Back to Journals » Journal of Inflammation Research » Volume 16
Preoperative Fibrinogen Albumin Ratio is an Effective Biomarker for Prognostic Evaluation of Gallbladder Carcinoma After Radical Resection: A 10-Year Retrospective Study at a Single Center
Authors Li Q, Zhang J, Gao Q, Fu J, Li M, Liu H, Chen C, Zhang D, Geng Z
Received 11 December 2022
Accepted for publication 11 February 2023
Published 18 February 2023 Volume 2023:16 Pages 677—689
DOI https://doi.org/10.2147/JIR.S399586
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Qi Li,1 Jian Zhang,1 Qi Gao,1 Jialu Fu,1,2 Mengke Li,1 Hengchao Liu,1 Chen Chen,1 Dong Zhang,1 Zhimin Geng1
1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2Department of Pediatric Surgery, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China
Correspondence: Zhimin Geng, Department of Hepatobiliary Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China, Email [email protected]
Background: To explore and screen preoperative serum immune response level-related biomarkers with better prognostic ability and developed a prognostic model for decision-making in clinical practice for gallbladder carcinoma (GBC) patients.
Methods: A total of 427 patients who underwent radical resection for GBC in the Department of Hepatobiliary Surgery of the First Affiliated Hospital of Xi’an Jiaotong University from January 2011 to December 2020 were retrospectively analyzed. Time-dependent receiver operating characteristic (time-ROC) was performed to determine the prognostic predictive power of preoperative biomarkers. A nomogram survival model was established and validated.
Results: Time-ROC indicated that the preoperative fibrinogen-to-albumin ratio (FAR) had a better predictive ability for overall survival among preoperative serum immune response level-related biomarkers. Multivariate analysis indicated that FAR was an independent risk factor (P< 0.05). The proportion of clinicopathological characteristics of poor prognosis (such as advanced T stage, and N1-2 stage) was significantly higher in high FAR group (P< 0.05). Subgroup analyses indicate the prognostic discrimination ability of FAR depended on CA19-9, CA125, liver involvement, major vascular invasion, perineural invasion, T stage, N stage, and TNM stage (all P < 0.05). A nomogram model was established based on the prognostic independent risk factors with the C-index of 0.803 (95% CI:0.771~0.835) and 0.774 (95% CI:0.696~0.852) in the training and testing sets, respectively. The decision curve analysis indicated the nomogram model had a better predictive ability than the FAR and TNM staging system in the training and testing sets.
Conclusion: Preoperative serum FAR has a better predictive ability for overall survival among preoperative serum immune response level-related biomarkers, and it can be used for survival assessment of GBC and guide clinical decision-making.
Keywords: gallbladder carcinoma, fibrinogen to albumin ratio, inflammation, prognosis, nomogram
Introduction
Gallbladder carcinoma (GBC) is the most common malignant tumor of the biliary system and ranks sixth among all digestive tract malignancies, which is characterized by a low early diagnosis rate, a high degree of malignancy, and a poor prognosis.1,2 The incidence rate of GBC has been increasing worldwide in recent years, and it is (1.00~1.30)/100,000 in China.3 The incidence, diagnosis, and treatment of GBC have not made major strides in the past 10 years, which has resulted in a stagnant 5-year survival rate for patients. A preoperative assessment of the prognosis of GBC patients can help to select appropriate treatment options for the patient.4 As a result, developing novel biomarkers is critical for the prognostic assessment of GBC.
Numerous studies have demonstrated that nutritional status and systemic inflammation of patients before surgery influence tumor cell proliferation, apoptosis, and angiogenesis, which can be used to evaluate the prognosis of patients with malignant tumors.5–7 Currently, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), prognostic nutritional index (PNI), platelet-to-albumin ratio (PAR), fibrinogen-to-albumin ratio (FAR), albumin-to-γ-glutamyl transpeptidase ratio (AGR) and albumin-to-alkaline phosphatase ratio (AAPR) were useful tests used to evaluate immune and inflammatory status in patients with malignant tumors, and they had been used to predict the prognosis of GBC patients.8–15 Nevertheless, there has been no comparative study of the above-mentioned serum biomarkers on the prognostic prediction of patients with GBC after radical resection. In this study, we aimed to explore and screen preoperative serum immune response level-related biomarkers with better prognostic ability and developed a prognostic model for decision-making in the clinical practice of GBC patients.
Methods
Patients and Design
We included all histologically confirmed GBC patients treated at the First Affiliated Hospital of Xi’an Jiaotong University between 2011 and 2020. The inclusion criteria were as follows: (1) postoperative pathologically confirmed GBC; (2) margin status recorded microscopically negative (R0); (3) preoperative serum albumin, lymphocytes, platelets, and other indicators were available; (4) clinicopathological characteristics and follow-up data were all available. The exclusion criteria were as follows: (1) patients with preoperative infection; (2) patients with preoperative severe chronic wasting disease; (3) patients with preoperative coagulation abnormality; (4) patients with preoperative anticoagulation or albumin infusion; (5) patients who received neoadjuvant therapy or other treatments for malignant tumors before surgery; (6) patients died within 30 days after surgery. In total, 427 patients met the inclusion/exclusion criteria and were included in the study. Through January 2022, all included patients were evaluated using the 8th edition AJCC staging system.
Study Variables
Table 1 showed the serological biomarkers variables and calculation methods for this study, and the best cut-off values determined using the X-tile software were also displayed. We defined NLR, PLR, LMR, SII, SIRI, PNI, PAR, FAR, AGR, and AAPR as low-risk and high-risk groups based on optimal cut-off values. Moreover, time-dependent receiver operating characteristic (time-ROC) analysis was performed using R software version 3.6.1 (http://www.r-project.org/) to determine the prognostic predictive power.
 |
Table 1 Calculation Method and Cut-off Value of Preoperative Serological Biomarkers |
Follow-Up
A routine follow-up was performed in outpatient and telephone settings for all patients included in the study. During the first year following surgery, liver function, tumor biomarkers (CEA, CA19-9, CA125), and ultrasound, contrast-enhanced CT or MRI examination were reviewed every 2–3 months, and over a one-year period, follow-ups were conducted once every 3–6 months. The OS was calculated from the date of radical resection until the date of death or the most recent follow-up of the patient, and clinical evidence of recurrence of the tumor. The follow-up ended in January 2022.
Statistical Analysis
All statistical analyses were performed using SPSS version 25. Analyses of categorical variables were conducted using the χ2 test. Univariate analysis was conducted using the Kaplan-Meier method and Log rank test, and multivariate analysis was conducted using the Cox proportional hazard regression model. The Kaplan-Meier curves were conducted by GraphPad Prism (version 8.0, San Diego, California, USA). Variables with P<0.05 were considered statistically significant.
Nomogram Development and Assessment
A training set (N=300) and a testing set (N=127) were created from all included patients in a 7:3 ratio (Baseline characteristics comparison between the training and testing sets shown in Supplementary Table 1). The nomogram prediction model was developed using R software based on the independent variables and an online calculator was developed. The concordance index (C-index), calibration plot, area under ROC (AUC), and decision curve analysis were used to evaluate the performance of the nomogram model.
Results
The study included 427 patients undergoing radical resection for histologically confirmed GBC between 2011 and 2020. The median survival time was 49.0 months, and overall survival rates were 78.0%, 54.8%, and 45.6% at 1, 3, and 5 years, respectively.
Time-Dependent Receiver Operating Characteristic (Time-ROC) Analysis
Time-dependent ROC curves are shown in Figure 1A and B, indicating that FAR outperformed other indicators in training and testing sets as a prognostic biomarker.
An Analysis of the Correlation Between FAR and Clinicopathological Characteristics
The patients with preoperative FAR>0.08 were compared with FAR≤0.08, the proportion of age>60 years, NLR≥2.2, PLR>155.3, LMR≤3.8, SII>399.7, SIRI>0.95, PNI≤44.0, PAR>6.25, AGR≤2.03, AAPR≤0.38, CA19-9>39.0U/mL, CA125>35.0U/mL, TBIL>34.1μmol/L, combined with gallbladder stones, without gallbladder polyps, tumor morphology with infiltrative type, poor tumor differentiation, liver invasion, macrovascular invasion, T stage (stage T3 and T4), N1-2 stage, TNM stage (stage III and IVA) was significantly higher than the latter (Table 2, P<0.05).
 |
Table 2 The Relationship of FAR with Clinical Characteristics for GBC After Radical Resection |
Subgroup Analyses Between Low FAR and High FAR Groups
Forest plots indicated the FAR has an excellent ability to distinguish the prognosis of CA19-9, CA125, liver involvement, major vascular invasion, perineural invasion, T stage, N stage, and TNM stage (Figure 2, all P <0.05).
 |
Figure 2 Forest plot of the association between fibrinogen-to-albumin ratio (FAR) and overall survival in the training set. (A) Forest plot of low FAR group. (B) Forest plot of high FAR group. |
Survival Analysis in the Whole Cohort
Univariate analysis showed that the overall survival of patients in the low LMR, PNI, AGR and AAPR, high NLR, PLR, SII, SIRI, PAR and FAR groups were significantly worse than those in the high LMR, PNI, AGR and AAPR, low NLR, PLR, SII, SIRI, PAR and FAR groups (P<0.05), which demonstrated that serum biomarkers may be used to predict GBC prognosis. In the testing set, by using the same cut-off value, the overall survival of patients in the low LMR, PNI, AGR and AAPR groups, and the high NLR, SIRI, and FAR groups were worse than the high LMR, PNI, AGR and AAPR groups, and the low NLR, SIRI, and FAR groups (P<0.05), while there no statistical difference between the high PLR, SII, PAR and low PLR, SII, PAR groups in overall survival, which was considered to be related to the difference in the overall distribution of patients in the training and testing sets (Supplementary Figure 1 and Figure 2).
The multivariate analysis included only FAR among preoperative immune response level-related markers to avoid multicollinearity, which indicated that FAR was an independent risk factor. Detailed results of the univariate and multivariate analysis are shown in Table 3.
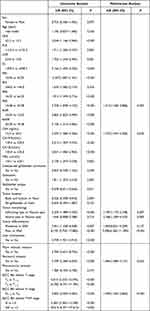 |
Table 3 Univariate and Multivariate Analysis of Prognosis for GBC After Radical Resection |
Nomogram Development and Online
A nomogram prediction model for OS was established based on the independent risk factors, including FAR, CEA, CA-125, N stage, major vascular invasion, perineural invasion, and tumor differentiation. The online calculator is available at https://doczj.shinyapps.io/nom_far_gbc/, which is convenient and effective for clinicians. In addition, the online calculator demonstrates that FAR has a good prognostic discrimination ability. Detailed results of the Cox regression are shown on the right-hand side of Table 2, and the nomogram is shown in Figure 3.
 |
Figure 3 Nomogram prognosis prediction model for gallbladder carcinoma after radical resection. |
Nomogram Assessment
The C-index of the nomogram model was 0.803 (95% CI:0.771~0.835) and 0.774 (95% CI:0.696~0.852) in the training and testing sets, respectively. The AUCs of the nomogram to predict the 1-year, 3-year, and 5-year prognosis were 0.858, 0.858, and 0.837 in the training set, respectively, and the AUCs of the nomogram to predict the 1-year, 3-year, and 5-year prognosis were 0.779, 0.803, and 0.873 in the testing set, respectively (Figure 4A and B).
Further, we also assigned scores to different variable statuses of each patient, stratified them based on X-tile software, and divided patients into a low-risk group with 0–128 points, a medium-risk group with 129–198 points, and a high-risk group with 200–340 points, according to the results, overall survival was statistically significantly different among the three groups (P<0.0001, Figure 4C); the testing set also revealed a statistically significant difference in overall survival among the three groups, based on the same stratification method (P<0.0001, Figure 4D).
In Figure 5A and B, the calibration plots illustrated that the nomogram model performed better in the training and testing sets, and decision curve analysis demonstrated the nomogram model has a better predictive ability than the FAR and TNM staging system in both training and testing sets (Figure 5C and D).
Discussion
Neutrophils, lymphocytes, monocytes, and platelets are important components in the tumor microenvironment. Malignant tumors trigger a non-specific inflammatory response that is characterized by elevated neutrophil and platelet levels and decreased lymphocytes. Neutrophils in tumor tissue promote tumor proliferation and angiogenesis by secreting TNF-α, VEGF, and interleukin; platelets promote tumor proliferation and differentiation by secreting TNF-β, VEGF, and platelet-derived factors; and monocytes can differentiate into tumor-associated macrophages to promote tumor cell growth, infiltration and angiogenesis, while cytokines are released by lymphocytes and mediate cytotoxic responses to inhibit the growth, proliferation, and metastasis of tumors.16–19 Therefore, preoperative peripheral blood inflammatory markers can reflect the balance between tumor inflammatory response and immune antitumor function. In this study, the overall survival of patients in the low LMR, high NLR, PLR, SII, SIRI groups were significantly worse than those in the high LMR, low NLR, PLR, SII, SIRI groups, which indicated that serum biomarkers may be used to predict the prognosis of GBC. Based on the biological importance of inflammatory cells within the tumor microenvironment, NLR, PLR, LMR, SII, and SIRI are likely to play a prognostic role, as reported.8,9
The serum albumin level is a common serological biomarker of nutrient status and liver function. It has been found that albumin acts as an antioxidant to scavenge reactive oxygen species and nitrogen species that cause systemic inflammation, while inhibiting tumor progression by reducing the phosphorylation of Rb protein in cellular pathways.20,21 Malignant tumors are often associated with abnormal activation of the coagulation system. Fibrinogen, an important component of the coagulation system, is related to the level of inflammatory response in the body. Several studies have demonstrated that tumor cells can synthesize and secrete fibrinogen into the microenvironment to promote their growth, while platelets can induce fibrinogen aggregation around cancer cells by forming thrombin, which helps to avoid the clearance of natural killer cells.22,23 γ-GGT is essential for glutathione metabolism and plays a determinant role in protecting against oxidative stress, and the increase of γ-GGT reflects high tumor risk and poor prognosis associated with strong oxidative stress in patients.24,25 Therefore, the combination of parameters such as albumin, fibrinogen, and γ-GGT can be effectively used for the prognostic assessment of GBC patients. The study also showed that the overall survival of patients in the low PNI, AGR, AAPR, high PAR, FAR groups were significantly worse than those in the high PNI, AGR, AAPR, low PAR, FAR groups, which indicated the prognostic value of FAR and other biomarkers for GBC.
In this study, a comparison of the prognostic ability of different preoperative biomarkers showed that FAR was superior to the rest of the biomarkers in assessing prognosis. Based on multivariate analysis, FAR was also identified as an independent risk factor; its cut-off value for the prognosis of GBC was 0.08, consistent with the results of Xu et al13 According to further analysis of the FAR and clinicopathological characteristics of patients, patients with high FAR had a certain correlation with the clinicopathological features of poor prognosis, such as age>60 years, elevated tumor biomarkers, combined with jaundice, poor tumor differentiation, liver invasion, macrovascular invasion, and advanced T stage and lymph node metastasis. The correlation analysis revealed that high FAR levels were positively correlated with poor prognostic characteristics of GBC patients, which would demonstrate that high FAR was associated with poor prognosis. Moreover, the prognostic discrimination ability of FAR depended on CA19-9, CA125, liver involvement, major vascular invasion, perineural invasion, T stage, N stage, and TNM stage.
A single-center analysis of 154 GBC cases by Xu et al13 evaluated prognostic predictive value of FAR, and they demonstrated that FAR was an independent risk factor for prognosis, but did not validate the prognostic prediction ability of FAR and establish a survival prediction model. The nomogram, as a predictive statistical model for individual patients, has proven to have advantages over the traditional TNM staging system in terms of predicting long-term survival outcomes, which has been proposed as a practical tool to guide cancer treatment.26–30
In this study, a nomogram model was developed based on independent risk factors, including FAR, CEA, CA-125, N stage, major vascular invasion, perineural invasion, and tumor differentiation. A good prediction ability was demonstrated by the C-index of our model of 0.803 in the training set and 0.774 in the testing set, which could effectively promote the clinical application of FAR. Sun et al14 established a nomogram model that considered AGR, T stage, surgical margin, body mass index, and CA19-9 with the C-index of 0.780 and 0.762 in the training and testing set, respectively. Liu et al8 analyzed 1072 cases from China Gallbladder Cancer Research Group (CRGGC) and established a nomogram prediction model based on SII, T stage, N stage, CA19-9, and surgical margins with the C-index of 0.735 and 0.686 in the training and testing set, respectively. Ma et al31 also developed a nomogram model based on tumor size, liver invasion, surgical margins, and nerve invasion with the C-index of 0.777. In comparison to the above nomogram models, our nomogram, by incorporating preoperative FAR, had a better predictive ability. Moreover, the predictive power of our nomogram model was significantly higher than that of TNM staging system according to decision curve analysis. In turn, we developed the first online prediction model that will be convenient and effective for clinicians.
There were several limitations in our study, even though preoperative FAR showed some predictive value. The sample size was the largest in a single Chinese center, but it was still relatively small. Furthermore, we failed to validate the prognostic predictive capability of FAR and our nomogram externally. Additionally, no postoperative assessment of recurrence was performed. The development of preoperative non-invasive serological biomarkers with greater prognostic potential needs to be studied in a multicenter, prospective, and large-scale study in the near future, to provide decision support for the clinical diagnosis and treatment of GBC.
Conclusions
In summary, preoperative serum FAR has a better predictive ability for overall survival among preoperative serum immune response level-related biomarkers, and FAR represents an independent risk factor affecting postoperative prognosis. Our novel nomogram established based on FAR can be used in predicting survival probability and stratifying risk to guide clinical decision-making.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by correspondence author, without undue reservation.
Ethics Statement
The study was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University (No: XJTU1AF2022LSK-089), Xi’an, China. Written informed consent was obtained from all included patients and their families before study enrollment, and adhering to the principles of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Supported by the National Natural Science Foundation of China (No. 62076194); Key Research and Development Program of Shaanxi Province (2021-SF-016, 2022-SF-410, and 2022-SF-606); Clinical Research Fund of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF-CRF-2018-022).
Disclosure
The authors have no conflicts of interest in relation to this work to declare.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi:10.2147/CLEP.S37357
3. Huang J, Patel HK, Boakye D, et al. Worldwide distribution, associated factors, and trends of gallbladder cancer: a global country-level analysis. Cancer Lett. 2021;521:238–251. doi:10.1016/j.canlet.2021.09.004
4. Lindnér P, Holmberg E, Hafström L. Gallbladder cancer - no improvement in survival over time in a Swedish population. Acta Oncol. 2018;57(11):1482–1489. doi:10.1080/0284186X.2018.1478124
5. Munn LL. Cancer and inflammation. Wiley Interdiscip Rev Syst Biol Med. 2017;9(2):10. doi:10.1002/wsbm.1370
6. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi:10.1038/s41591-018-0014-x
7. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
8. Li L, Ren T, Liu K, et al. Development and validation of a prognostic nomogram based on the systemic immune-inflammation index for resectable gallbladder cancer to predict survival and chemotherapy benefit. Front Oncol. 2021;11:692647. doi:10.3389/fonc.2021.692647
9. Sun L, Hu W, Liu M, et al. High systemic inflammation response index (SIRI) indicates poor outcome in gallbladder cancer patients with surgical resection: a single institution experience in China. Cancer Res Treat. 2020;52(4):1199–1210. doi:10.4143/crt.2020.303
10. Cao P, Hong H, Yu Z, et al. A novel clinically prognostic stratification based on prognostic nutritional index status and histological grade in patients with gallbladder cancer after radical surgery. Front Nutr. 2022;9:850971. doi:10.3389/fnut.2022.850971
11. Wang J, Bo X, Li M, et al. Prediction efficacy for clinical outcome of prognostic nutritional index in patients with resectable biliary tract cancer depends on sex and obstructive jaundice status. Ann Surg Oncol. 2021;28(1):430–438. doi:10.1245/s10434-020-08728-8
12. Li C, Peng W, Zhang X-Y, et al. The preoperative platelet to albumin ratio predicts the prognosis of hepatocellular carcinoma patients without portal hypertension after liver resection. Medicine. 2019;98(45):e17920. doi:10.1097/MD.0000000000017920
13. Xu WY, Zhang HH, Xiong JP, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. 2018;24(29):3281–3292. doi:10.3748/wjg.v24.i29.3281
14. Sun L, Ke X, Wang D, et al. Prognostic value of the albumin-to-γ-glutamyltransferase ratio for gallbladder cancer patients and establishing a nomogram for overall survival. J Cancer. 2021;12(14):4172–4182. doi:10.7150/jca.49242
15. Li H, Li J, Wang J, et al. Assessment of liver function for evaluation of long-term outcomes of intrahepatic cholangiocarcinoma: a multi-institutional analysis of 620 patients. Front Oncol. 2020;10:525. doi:10.3389/fonc.2020.00525
16. Jabłońska E, Kiluk M, Markiewicz W, et al. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunol Ther Exp. 2001;49(1):63–69.
17. Treffers LW, Hiemstra IH, Kuijpers TW, et al. Neutrophils in cancer. Immunol Rev. 2016;273(1):312–328. doi:10.1111/imr.12444
18. Szebeni GJ, Vizler C, Kitajka K, et al. Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflamm. 2017;2017:9294018. doi:10.1155/2017/9294018
19. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.017
20. Nojiri S, Joh T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int J Mol Sci. 2014;15(3):5163–5174. doi:10.3390/ijms15035163
21. Taverna M, Marie AL, Mira JP, et al. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3(1):4. doi:10.1186/2110-5820-3-4
22. Sahni A, Simpson-Haidaris PJ, Sahni SK, et al. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost. 2008;6(1):176–183. doi:10.1111/j.1538-7836.2007.02808.x
23. Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100(5):859–865. doi:10.1111/j.1349-7006.2009.01115.x
24. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263–355. doi:10.1080/20014091084227
25. Kunutsor SK, Apekey TA, Van Hemelrijck M, et al. Gamma glutamyltransferase, alanine aminotransferase and risk of cancer: systematic review and meta-analysis. Int J Cancer. 2015;136(5):1162–1170. doi:10.1002/ijc.29084
26. Kattan MW, Scardino PT. Evidence for the usefulness of nomograms. Nat Clin Pract Urol. 2007;4(12):638–639. doi:10.1038/ncpuro0968
27. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi:10.1200/JCO.2012.41.5984
28. Li Q, Chen C, Zhang J, et al. Prediction efficacy of prognostic nutritional index and albumin-bilirubin grade in patients with intrahepatic cholangiocarcinoma after radical resection: a multi-institutional analysis of 535 Patients. Front Oncol. 2021;11:769696. doi:10.3389/fonc.2021.769696
29. Li Q, Zhang J, Chen C, et al. A nomogram model to predict early recurrence of patients with intrahepatic cholangiocarcinoma for adjuvant chemotherapy guidance: a multi-Institutional analysis. Front Oncol. 2022;12:896764. doi:10.3389/fonc.2022.896764
30. Yang J, Song X, Lai Y, et al. Development and validation of a postoperative nomogram for predicting overall survival after endoscopic surgical management of olfactory neuroblastoma. EClinicalMedicine. 2020;29–30:100577. doi:10.1016/j.eclinm.2020.100577
31. Ma Z, Dong F, Li Z, et al. A novel prognostic nomogram for gallbladder cancer after surgical resection: a single-center experience. J Oncol. 2021;2021:6619149. doi:10.1155/2021/6619149
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



