth,29 we used HEPES and citrate; we also added a small amount of SDS (10 times below the critical micelle concentration) to the reaction mixture in an attempt to shield the hydrophobic sites that are known to be exposed on the MNT molecule at mildly acidic pH. Using the standardized set of labeling conditions outlined in the “Materials and methods” section, the 111In-NOTA–MNT labeling had moderately fast reaction kinetics at 37°C (Figure 2A). Yields for labeling these NOTA–MNT conjugates with 111In were relatively constant over a broad range of SDS concentration (Figure 2B), citrate concentration (Figure 2C), and pH (Figure 2D).
SDS-PAGE autoradiographs of the three 111In-NOTA–MNT conjugates are presented in Figure 3A. Native PAGE of 111In-NOTA–MNT-EGF is presented in Figure S4. Initially, we used a standard commercial 111In preparation; however, this limited us to a maximal 0.2 GBq/mg specific activity for 111In-NOTA–MNT. It was possible to increase the 111In-NOTA–MNT specific activity to a maximal value of 2.7 GBq/mg using custom-made batches of 111In (Figure 3B). Because labeling yields were consistently nearly quantitative (96.5%±0.1%, n=19), the 111In-NOTA–MNT were not purified before use. The same labeling process performed with conventionally used DOTA–MNT demonstrated rather slow reaction kinetics at 37°C, reaching a plateau at approximately 3.5 h (Figure S5), limiting its further potential clinical translation; thus, all the studies were performed on NOTA–MNT.
Cytotoxicity studies
The cytotoxicity of 111In-NOTA–MNTs compared to control 111InCl3 was enhanced significantly and as would be expected for saturable, receptor-mediated targeting, the cytotoxic potency for these 111In-NOTA–MNT constructs was dependent on the specific activity of the labeled MNT. For example, significantly more efficient colony formation inhibition after 111In-NOTA–MNT-EGF treatment was observed at 2.7 GBq/mg (A10, activity to give 10% survival =0.13 MBq/mL) compared with 0.2 GBq/mg (A10=0.38 MBq/mL; Figure 4). When 111In was delivered by MNT-FA to FR-positive cancer cells (HeLa, SK-OV-3), their ability to form colonies was reduced significantly, compared with nontargeted 111InCl3 (Figure 5A and B). Moreover, this effect was abrogated when an excess of free FA was added to the cells, confirming the specificity of 111In-NOTA–MNT-FA cytotoxicity on FR-positive target cells (Figure 5A). Similar cytotoxic effects were observed for 111In delivered by MNT-MSH (Figure 5C) and MNT-EGF (Figure 5D) to the cancer cells overexpressing MC1R (B16-F1) or EGFR (U87.wtEGFR), respectively. Both these EGFR-targeted and MC1R-targeted MNTs have been previously demonstrated to act specifically on the target cells overexpressing EGFR or MC1R receptors, respectively.13,18 Manifold advantage of full-size MNT over MNTs lacking any of the functional domains for delivery of cytotoxic drug has been demonstrated previously.18
Animal studies
SPECT/CT imaging
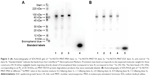
Following i.t. injection, 111In-NOTA–MNT-MSH demonstrated good intratumoral retention of 111In activity (Figure 6). When fit to a single exponential equation, the decay-corrected clearance half-life from the B16-F1 tumor was calculated to be 3.7 days (95% confidence interval: 2.8–5.3); however, the data were better (P=0.0003, F-test) fit to a two-exponential equation with half-lives of about 0.5 and 11 days. Animated three-dimensional tomograms are presented in Video S1. Detection of radioactivity following intratumoral administration of 111In-NOTA–MNT-MSH in normal tissues was quite low and limited to nearly undetectable signal observed only in kidneys and liver (Figure 6).
Therapy studies
The potential utility of 111In-NOTA–MNT-MSH as a strategy for molecularly targeted radiotherapy was investigated after intratumoral injection in mice with subcutaneous B16-F1 melanoma tumors. Dose-dependent tumor growth delay was observed in three experiments (2.6, 5.2, and 10.4 MBq, respectively) with 111In-NOTA–MNT-MSH (Figure 7). The highest dose was the most efficient resulting in 82% tumor growth inhibition, compared to control animals receiving saline. The corresponding dosages of either control 111In or nonlabeled NOTA–MNT-MSH did not result in any tumor growth inhibition.
Discussion
The main advantage of using Auger electrons for cancer treatment – the strong local damage of DNA – can be best achieved when the radionuclide is transported into the nuclei of cancer cells, but not into neighboring normal cells. Like many targeted radiotherapeutics, MNTs utilize receptor recognition to impart cancer cell specificity and internalization; however, these events lead to translocation to endosomes rather than the cell nucleus.30,31 Because nuclear import machinery resides in the cytoplasm, the MNT must escape the endosomes before its NLS component can activate nuclear transport. This is accomplished by its endosomolytic module, which increases its hydrophobicity to provide penetration through the endosomal membrane in response to the decreased pH of acidifying endosomes.26 The ability of MNTs to escape from acidifying endosomes as well as the necessity of tDTox inclusion into MNT molecule have been demonstrated by us previously,26 along with their ability to accumulate efficiently in the nuclei of target cancer cells in vitro13,16,17 and in vivo.14 Unfortunately, this property of endosomolytic module is problematic from the perspective of 111In labeling because it makes the MNTs prone to aggregation in the 4–6.5 pH range that is typically used for efficient 111In labeling.32,33 However, thorough optimization of the reaction conditions yielded >96% labeling of all three NOTA–MNT conjugates, obviating the need for further purification, and at sufficient specific activity of 2.7 GBq/mg (222 GBq/μmol). This 111In labeling approach should be adaptable to other nanomedicines with biophysical characteristics incompatible with standard 111In protein labeling procedures.
Specific activity has been shown to be important for maximizing the therapeutic potential of a radiolabeled molecule even when labeled with α-emitters that only require <10 atoms bound per cell to achieve effective cell kill.34 Because the number of Auger electron emitters needed to accomplish a similar degree of cytotoxicity is two to three orders of magnitude higher,16,17 maximizing the specific activity is a critical parameter for Auger emitter radiotherapy. Indeed, our studies with 111In-NOTA–MNT-EGF (Figure 4) demonstrated a profound increase in cytotoxicity when its specific activity was increased from 0.2 to 2.7 GBq/mg, where about 12% of MNT was labeled. Beyond decreasing MNT mass and increasing 111In activity, if higher specific activity for these MNTs is required, it might be possible to modify the MNTs with a metal chelating polymer, which permitted site-specific addition of 24 or 29 DTPA groups to trastuzumab.35 Although this strategy was very effective in increasing the specific activity, this modification compromised NLS functionality and its effect on the in vivo behavior of the 111In–trastuzumab conjugate might be problematic.
With regard to the in vivo studies of molecularly targeted Auger electron radiotherapeutics, the current study is one of the few studies evaluating therapeutic efficacy. Kassis5 utilized 5-[125I]iodo-2′-deoxyuridine in a variety of animal models, generally by loco-regional administration, and with multiple doses of the therapeutic. Recently, Kiess et al10 reported significant growth delay of prostate-specific membrane antigen + PC3 PIP tumors after intravenous injection of 125I-DCIBzL, a small prostate-specific membrane antigen–avid molecule. The therapeutic efficacy of several 111In-labeled monoclonal antibodies including those targeting HER29 and CD7436 has been evaluated, with encouraging responses observed in some cases.9,36 Potentially germane to the current studies with 111In-NOTA–MNT-EGF, Chen et al37 reported significant growth inhibition of 10–15 mm3 subcutaneous human breast carcinoma xenografts after they received five weekly doses of 111In-DTPA-EGF at a cumulative dose of 92.5 MBq. Direct comparisons were not made between an 111In-EGF and an 111In-MNT-EGF construct in the present article; however, we do note that in a previous study using residualizing 125I labeling, the in vitro cytotoxicity of [125I]SGMIB-MNT-EGF was 5–18 times higher than that of [125I]SGMIB-EGF.16
For in vivo proof-of-principle experiments, we selected MNT-MSH, targeted to melanoma cells, which overexpress MC1R.38 This MNT has been regarded recently as a promising approach for the treatment of malignant melanoma,39–41 including uveal melanoma.42 Although skin melanomas are usually managed by surgical excision, ocular melanomas are routinely treated with brachytherapy.43,44 While effective γ-emitting (125I, 103Pd) or β-emitting (106Ru) eye plaques are utilized for brachytherapy, this treatment can lead to nonspecific damage of healthy eye tissues.44,45 111In-NOTA–MNT-MSH might have an advantage over conventional brachytherapy in this setting due to its specific action on MC1R-expressing melanoma cells.
As a first step, the tissue distribution and therapeutic efficacy of 111In-NOTA–MNT-MSH was evaluated after loco-regional administration in the mouse melanoma B16-F1 subcutaneous tumor model. SPECT imaging revealed prolonged retention of 111In in the tumor and minimal activity in normal tissues. Although catabolite analyses were not performed, the lack of normal tissue activity suggests that 111In escaped from the tumor either as, or was rapidly converted to, a rapidly excreted form. Moreover, the results from single-dose 111In-NOTA–MNT-MSH tumor growth delay experiments were promising and compared favorably to those obtained with other Auger electron emitting therapeutics. Based on studies with other Auger emitting therapeutics such as 111In-DTPA-EGF37 as well as our own with MNT-MSH photosensitizer conjugates,14,18 it should be possible to further enhance therapeutic efficacy through the use of multidose 111In-NOTA–MNT-MSH strategies, and studies are planned to investigate this possibility.
Conclusion
Using an 111In labeling procedure modified for compatibility with MNT biophysical properties, MNTs targeting EGFR, FR, and MC1R were labeled in excellent yield providing suitable specific activity for the efficient treatment. The resultant 111In-NOTA–MNT conjugates were shown to be highly cytotoxic against cancer cells overexpressing these receptors, and one of these, 111In-NOTA–MNT-MSH, exhibited encouraging therapeutic potential in a murine model of melanoma. Taken together, these results suggest that MNTs warrant further evaluation as a platform technology for 111In Auger electron molecular radiotherapy with the vehicles for targeted intracellular delivery into the nuclei of cancer cells.
Acknowledgments
This work was supported in part (the development of labeling of NOTA–MNTs with 111In, and all experiments performed on 111In-NOTA–MNT-FA) by the Russian Science Foundation Grant 14-14-00874. The rest of this work was supported by the State Assignment Grant 0100-2014-0013 and the State Contract 13411.1008799.13.135. We thank YV Khramtsov for the excellent help with atomic force microscopy experiments. We thank YuR Sharapova, DP Sianova, and AS Abrosimov for the excellent technical assistance. Experiments were carried out with the use of the equipment from MV Lomonosov Moscow State University Program of Development and the Center of Collective Usage of the Institute of Gene Biology.
Author contributions
TAS, AAR, and ASS designed and evaluated the study. RIY and NBM designed and evaluated the therapeutic experiment with mice. TAS, AAR, NBM, MSV, VMP, TNL, AVU, and RIY performed the study and data analysis. TAS, AAR, MRZ, and ASS wrote the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Rajendran L, Knolker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov. 2010;9(1):29–42. | ||
Li Y, Wang J, Wientjes MG, Au JL. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev. 2012;64(1):29–39. | ||
Wei H, Zhuo RX, Zhang XZ. Design and development of polymeric micelles with cleavable links for intracellular drug delivery. Progr Polymer Sci. 2013;38:503–535. | ||
Tammam SN, Azzazy HM, Lamprecht A. How successful is nuclear targeting by nanocarriers? J Control Release. 2016;229:140–153. | ||
Kassis AI. The amazing world of auger electrons. Int J Radiat Biol. 2004;80(11–12):789–803. | ||
Buchegger F, Perillo-Adamer F, Dupertuis YM, Delaloye AB. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging. 2006;33(11):1352–1363. | ||
Cornelissen B, Vallis KA. Targeting the nucleus: an overview of Auger-electron radionuclide therapy. Curr Drug Discov Technol. 2010;7(4):263–279. | ||
Costantini DL, Chan C, Cai Z, Vallis KA, Reilly RM. 111In-labeled trastuzumab (Herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48(8):1357–1368. | ||
Costantini DL, McLarty K, Lee H, Done SJ, Vallis KA, Reilly RM. Antitumor effects and normal-tissue toxicity of 111In-nuclear localization sequence-trastuzumab in athymic mice bearing HER-positive human breast cancer xenografts. J Nucl Med. 2010;51(7):1084–1091. | ||
Kiess AP, Minn I, Chen Y, et al. Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J Nucl Med. 2015;56(9):1401–1407. | ||
Hoang B, Reilly RM, Allen C. Block copolymer micelles target auger electron radiotherapy to the nucleus of HER2-positive breast cancer cells. Biomacromolecules. 2012;13(2):455–465. | ||
Panosa C, Fonge H, Ferrer-Batalle M, et al. A comparison of non-biologically active truncated EGF (EGFt) and full-length hEGF for delivery of Auger electron-emitting 111In to EGFR-positive breast cancer cells and tumor xenografts in athymic mice. Nucl Med Biol. 2015;42(12):931–938. | ||
Gilyazova DG, Rosenkranz AA, Gulak PV, et al. Targeting cancer cells by novel engineered modular transporters. Cancer Res. 2006;66(21):10534–10540. | ||
Slastnikova TA, Rosenkranz AA, Gulak PV, et al. Modular nanotransporters: a multipurpose in vivo working platform for targeted drug delivery. Int J Nanomedicine. 2012;7:467–482. | ||
Rosenkranz AA, Vaidyanathan G, Pozzi OR, Lunin VG, Zalutsky MR, Sobolev AS. Engineered modular recombinant transporters: application of new platform for targeted radiotherapeutic agents to alpha-particle emitting 211At. Int J Radiat Oncol Biol Phys. 2008;72(1):193–200. | ||
Slastnikova TA, Koumarianou E, Rosenkranz AA, et al. Modular nanotransporters: a versatile approach for enhancing nuclear delivery and cytotoxicity of Auger electron-emitting 125I. EJNMMI Res. 2012; 2(1):59. | ||
Koumarianou E, Slastnikova TA, Pruszynski M, et al. Radiolabeling and in vitro evaluation of 67Ga-NOTA-modular nanotransporter: potential Auger electron emitting EGFR-targeted radiotherapeutic. Nucl Med Biol. 2014;41(6):441–449. | ||
Rosenkranz AA, Lunin VG, Gulak PV, et al. Recombinant modular transporters for cell-specific nuclear delivery of locally acting drugs enhance photosensitizer activity. FASEB J. 2003;17(9):1121–1123. | ||
Howell RW. Radiation spectra for Auger-electron emitting radionuclides: report No 2 of AAPM Nuclear Medicine Task Group No 6. Med Phys. 1992;19(6):1371–1383. | ||
Goddu SM, Howell RW, Bouchet LG, Bolch WE, Rao DV. MIRD Cellular S Values. Reston, VA: Society of Nuclear Medicine; 1997. | ||
Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci U S A. 1991;88(13):5572–5576. | ||
Sun Y, Martell AE, Welch MJ. Synthesis of a new diaminodithiol bifunctional chelator for radiolabeling biomolecules with indium (III). Tetrahedron. 2000;56(29):5093–5103. | ||
Solca F, Siegrist W, Drozdz R, Girard J, Eberle AN. The receptor for alpha-melanotropin of mouse and human melanoma cells. Application of a potent alpha-melanotropin photoaffinity label. J Biol Chem. 1989;264(24):14277–14281. | ||
Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66(6):1406–1414. | ||
Siu MK, Kong DS, Chan HY, et al. Paradoxical impact of two folate receptors, FR+- and RFC, in ovarian cancer: effect on cell proliferation, invasion and clinical outcome. PLoS One. 2012;7(11):e47201. | ||
Khramtsov YV, Rokitskaya TI, Rosenkranz AA, et al. Modular drug transporters with diphtheria toxin translocation domain form edged holes in lipid membranes. J Control Release. 2008;128(3):241–247. | ||
Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem Rev. 2010;110(5):2858–2902. | ||
Brom M, Joosten L, Oyen WJ, Gotthardt M, Boerman OC. Improved labelling of DTPA-and DOTA-conjugated peptides and antibodies with 111In in HEPES and MES buffer. EJNMMI Res. 2012;2:4. | ||
Morfin JF, Toth E. Kinetics of Ga (NOTA) formation from weak Ga-citrate complexes. Inorg Chem. 2011;50(20):10371–10378. | ||
Rosenkranz AA, Ulasov AV, Slastnikova TA, Khramtsov YV, Sobolev AS. Use of intracellular transport processes for targeted drug delivery into a specified cellular compartment. Biochemistry (Mosc). 2014;79(9):928–946. | ||
Sobolev AS. Modular transporters for subcellular cell-specific targeting of anti-tumor drugs. Bio Essays. 2008;30(3):278–287. | ||
Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43(1):260–290. | ||
Sosabowski JK, Mather SJ. Conjugation of DOTA-like chelating agents to peptides and radiolabeling with trivalent metallic isotopes. Nat Protoc. 2006;1(2):972–976. | ||
Akabani G, Carlin S, Welsh P, Zalutsky MR. In vitro cytotoxicity of 211At-labeled trastuzumab in human breast cancer cell lines: effect of specific activity and HER2 receptor heterogeneity on survival fraction. Nucl Med Biol. 2006;33(3):333–347. | ||
Ngo Ndjock Mbong G, Lu Y, Chan C, et al. Trastuzumab labeled to high specific activity with 111In by site-specific conjugation to a metal-chelating polymer exhibits amplified auger electron-mediated cytotoxicity on HER2-positive breast cancer cells. Mol Pharm. 2015;12(6):1951–1960. | ||
Michel RB, Rosario AV, Andrews PM, Goldenberg DM, Mattes MJ. Therapy of small subcutaneous B-lymphoma xenografts with antibodies conjugated to radionuclides emitting low-energy electrons. Clin Cancer Res. 2005;11(2 Pt 1):777–786. | ||
Chen P, Cameron R, Wang J, Vallis KA, Reilly RM. Antitumor effects and normal tissue toxicity of 111In-labeled epidermal growth factor administered to athymic mice bearing epidermal growth factor receptor-positive human breast cancer xenografts. J Nucl Med. 2003;44(9):1469–1478. | ||
Rosenkranz AA, Slastnikova TA, Durymanov MO, Sobolev AS. Malignant melanoma and melanocortin 1 receptor. Biochemistry (Mosc). 2013;78(11):1228–1237. | ||
Simoes MC, Sousa JJ, Pais AA. Skin cancer and new treatment perspectives: a review. Cancer Lett. 2015;357(1):8–42. | ||
Silva CO, Martinho N, Aniceto N, Reis CP. Melanoma prevention: challenges and progresses in nanotechnology for melanoma prevention and treatment. In: Kharisov BI, Kharissova OV, Ortiz-Mendez U, editors. CRC Concise Encyclopedia of Nanotechnology. Boca-Raton, London, New York: CRC Press; 2015:453–470. | ||
Slastnikova TA, Rosenkranz AA, Lupanova TN, Gulak PV, Gnuchev NV, Sobolev AS. Study of efficiency of the modular nanotransporter for targeted delivery of photosensitizers to melanoma cell nuclei in vivo. Dokl Biochem Biophys. 2012;446(1):235–237. | ||
You S, Luo J, Grossniklaus HE, Gou ML, Meng K, Zhang Q. Nanomedicine in the application of uveal melanoma. Int J Ophtalmol. 2016;9(8):1215–1225. | ||
Jiang P, Kanzia G, Neumann D, Roider J, Dunst J. 106Ruthenium eye plaque brachytherapy in the management of medium sized uveal melanoma. Int J Radiat Oncol Biol Phys. 2015;93:E77–E78. | ||
Wisely CE, Hadziahmetovic M, Reem RE, et al. Long-term visual acuity outcomes in patients with uveal melanoma treated with 125I episcleral OSU-Nag plaque brachytherapy. Brachytherapy. 2016;15(1):12–22. | ||
ABS-OOTF Committee. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13(1):1–14. |
Supplementary materials
Video S1 SPECT visualization of 111In-labeled MC1R-targeted MNT retention in mice.
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.