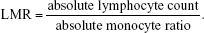Back to Journals » OncoTargets and Therapy » Volume 10
Preoperative lymphocyte-to-monocyte ratio predicts survival in primary hepatitis B-virus positive hepatocellular carcinoma after curative resection
Authors Li G, Ji J, Yang F, Xu H, Bai Y
Received 12 April 2016
Accepted for publication 23 August 2016
Published 23 February 2017 Volume 2017:10 Pages 1181—1189
DOI https://doi.org/10.2147/OTT.S110411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Min Li
Guang-Jun Li,1 Juan-Juan Ji,2 Fang Yang,2 Hong-Wei Xu,1 Yu Bai3
1First Department of General Surgery, 2Department of Gastroenterology, 3Department of Pathology, The First Affiliated Hospital of Xin-Xiang Medical University, Henan, People’s Republic of China
Background: Both inflammation and immunity are associated with the development of malignancy. The lymphocyte-to-monocyte ratio (LMR) has been confirmed as a prognostic factor for several malignant diseases. The purpose of our study was to analyze prognostic significance of preoperative LMR in hepatitis B virus (HBV)-related hepatocellular carcinoma after curative resection.
Patients and methods: A total of 253 patients with primary HBV-positive hepatocellular carcinoma who underwent a curative operation were enrolled in this retrospective study. The relationship between preoperative LMR and survival outcomes was analyzed through Kaplan–Meier curves and multivariate Cox regression analyses.
Results: Patients with a high LMR had a significantly higher mean overall survival than those with a low LMR (67 months vs 55 months, P=0.023), and high LMR remained significant for longer survival in the multivariate analysis (hazard ratio, 0.147; 95% confidence interval [CI]: 0.085–0.253; P=0.021). Furthermore, patients with a high LMR also had a higher median recurrence-free survival than those with a low LMR in univariate analyses (60 months vs 48 months, P=0.026) and multivariate analyses (hazard ratio, 0.317; 95% CI: 0.042–1.023; P=0.032). However, the survival benefit was limited to patients with advanced cancer.
Conclusion: LMR was confirmed as an independent prognostic biomarker for primary HBV-positive hepatocellular carcinoma after curative resection.
Keywords: lymphocyte-to-monocyte ratio, survival, HBV, hepatocellular carcinoma
Introduction
In the People’s Republic of China, recent health statistics data have revealed the increasing trend of morbidity and mortality of people with liver cancer in a setting of an estimated 100 million carriers of hepatitis B virus (HBV), accounting for the most common cancer and the second leading cause of cancer-related death.1 Although the development of comprehensive treatment and advances in surgery have benefited liver cancer patients, the prognosis remains poor mainly due to high recurrence and metastasis rates after surgical resection, especially for HBV-positive hepatocellular carcinoma.2,3 Classification of patients facilitates predicting survival, thus helping to provide individualized therapies and improve clinical survival outcomes; therefore, the importance of prognostic and predictive biomarkers has been increasingly recognized.4 However, biomarkers definitely associated with prognosis are currently limited to clinicopathological characteristics which can only be assessed through postoperative histological studies and findings during surgery, such as histological stage, the pathological type, or TNM stage.5–9 So novel pretreatment biomarkers characterized by affordability and technical feasibility are required.
Inflammation plays an important role in the development of various malignant tumors.10 Inflammatory cells can secrete various proangiogenic factors and inflammatory mediators, thus prompting a favorable cancer-related inflammatory microenvironment, which can further promote vascular invasion and suppress the host immune system.11–13 Otherwise, various cytokines secreted by tumors can influence the blood cells associated with host inflammatory and immunity, such as the neutrophilic, lymphocytic, and monocyte cells and blood platelets.14,15
It has been confirmed that several inflammatory response-related biomarkers can potentially predict prognosis of various types of malignancies, such as the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio (LMR).16–20 Otherwise, we have analyzed the prognostic significance of LMR in 144 patients with pancreatic adenocarcinoma, and found higher preoperative LMR was significantly associated with better survival.21 However, there was still no report on the association of preoperative LMR with survival of resected HBV-positive hepatocellular carcinoma. In this study, we try to evaluate the prognostic significance of preoperative LMR in HBV-positive hepatocellular carcinoma patients who underwent curative surgery.
Patients and methods
Patients
Two hundred and fifty-three patients with primary hepatocellular carcinoma who underwent curative resection at the First Department of General Surgery in The First Affiliated Hospital of Xin-Xiang Medical University were enrolled in this study between July 1, 2008 and July 1, 2014. Inclusion criteria included histologically confirmed hepatocellular carcinoma and positive HBsAg, age more than 18 years, and life expectancy more than 6 months. The exclusion criteria included acute and severe preoperative lymphatic system or malignant hematologic conditions or adjuvant treatments. The standard of curative resection was consistent with the National Comprehensive Cancer Network guidelines for hepatobiliary cancer. Clinicopathologic characteristics of all patients were collected from the medical records by one surgeon and checked by another surgeon, including sex, age, tumor size, liver cirrhosis, Child-Pugh stage, HBsAg, AFP level, Barcelona Clinic Liver Cancer (BCLC) stage, TNM stage, tumor site an size, pathological differentiation, and operation type. Histopathological and TNM staging were assessed through postoperative histopathological examination and clinical assessment, respectively. Absolute lymphocyte count and monocyte count were obtained from a routine blood test on the day before surgery. LMR was calculated using the following equation:
|
Follow-up
All patients were followed-up through regular outpatient visits; physical examination and laboratory examinations including AFP every 3 months for the first 2 years, every 6 months for the next 3 years, and once annually thereafter. Enhanced abdominal computed tomography (CT) or magnetic resonance imaging (MRI) scans were obtained generally every year. Clinical follow-up lasted from surgery to either death or the endpoint of study (ie, June 2015). This study had been approved by the Ethics Committee of The First Affiliated Hospital of Xin-Xiang Medical University. Written informed consent was obtained from all patients.
Statistical analysis
Statistical analyses were performed by SPSS 20.0 (IBM Corporation, Armonk, NY, USA). P<0.05 (two sided) was considered as statistically significant. The optimal cutoff values for the LMR were confirmed through receiver operating characteristic (ROC) curve analysis. Overall survival (OS) was accurately defined as the duration from surgery to death, whereas recurrence-free survival (RFS) was calculated as the duration from surgery to tumor recurrence. Qualitative variables were analyzed through the χ2 test or Fisher’s exact test, while quantitative values were evaluated by independent Student’s t-test. The OS and RFS curves were studied in Kaplan–Meier analyses through the log-rank test. The Cox regression model was used to evaluate the hazard ratio (HR) and multivariate analysis.
Results
A total of 253 patients with primary localized hepatocellular carcinoma in our study consisted of 165 males and 88 females, with an average age of 60±1.8 years. According to the TNM staging, there were 122 patients with stage I or II tumors, 131 patients with stage III or IV tumors. In view of pathology, 128 patients had well/moderately differentiated adenocarcinoma, while 125 patients with poor differentiation. Moreover, there was no statistically significant association between LMR and clinicopathologic characteristics, except TNM stage. Baseline clinicopathologic characteristics are shown in Table 1.
  | Table 1 Correlation between the percentage of LMR and clinicopathologic characteristics |
Prognostic significance of LMR for hepatocellular carcinoma
In the ROC curve analysis, we confirmed that the optimal cutoff levels for the LMR was 3.0 for OS and 3.2 for RFS. The area under the curve was 0.566 for OS and 0.652 for RFS (Figure 1). The median follow-up duration was 33 months (range: 6–85 months). There were significant associations between BCLC stage, TNM stage, pathological differentiation, and LMR with OS and RFS (Table 2).
At the end of this study, 79 (31.2%) out of the 253 patients studied had died, including 18 (25.0%) out of 72 patients with an LMR ≥3.0 and 61 (33.7%) out of 181 patients with an LMR <3.0. Tumor recurrence occurred in 85 (48.6%) out of 175 patients with an LMR <3.2, and 27 (34.7%) out of 78 patients with an LMR ≥3.2. Kaplan–Meier survival analysis showed that LMR ≥3.0 was related to a shorter OS. The mean OS of patients with an LMR ≥3.0 in peripheral blood was 67 months, which is statistically significantly higher than those with an LMR <3.0 (55 months) (67 months vs 55 months, P=0.023), (Table 2; Figure 2). Otherwise, this result remained statistically significant in the multivariate analysis for OS (HR, 0.147; 95% confidence interval [CI]: 0.085–0.253; P=0.021) (Table 2).
In Kaplan–Meier univariate analysis of RFS, patients with high LMR (≥3.2) had higher mean RFS than patients with low LMR (<3.2) (60 months vs 48 months, P=0.026). In Cox multivariate analysis, high LMR was also confirmed as a significant predictor for RFS (HR, 0.317; 95% CI: 0.042–1.023; P=0.032) (Table 3).
For advanced hepatocellular carcinoma (TNM stage III), patients with high LMR (≥3.0) had significantly longer overall mean survival than those with an LMR <3.0 (60 months vs 45 months, P=0.029). Furthermore, this result remained significant for RFS (56 months vs 38 months, P=0.029). However, there was no statistically significant association between LMR and OS and RFS of patients with stage I and stage II cancer (for OS, 75 months vs 71 months, P=0.579; for RFS, 63 months vs 62 months, P=0.816) (see Figure 3).
In subgroup analysis of age, we found that the high LMR provided an OS (70 months vs 56 months, P=0.036) and RFS (64 months vs 50 months, P=0.041) benefit for patients aged <60 years. In patients ≥60 years, there was no significant difference between high LMR and low LMR, for OS (52 months vs 49 months, P=0.280) and RFS (45 months vs 41 months, P=0.279) (see Figure 4).
A multivariate analysis entering variables such as gender, tumor size, histological differentiation type, Child-Pugh stage, LMR, TNM stage and BCLC stage, into the Cox regression model to determine independent prognostic predictors for operable hepatocellular carcinoma was performed. The result showed that LMR (HR, 0.147; 95% CI: 0.085–0.253; P<0.05), TNM (HR, 5.432; 95% CI: 3.168–9.283; P<0.05), pathological differentiation (HR, 0.626; 95% CI: 0.412–0.952; P<0.05), and BCLC stage (HR, 6.532; 95% CI: 3.874–11.562; P<0.05) were the independent prognostic factors for OS (Table 2). Otherwise, LMR (HR, 0.317; 95% CI: 0.042–1.023; P<0.05), TNM (HR, 6.513; 95% CI: 3.167–8.522; P<0.05), and BCLC stage (HR, 3.622; 95% CI: 1.374–6.542; P<0.05) were the independent prognostic factors for RFS (Table 3).
Discussion
Both experimental and clinical data indicate that inflammation plays an important role in progression and metastasis of malignancies, which are also associated with host immunity.22 Lymphocytes were considered as the main components of the antitumor immune system, which were also the cellular basis of immunoediting and immunosurveillance, which can reflect antitumor immune response.23 Patients with advanced malignancy present with significant lymphocytopenia, suggesting local and systemic immunosuppression, which has been confirmed as an independent prognostic factor for OS in malignancy.24–26
Tumor-infiltrating leukocytes also have an important role in the development and progression of tumor, such as neutrophils and monocytes.27 The monocytes in peripheral blood includes the dendritic cells and macrophages.28 Dendritic cells have an immune regulatory function, which can cause immunosuppression of patients with malignant tumors through activating Treg cells, while macrophages can kill tumor cells.29,30 Otherwise, the role of macrophages/monocytes in the development and progression of cancer is controversial, presenting as inhibiting as well as enhancing potential of monocytes in human cancer.31 However, there is increasing evidence that the tumor-associated macrophages can prompt tumor progression.32,33 Furthermore, several published studies showed that increased preoperative peripheral blood monocyte count was related to poor survival in patients with cancer.34,35
LMR, a biomarker related to both inflammation and immunity, can reflect the unbalanced status of inflammation and immunity, and has also been confirmed as a significant prognostic factor for several malignancies.36–38 In the present study, we respectively analyzed 253 patients who underwent a curative operation for primary HBV-positive hepatocellular carcinoma, and found LMR was not only an independent prognostic factor of primary hepatocellular carcinoma, but also significantly related to TNM stage. However, the association was limited to patients with advanced hepatocellular carcinoma in subgroup analyses. It has been confirmed that advanced cancer patients who were characterized by severe inflammatory status can present as having a lower LMR.39 Otherwise, LMR was highly correlated with TNM stage, thus the prognosis.
Otherwise, we found that high LMR did not represent better prognosis for patients aged less than 60 years, but for patients aged ≥60 years. It has been confirmed that the immune system deteriorates in the elderly, a phenomenon known as immunosenescence. In the setting of aging, the quality and quantity of lymphocytes decline.40 Furthermore, the LMR of the elderly may be influenced, therefore not inflecting actual inflammatory status. Therefore, this study confirmed that preoperative LMR can be applied to enable pretreatment risk stratification for individual patients, and to predict post-curative resection survival for HBV-positive hepatocellular carcinoma patients. Otherwise, LMR, as a preoperative prognostic predictive marker, can be used to plan individual treatment through patient classification. In other words, patients with a high LMR need be more aggressively treated and more frequently followed-up.
Limitations
There were several limitations in this study. The outcome of the retrospective design of the study cannot fully confirm the significance of LMR. It will take a larger scale multicenter prospective study to confirm the role of LMR. Furthermore, we estimated the cutoff LMR values for OS and RFS through ROC curves, which may not be applied to other studies. We are doing a meta-analysis including various LMR validation studies to confirm the optimal cutoff value for LMR.
Conclusion
In conclusion, LMR, characterized as easy to perform, with general availability and affordability, could be considered as an independent prognostic predictive biomarker for operable hepatocellular carcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27(1):1. | ||
Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54(3):757–759. | ||
Tralhao JG, Dagher I, Lino T, Roudie J, Franco D. Treatment of tumour recurrence after resection of hepatocellular carcinoma. Analysis of 97 consecutive patients. Eur J Surg Oncol. 2007;33(6):746–751. | ||
McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. | ||
Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin-bilirubin (ALBI) grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(5):1031–1036. | ||
Liu PH, Hsu CY, Hsia CY, et al. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 2016;64(3):601–608. | ||
Schwarz L, Katz MH. Diagnosis and Management of Borderline Resectable Pancreatic Adenocarcinoma. Hematol Oncol Clin North Am. 2015;29(4):727–740. | ||
Sharma G, Whang EE, Ruan DT, Ito H. Efficacy of Neoadjuvant Versus Adjuvant Therapy for Resectable Pancreatic Adenocarcinoma: A Decision Analysis. Ann Surg Oncol. 2015;22 Suppl 3:S1229–S1237. | ||
Martin RC 2nd, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262(3):486–494. | ||
Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–233. | ||
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. | ||
Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33 Suppl 1:S79–S84. | ||
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. | ||
Lippi G, Meschi T, Nouvenne A, Mattiuzzi C, Borghi L. Neutrophil gelatinase-associated lipocalin in cancer. Adv Clin Chem. 2014;64:179–219. | ||
Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb). 2011;21(3):264–275. | ||
Harimoto N, Shirabe K, Nakagawara H, et al. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation. 2013;96(11):1008–1012. | ||
Liao W, Zhang J, Zhu Q, et al. Preoperative Neutrophil-to-Lymphocyte Ratio as a New Prognostic Marker in Hepatocellular Carcinoma after Curative Resection. Transl Oncol. 2014;7(2):248–255. | ||
Yang Z, Zhang J, Lu Y, et al. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget. 2015;6(40):43090–43098. | ||
Lin ZX, Ruan DY, Li Y, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015;21(38):10898–10906. | ||
Fan ZC, Yan J, Liu GD, et al. Real-time monitoring of rare circulating hepatocellular carcinoma cells in an orthotopic model by in vivo flow cytometry assesses resection on metastasis. Cancer Res. 2012;72(10):2683–2691. | ||
Li GJ, Xu HW, Ji JJ, Yang F, Gao BQ. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther. 2016;9:1085–1092. | ||
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. | ||
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. | ||
Romano F, Uggeri F, Crippa S, et al. Immunodeficiency in different histotypes of radically operable gastrointestinal cancers. J Exp Clin Cancer Res. 2004;23(2):195–200. | ||
von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7(3 Suppl):925s–932s. | ||
Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32(1):22–28. | ||
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. | ||
Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267(2):204–215. | ||
Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10(12):1969–1980. | ||
Fidler IJ, Schroit AJ. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988;948(2):151–173. | ||
Mytar B, Baj-Krzyworzeka M, Majka M, Stankiewicz D, Zembala M. Human monocytes both enhance and inhibit the growth of human pancreatic cancer in SCID mice. Anticancer Res. 2008;28(1A):187–192. | ||
Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. | ||
Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. | ||
Sasaki A, Kai S, Endo Y, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11(5):596–602. | ||
Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139(6):755–764. | ||
Zhang GM, Zhu Y, Luo L, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol. 2015;36(11):8537–8543. | ||
Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. | ||
Zhou X, Du Y, Xu J, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcomes in patients with stage II/III gastric cancer. Tumour Biol. 2014;35(11):11659–11666. | ||
Stark T, Livas L, Kyprianou N. Inflammation in prostate cancer progression and therapeutic targeting. Transl Androl Urol. 2015;4(4):455–463. | ||
Tomihara K, Curiel TJ, Zhang B. Optimization of immunotherapy in elderly cancer patients. Crit Rev Oncog. 2013;18(6):573–583. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.