Back to Journals » International Journal of Nanomedicine » Volume 15
Preparation, Characterization, and in vivo Evaluation of NK4-Conjugated Hydroxycamptothecin-Loaded Liposomes
Authors Zhou T, Zhang W, Cheng D, Tang X, Feng J, Wu W
Received 25 December 2019
Accepted for publication 9 March 2020
Published 31 March 2020 Volume 2020:15 Pages 2277—2286
DOI https://doi.org/10.2147/IJN.S243746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Ting Zhou,1,* Wei Zhang,1,* Dongliang Cheng,1 Xin Tang,2 Jianfang Feng,3 Wei Wu1
1School of Pharmacy, Guilin Medical University, Guilin 541004, People’s Republic of China; 2School of Public Health, Guilin Medical University, Guilin 541004, People’s Republic of China; 3School of Pharmacy, Guangxi University of Chinese Medicine, Nanning 530200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Wu
School of Pharmacy, Guilin Medical University, 109 North 2nd Huancheng Road, Guilin 541004, People’s Republic of China
Email [email protected]
Jianfang Feng
School of Pharmacy, Guangxi University of Chinese Medicine, 13 Wuhe Avenue, Nanning 530200, People’s Republic of China
Email [email protected]
Purpose: In this study, NK4-conjugated hydroxycamptothecin liposomes (NK4-HCPT-Lips) were prepared with the aim of improving drug targeting to the liver.
Methods: NK4-HCPT-Lips were prepared using the thin-film dispersion method. In vitro antitumor activities were evaluated by MTT assay. HCPT levels in plasma and tissues were determined via high-performance liquid chromatography (HPLC) with camptothecin as the internal standard, and the characteristics, pharmacokinetics, and bio-distribution of NK4-HCPT-Lips were evaluated.
Results: The liposomes showed a regular spherical-shaped morphology, and the entrapment efficiency and drug loading capacity reached 82.5 ± 2.4% and 3.01 ± 0.23%, respectively, with a particle size of 155.6 ± 2.6 nm and a zeta potential of − 24.8 ± 3.3 mV. Inhibition effect experiments found that NK4-HCPT-Lips had a good inhibition on the HepG2 cells. Pharmacokinetic studies revealed an increase in the area under the curve and mean residence time as well as a decrease in plasma clearance (p < 0.05) of the NK4-HCPT-Lips compared to those of HCPT liposomes and a commercial HCPT injection. Tissue distribution studies showed that NK4-HCPT-Lips were present at high levels in the liver but were cleared from the kidneys.
Conclusion: These results demonstrate that NK4-HCPT-Lips possess excellent liver-targeting attributes, which could enhance the therapeutic effects of drug treatments for hepatic diseases.
Keywords: hydroxycamptothecin, NK4, liposomes, pharmacokinetics, bio-distribution
Introduction
Hepatocellular carcinoma (HCC) is a common and highly invasive cancer, with the fifth-highest morbidity worldwide.1 Furthermore, due to its poor prognosis, the mortality rate is very high. The regions of the highest incidence cover Eastern (more than 50% of the cases occurring in China) and Southern Asia and sub-Saharan Africa, involving approximately 841,000 new cases and 782,000 deaths annually.2,3 Therefore, there is an urgent need for safe and effective anticancer drugs or treatment methods for HCC in the clinic. Hydroxycamptothecin (HCPT) is an alkaloid isolated from Camptotheca involucrata, a special plant of Davidia grown in China. HCPT is a cell cycle-specific drug that selectively inhibits topoisomerase Ⅰ and interferes with DNA replication. It has broad-spectrum anticancer effects and has been shown to have a good curative effect on liver, gastric, bladder, rectal, and ovarian cancer, as well as head and neck tumors and leukemia.4,5 HCPT is a natural anticancer drug with an application value similar to that of paclitaxel.6,7 HCPT is an insoluble drug, and sodium salt is typically used as the injection vehicle in clinical applications. However, the α-hydroxylactone ring, an active group of HCPT, opens easily in water, causing it to lose topoisomerase I activity, and thus reducing its antitumor activity. In addition, a short half-life and strong adverse reactions are potential problems associated with HCPT sodium salt injection.8,9 Thus, prolonging the half-life of HCPT and maintaining a closed ring α-hydroxylactone structure is a key to improving its antitumor effects.
One of the most effective ways to enhance the efficacy and reduce the side effects is to deliver drugs directly to the lesion. Targeted drug delivery is mainly achieved through carrier conduction and receptor mediation.10 Liposome microparticle delivery systems have been widely studied because of their excellent biocompatibility and biodegradability. When liposomes enter the body through intravenous administration, they are mainly engulfed and eliminated by reticuloendothelial cells, and passively target the reticuloendothelial system of the liver. When using liposomes as drug carriers, the α-hydroxylactone ring structure of hydroxycamptothecin can be embedded into the bilayer phospholipid membrane of the liposomes, avoiding loss of biological activity due to the opening of the α-hydroxylactone ring.11 In recent years, attempts have been made to modify the liposome surface in order to improve targeting efficiency and stability.12–16
Hepatocyte growth factor (HGF) is a cytokine produced by stromal cells (such as fibroblasts and macrophages) that acts on the c-met receptor on the surface of tumor cells.17,18 HGF/c-Met signaling plays an important role in promoting tumor growth, invasion, metastasis, and tumor angiogenesis.19,20 Therefore, HGF/c-Met is a potential target site for antitumor therapy. NK4 is a specific HGF antagonist that is composed of the α-chain N-terminal and four Kringle regions of HGF.21 NK4 binds to the c-Met receptor but does not activate it. Thus, activation of the c-Met receptor by HGF is competitively inhibited by NK4, blocking the HGF/c-Met signal transduction system, which in turn restrains tumor growth, invasion, metastasis, and tumor angiogenesis, and promote tumor cell apoptosis.22,23 Studies have shown that NK4 inhibits the growth and development of tumor cells mediated by HGF in vitro, and also restrains tumor growth, invasion, and metastasis in nude mice.24,25 Research into anticancer treatments that target the NK4 pathway has become a popular topic.17,26 Therefore, using NK4 as a liver-targeting ligand would not only actively recognize and bind to hepatocytes but also blocks the HGF/c-Met signaling pathway to play a synergistic anticancer role (Figure 1). In recent years, reports have described decoration of liposomes with the HGF single-chain variable fragment (scFv),27 and drug delivery systems mediated by scFv have shown great success as a potential in tumor-targeting therapy.
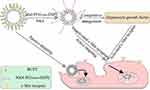 |
Figure 1 Schematic of cellular uptake of NK4-HCPT-Lips. |
In the present study, hydroxycamptothecin, an effective anticancer component of traditional Chinese medicine, was used as a model drug, and NK4-modified hydroxycamptothecin-loaded liposomes (NK4-HCPT-Lips) were prepared. The physicochemical properties of NK4-HCPT-Lips were characterized, and the tissue drug distribution was investigated to evaluate the liver-targeting effects of NK4-HCPT-Lips in vivo.
Materials and Methods
Materials
10-Hydroxycamptothecin (HCPT, purity ≥ 98%), soybean phospholipids (SPC, purity > 98%), cholesterol (Chol, purity > 95%), HEPES buffer, hydroxycamptothecin standard (98%, Lot No.: H1524105) and camptothecin (CPT, purity ≥ 98%, Lot No.: H1810045) as the internal standard (I.S.) were purchased from Aladdin Industrial Corporation (Shanghai, China). Hydroxycamptothecin injection was obtained from Shenzhen Main Luck Pharmaceuticals Inc. (Shenzhen, China). DSPE-PEG2000 - Mal were purchased from Shang Hai Ponsure Biotech Inc. NK4 protein was obtained from Detai Bio-Tech Co., Ltd. (Nanjing, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (Taufkirchen, Germany), Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Sigma-Aldrich (St Louis, MO, USA). Methanol was HPLC grade. All other reagents were analytical grade, and purified water was used throughout.
The HepG2 hepatoma cell line was obtained from the Shanghai Cell Center of the Chinese Academy of Medical Science (Shanghai, PRC). Sprague-Dawley (SD) rats (220–250 g) and Kun-Ming (KM) mice (20–30 g) were obtained from Hunan Silaikejingda Laboratory Animal Co., Ltd., Hunan, China (Certificate No. SCXK 2016–0002). Experiments were conducted in accordance with the guidelines issued by the State Food and Drug Administration (SFDA of China). Animals were housed and cared for in accordance with the guidelines established by the National Science Council of the Republic of China. All animal experiments were approved by the Animal Ethics Committee of Guilin Medical University and carried out in accordance with guidelines. All efforts were made to minimize animal suffering.
Preparation of Liposome-Encapsulating Hydroxycamptothecin
Preparation of Hydroxycamptothecin Liposomes
Liposomes were prepared using the lipid film hydration method.28 Hydroxycamptothecin liposomes (HCPT-Lips) composed of SPC, Chol and HCPT (130, 40, or 7 mg) were dissolved in 10 mL chloroform, and a thin lipid film was formed by removing the chloroform in a rotary evaporator at 50°C and drying under vacuum for 1 h. The lipid film was hydrated with 5 mL 10 mM HEPES buffer (pH 6.8) at 50°C for 2 h. Liposomes were sonicated for 5 min at 300 W in an ice bath using an ultrasonic cell disruptor (SCIENTZ-IID, Scientz, China), then passed through a 200 nm polycarbonate membrane 21 times using a LiposoEast extruder (LE-1, Morgec, Shanghai, China).
Preparation of NK4-Modified Hydroxycamptothecin Liposomes
Mal-PEG2000-DSPE was dissolved in HEPES buffer and added to HCPT-Lips at 1% of the liposome phospholipids weight. The mixture was incubated at 50°C for 2 h with gentle shaking to introduce maleimide functional groups to conjugate antibodies.27 NK4 was then incubated with Mal-PEG2000-DSPE-inserting HCPT-Lips at 4°C overnight to allow conjugation. The final concentration of NK4 was 80 µg/mL. Free HCPT and NK4 were removed by gel filtration using a Sepharose CL4B column (Solaibio, Beijing, China). Liposomes were stored at 4°C.
Liposome Characterization
Transmission Electron Microscopy
HCPT-Lips and NK4-HCPT-Lips morphology were observed using Transmission electron microscopy (TEM, HT7700, HITACHI, Japan). The liposomes were then diluted 10 times and placed on copper grids with 2% phosphotungstic acid staining for 2 min for further analysis.
Z-Average Size, Zeta Potential, and Polydispersity Index
The average size, zeta potential, and polydispersity index (PDI) of HCPT-Lips and NK4-HCPT-Lips were measured using a Zetasizer instrument (Nano-ZS90, Malvern Instruments, Malvern, UK). Prior to measurement, liposomes were diluted 10 times in distilled water.
Encapsulation Efficiency and Drug Loading
Free HCPT was separated from liposomes using a Sepharose CL-4B gel column for measurement of Encapsulation efficiency (EE) and drug loading (DL). Briefly, 0.2 mL of liposomes was loaded onto a Sepharose CL-4B gel column and eluted with HEPES buffer (pH 6.8), followed by separation of liposomes and free drug. The quantity of the entrapped drug was determined by disrupting the liposome fraction with methanol. HCPT concentration was quantified using high-performance liquid chromatography (HPLC; 20A, SHIMADZU, Japan) at 370 nm with a reversed-phase InertSustain-C 18 analytical column (250 mm×4.6 mm, 5 µm). The mobile phase was a mixture of methanol–water at 55:45 (v/v). Flow rate was 1 mL/min. Injection volume was 20 µL. The EE of liposomes was calculated using Equation (1), and the DL was calculated using Equation (2).
(1) (2)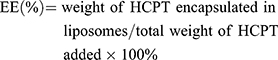

In vitro Drug Release
In vitro drug release from HCPT-Lips and NK4-HCPT-Lips was analyzed via dialysis against PBS (pH=7.4) at 37°C under sink conditions.16 Briefly, 1 mL of HCPT-Lips, NK4-HCPT-Lips, or free HCPT containing 1.4 mg of the drug were placed in a dialysis bag with a molecular weight cutoff of 8–14 kDa, sealed and immersed in 200 mL phosphate-buffered saline (pH=7.4), separately. The medium was shaken at 200 rpm at 37°C. At predetermined time intervals (0.5, 1, 2, 4, 8, 12, 24, 48, and 72 h) 2 mL of medium was withdrawn and replaced with an equal amount of fresh medium. Samples were filtered through a 0.22 μm syringe filter, injected into the HPLC system, and analyzed by the aforementioned HPLC method. All measurements were taken in triplicate.
Stability Study
Liposome stability under in vitro storage conditions is an important criterion for both in vitro and in vivo biomedical applications. The stability of the liposomes was evaluated 7, 15, and 30 days after preparation and storage at 4°C.
In vitro Cytotoxicity Study
The in vitro cytotoxicity of HCPT-Inject, HCPT-Lips, or NK4-HCPT-Lips were tested on HepG2 through MTT assay. Approximately 1×104 cells per well were seeded in 96-well plates containing DMEM media supplemented with 10% FBS and incubated in an incubator containing 5% CO2 at 37°C. After 80% confluency, the medium was replaced with 200 μL fresh DMEM medium alone (as control) or containing different concentrations of HCPT-Inject, HCPT-Lips, NK4-HCPT-Lips, viz., 0.01, 0.1, 1, 10, 20 μg/mL and subsequently cultured for 24 and 48 hrs at 37°C. Twenty μL of 5 mg/mL MTT in PBS was added to each well, and the cells were incubated for another 4 hrs at 37°C in 5% CO2. Blank liposomes were also tested at an equal concentration of the drug-loaded liposomes for 24 and 48 hrs. The medium was replaced with 200 μL DMSO to dissolve MTT formazan crystals. After that, the optical density was read at 490 nm using a microplate reader (iMark, Bio-Rad, USA), and the half-maximal inhibitory concentration (IC50) values were calculated by Graph Pad software (Version 5, Graph Pad Software Inc, USA).
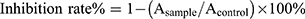
In vivo Analytical Method
To determine the amount of drug accumulation, a 200 μL aliquot of each sample was combined with 20 μL CPT (50 μg/mL) solution (IS) and 10 μL glacial acetic acid, and incubated in the dark for 2 hrs. Then, 1 mL methanol was added, and the mixture was vortexed for 3 min, followed by centrifugation at 13,000 rpm for 10 min. Finally, 20 μL of the supernatant was filtered through a 0.22-μm microfiltration membrane and injected into the HPLC system for analysis. Standard curves were constructed by plotting the ratio of HCPT to internal standard CPT peak areas as a function of known concentration.
In vivo Pharmacokinetic Study
An in vivo pharmacokinetic study was performed as previously reported.29 Briefly, SD rats were randomly divided into three groups, with five rats per group. HCPT-Inject, HCPT-Lips, or NK4-HCPT-Lips were injected into the tail vein (8 mg/kg). Blood samples were collected into heparinized tubes at predetermined intervals of 0.033, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 12 hrs post-dose, then centrifuged at 12,000 rpm for 10 min. Plasma samples stored at −20°C and analyzed within 3 days.
In vivo Bio-Distribution Study
Mice were randomly divided into three groups, and then administered HCPT-Inject, HCPT-Lips, or NK4-HCPT-Lips intravenously at a dose of 8 mg/kg HCPT, respectively. Mice (n = 5 per time point) were sacrificed after defined time periods (0.167, 1, 4, 8, and 12 h), and then the tissues (heart, liver, spleen, lung and kidney) were collected, weighed and homogenized (ratio of tissue-to-water 1:5, g/mL) in normal saline. Samples were immediately frozen at −20°C and analyzed within 3 days.
Statistical Analysis
Results are reported as mean ± standard deviation (SD, n = 3). Data were analyzed using the analysis of variance (ANOVA) and Student’s t test with SPSS software (version 20, SPSS Inc., Chicago, IL)., and p < 0.05 was considered significant. Pharmacokinetic parameters were analyzed using DAS 2.0 via compartmental analysis.
Results and Discussion
Characterization
The morphological characteristics of liposomes were directly investigated using TEM. As shown in Figure 2A and B, the particles were spherical in shape, ranging in size from 100 to 200 nm, with no aggregation or fusion. The average size, zeta potential, PDI, EE, and LD of the liposomes are shown in Table 1. The average size of HCPT-Lips and NK4-HCPT-Lips was 152.9 ± 3.8 nm and 155.6 ± 2.6 nm (Figure 2C), respectively. The sizes were eligible for the enhanced permeability and retention effect (EPR), which indicates an expectation to undergo passive and active targeting to tumor tissues.30 The PDI was below 0.2, indicating that the particle size distribution was relatively narrow. The zeta potentials, a key factor in evaluating the stability of liposome dispersion, were detected to be −25.7 ± 3.5 mV for HCPT-Lips and −24.7 ± 3.3 mV for NK4-HCPT-Lips (Figure 2D). Liposomes with an absolute zeta potential value greater than 20 mV have relatively high repulsive interaction and are considered stable.31 For the prepared liposomes, the EE of HCPT-Lips was 83.6 ± 3.3%, and DL was 3.19 ± 0.28%, whereas the EE of NK4-HCPT-Lips was 82.5 ± 2.4%, and DL was 3.01 ± 0.23%. From these results, it was concluded that the size and zeta potential of HCPT-Lips and NK4-HCPT-Lips prepared using the thin-film dispersion method are satisfactory. These results also show that the conjugation of NK4 has little influence on morphology, size distribution, or encapsulation efficiency.
 |
Table 1 Characterization of the Liposomes (n=3) |
 |
Figure 2 Characterization of liposomes. Notes: (A) TEM image of HCPT-Lips. (B) TEM image of NK4-HCPT-Lips. (C) Size distribution of NK4-HCPT-Lips. (D) Zeta potential of NK4-HCPT-Lips. |
In vitro Release
The in vitro release profiles of free-HCPT, HCPT-Lips, and NK4-HCPT-Lips are shown in Figure 3. The results were very similar, with cumulative HCPT release rates of 62.4 ± 4.2% for HCPT-Lips and 60.9 ± 4.5% for NK4-HCPT-Lips within 72 h. The cumulative release rate of liposomes within 12 h was less than 40%, whereas, the cumulative release percentage of free-HCPT was 91.8 ± 3.2%. This indicates that drug release is delayed after being loaded with liposomes, and that NK4-modified liposomes have little effect on release in vitro.
In vitro Stability
The liposomes were easily dispersed after being placed at 4°C for 1 month, with no obvious changes in appearance. The Z-average size and EE of the liposomes changed very little during storage (Table 2). There was no aggregation phenomenon between particles during the storage period, suggesting that the liposome solution is stable, mainly due to the fact that the surface potential is high (>20 mV), which is beneficial to the stability of the liposome solution.31
 |
Table 2 Stability Data of Liposomes (n=3) |
In vitro Cytotoxicity Studies
Over 95% of the cells survived during the incubation period, indicating that the blank liposomes did not exhibit cytotoxicity against HepG2 cells. As shown in Figure 4, HCPT-Inject and liposomes had a dose-dependent inhibitory effect on HepG2 cells proliferation. HCPT-inject showed stronger growth inhibition on HepG2 cells than HCPT-Lips and NK4-HCPT-Lips at 24 h, but NK4-HCPT-Lips exhibited the most significant cytotoxicity against HepG2 cancer cells at 48 h (Table 3). It is possible that HCPT were free molecules in HCPT-Inject, which could enter into tumor cells quickly and exerted antitumor activity, suppressed DNA replication and consequently induced apoptosis in tumor cell lines, while HCPT liposomes were internalized into cells via endocytosis in a time-dependent manner and the drug effect was relatively slow.32
 |
Table 3 The IC50 of HCPT Formulations (n=3) |
In vivo Pharmacokinetics
The plasma concentration of HCPT liposomes and commercial HCPT injection (HCPT-Inject) in SD rats over time is shown in Figure 5. At 2 min, the plasma concentration of HCPT-Inject and liposomes was at the maximum level, and then gradually decreased. The curve of the HCPT-Inject was steep, and the elimination rate of HCPT was rapid, such that it could not be detected in the blood 4 h later. In contrast, the curves for HCPT-lips and NK4-HCPT-Lips were relatively flat, and HCPT could still be detected by HPLC 12 h later. This indicates that HCPT-lips and NK4-HCPT-Lips significantly prolong drug retention time in rats, and thus have a sustained-release effect in vivo.
Pharmacokinetic results are shown in Table 4. The plasma concentration–time curve of the HCPT-Inject was consistent with the two-compartment model, whereas HCPT-Lips and NK4-HCPT-Lips fit best with the three-compartment model. The area under concentration–time curve (AUC) for NK4-HCPT-Lips was 17.279 µg·h/mL, 1.5 times higher than that for HCPT-Lips (11.561 µg·h/mL) and 11.4 times higher than that for HCPT-Inject (1.51 µg·h/mL). The half-life time (t1/2) of HCPT-Lips and NK4-HCPT-Lips was 1.086 h and 1.268 h, respectively, and was 0.349 h for HCPT-Inject, which is significantly lesser than that of the liposome preparations. The mean residence time (MRT) of NK4-HCPT-Lips was 3.103 h, and was 0.289 h and 2.007 h for HCPT-Injection and HCPT-Lips, respectively. In addition, plasma clearance (CL) of NK4-HCPT-Lips (0.373 L/kg/h) was significantly lower than that of HCPT-Inject (5.297 L/kg/h) and HCPT-Lips (0.665 L/kg/h). Thus, liposomes modified by NK4 not only achieve higher drug blood levels and longer circulation time in rats but also effectively reduce the in vivo clearance rate. It is possible that NK4 ligands are embedded into the surface membrane of liposomes, which would prevent drug leakage from the liposomes, thus improving the antitumor effects of drugs in vivo.
 |
Table 4 Pharmacokinetic Parameters |
In vivo Bio-Distribution
Drug concentrations in the heart, liver, spleen, lung, and kidney at different time points are shown in Figure 6. HCPT-Inject was mainly distributed in the kidney and liver. The drug content in other organs was relatively low and could not be detected 1 h later, indicating that it had been completely eliminated. Compared with the commercial HCPT-Inject, the concentration of HCPT loaded into liposomes was significantly increased in the liver and spleen, which may be due to macrophage uptake via the reticuloendothelial system in these organs. At all time points, the concentration of NK4-HCPT-Lips in the liver was always higher than that of HCPT-Inject and HCPT-Lips. Furthermore, although there was a downward trend over time, it remained at relatively high levels, indicating that the drug reached the liver quickly and remained there for some time (Figure 6B). At the same time, HCPT-Lips and NK4-HCPT-Lips also accumulated in the kidney and spleen, but then were quickly eliminated (Figure 6C and E). These results show that the disposition of liposomes in vivo is quite different from the free drug. In particular, the NK4-modified liposomes achieved the highest concentration and the longest retention time in the liver tissue of mice, indicating that NK4-HCPT-Lips manifest stronger liver targeting than HCPT-Inject and HCPT-Lips.
Conclusion
In this study, the average particle size of NK4-HCPT-Lips prepared using the thin-film dispersion method was less than 160 nm, PDI was less than 0.2, and liposomes were spherical vesicles of uniform size. The zeta potential of the liposome solution was less than −20 mV, indicating high stability. The drug encapsulation rate was above 80%, which meets the requirements of liposome preparation, and they showed sustained release in an in vitro release experiment. The NK4-HCPT-Lips exhibited excellent inhibition effect against the HepG2 after 48 h, when compared to the commercially available hydroxycamptothecin and conventional HCPT-Lips the anti-cancer effect being at a dose and time-dependent manner. Pharmacokinetic studies demonstrated that NK4-HCPT-Lips have higher AUC and longer in vivo circulation than HCPT-Lips in rats. The tissue distribution study suggests that the drug-loaded liposomes accumulate in the liver, and that NK4 ligand further enhances liver targeting of the liposomes. In conclusion, NK4-HCPT-Lips represents an excellent application prospect due to its accurate liver targeting. However, its therapeutic effect on pharmacodynamics study will require further study.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81560653, 81760718, 81573623) and Natural Science Foundation of Guangxi (No. 2016GXNSFAA380081).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Song P, Cai Y, Tang H, Li C, Huang J. The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines from 2001 to 2017. Biosci Trends. 2017;11(4):389–398. doi:10.5582/bst.2017.01202
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
3. Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
4. Wang TE, Ding Y, Yang YF, et al. Synergistic antitumor effects of triptolide plus 10-hydroxycamptothecin on bladder cancer. Biomed Pharmacother. 2019;115:108899. doi:10.1016/j.biopha.2019.108899
5. Xing H, Luo X, Li Y, et al. Effect of verapamil on the pharmacokinetics of hydroxycamptothecin and its potential mechanism. Pharm Biol. 2020;58(1):152–156. doi:10.1080/13880209.2020.1717550
6. Chen Y, Chen C, Xiao Y, Zhang X, Chen Y. Liposomes encapsulating 10-hydroxycamptothecin-cyclodextrin complexes and their in vitro anti-tumor activities. J Nanosci Nanotechnol. 2015;15(5):3786–3795. doi:10.1166/jnn.2015.9495
7. Zhang Y, Wu X, Mi Y, Li H, Hou W. Engineering of (10-hydroxycamptothecin intercalated layered double hydroxide)@liposome nanocomposites with excellent water dispersity. J Phys Chem Solids. 2017;108:125–132. doi:10.1016/j.jpcs.2017.04.018
8. Zhu HM, Gu JH, Xie Y, et al. Hydroxycamptothecin liposomes based on thermal and magnetic dual-responsive system: preparation, in vitro and in vivo antitumor activity, microdialysis-based tumor pharmacokinetics. J Drug Target. 2017;26(1):1–12. doi:10.1080/1061186X.2017.1339196
9. Yang J, Ni B, Liu J, Zhu L, Zhou W. Application of liposome-encapsulated hydroxycamptothecin in the prevention of epidural scar formation in New Zealand white rabbits. Spine J. 2011;11(3):218–223. doi:10.1016/j.spinee.2011.01.028
10. Brannon Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2012;64:206–212. doi:10.1016/j.addr.2012.09.033
11. Fang YP, Chuang CH, Wu YJ, et al. SN38-loaded <100 nm targeted liposomes for improving poor solubility and minimizing burst release and toxicity: in vitro and in vivo study. Int J Nanomedicine. 2018;13:2789–2802. doi:10.2147/IJN.S158426
12. Shi LL, Tang C, Yin CH. Glycyrrhizin-modified o-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials. 2012;33(30):7594–7604. doi:10.1016/j.biomaterials.2012.06.072
13. Chen JD, Jiang H, Wu Y, et al. A novel glycyrrhetinic acid-modified oxaliplatin liposome for liver-targeting and in vitro/vivo evaluation. Drug Des Devel Ther. 2015;9:2265–2275. doi:10.2147/DDDT.S81722
14. Xia Y, Zhong JY, Zhao MQ, et al. Galactose-modified selenium nanoparticles for targeted delivery of doxorubicin to hepatocellular carcinoma. Drug Deliv. 2019;26(1):1–11. doi:10.1080/10717544.2018.1556359
15. Shi C, Gao F, Gao X, Liu Y. A novel anti-VEGF165 monoclonal antibody-conjugated liposomal nanocarrier system: physical characterization and cellular uptake evaluation in vitro and in vivo. Biomed Pharmacother. 2015;69:191–200. doi:10.1016/j.biopha.2014.11.025
16. Liu D, Xing J, Xiong F, Yang F, Gu N. Preparation and in vivo safety evaluations of antileukemic homoharringtonine-loaded PEGylated liposomes. Drug Dev Ind Pharm. 2017;43(4):652–660. doi:10.1080/03639045.2016.1275670
17. Ghanaatgar-Kasbi S, Khorrami S, Avan A, et al. Targeting the c-MET/HGF signaling pathway in pancreatic ductal adenocarcinoma. Curr Pharm Des. 2019;24(39):4619–4625. doi:10.2174/1381612825666190110145855
18. Ariyawutyakorn W, Saichaemchan S, Varella-Garcia M. Understanding and targeting MET signaling in solid tumors - are we there yet? J Cancer. 2016;7(6):633–649. doi:10.7150/jca.12663
19. Guo JR, Li W, Wu Y, et al. Hepatocyte growth factor promotes proliferation, invasion, and metastasis of myeloid leukemia cells through PI3K-AKT and MAPK/ERK signaling pathway. Am J Transl Res. 2016;8:3630–3644.
20. Hao NB, Tang B, Wang GZ, et al. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-κB signaling pathway for gastric cancer metastasis. Cancer Lett. 2015;361(1):57–66. doi:10.1016/j.canlet.2015.02.043
21. Xie R, Li QP, Ge XX, et al. NK4 suppresses cholangiocarcinoma angiogenesis and invasion through targeting HIF-1α pathway. Int J Clin Exp Med. 2017;10:2345–2352.
22. Cai C, Hou LL, Zhang JS, et al. The inhibitory effect of mesenchymal stem cells with rAd-NK4 on liver cancer. Appl Biochem Biotechnol. 2017;183(1):444–459. doi:10.1007/s12010-017-2456-x
23. Deng XB, Xiao L, Wu Y, et al. Inhibition of mesothelioma cancer stem-like cells with adenovirus-mediated NK4 gene therapy. Int J Cancer. 2015;137(2):481–490. doi:10.1002/ijc.v137.2
24. Zhu Y, Cheng M, Yang Z, et al. Mesenchymal stem cell-based NK4 gene therapy in nude mice bearing gastric cancer xenografts. Drug Des Devel Ther. 2014;8:2449–2462. doi:10.2147/DDDT.S71466
25. Gao Z, Zheng X, Shen R, et al. NK4 growth inhibition of human Raji lymphoma xenografts by competitive interrupting HGF/Met signal pathway. Chin J Pathol. 2014;43:551–555.
26. Wang DD, Saga Y, Sato N, et al. The hepatocyte growth factor antagonist NK4 inhibits indoleamine-2, 3-dioxygenase expression via the c-Met-phosphatidylinositol 3-kinase-AKT signaling pathway. Int J Oncol. 2016;48(6):2303–2309. doi:10.3892/ijo.2016.3486
27. Lu RM, Chang YL, Chen MS, et al. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials. 2011;32(12):3265–3274. doi:10.1016/j.biomaterials.2010.12.061
28. Ye TT, Wu Y, Shang L, Deng X, Wang S. Improved lymphatic targeting: effect and mechanism of synthetic borneol on lymph node uptake of 7-ethyl-10-hydroxycamptothecin nanoliposomes following subcutaneous administration. Drug Deliv. 2018;25(1):1461–1471. doi:10.1080/10717544.2018.1482973
29. Zhou T, Tang X, Zhang W, Feng J, Wu W. Preparation and in vitro and in vivo evaluations of 10-hydroxycamptothecin liposomes modified with stearyl glycyrrhetinate. Drug Deliv. 2019;26(1):673–679. doi:10.1080/10717544.2019.1636422
30. Chen J, Chen YC, Cheng Y, Gao Y. Glycyrrhetinic acid liposomes containing mannose-diester lauric diacid-cholesterol conjugate synthesized by lipase-catalytic acylation for liver-specific delivery. Molecules. 2017;22(10):1598–1617. doi:10.3390/molecules22101598
31. Nayak D, Boxi A, Ashe S, Thathapudi NC, Nayak B. Stavudine loaded gelatin liposomes for HIV therapy: preparation, characterization and in vitro cytotoxic evaluation. Mater Sci Eng C. 2017;73:406–416. doi:10.1016/j.msec.2016.12.073
32. Li Y, Liu R, Yang J, et al. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials. 2015;41:1–14. doi:10.1016/j.biomaterials.2014.11.010
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




