Back to Journals » Cancer Management and Research » Volume 13
Preoperative Contrast-Enhanced Ultrasound (CEUS) Combined with 125I Seeds Localization in Sentinel Lymph Node Biopsy for Breast Cancer
Authors Zhou P, Zheng W, Liu Y, Wang Y
Received 7 December 2020
Accepted for publication 31 January 2021
Published 24 February 2021 Volume 2021:13 Pages 1853—1860
DOI https://doi.org/10.2147/CMAR.S296142
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Pengpeng Zhou,1,2,* Weizhen Zheng,3,* Yanbing Liu,2 Yongsheng Wang2
1Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, 250000, Shandong, People’s Republic of China; 2Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, 250000, Shandong, People’s Republic of China; 3Shandong Provincial Hospital, Jinan, 250000, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongsheng Wang
Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Ji Yan Road 440, Jinan, 250000, Shandong, People’s Republic of China
Email [email protected]
Objective: To assess the clinical value of contrast-enhanced ultrasound (CEUS) technology in predicting axillary lymph nodes status before surgery, and to explore the feasibility of sentinel lymph nodes (SLNs) localization guided by CEUS combined with 125I implantation for breast cancer.
Methods: From August 2017 to February 2019, 115 patients were included in this prospective study. Before surgery, a microbubble (SonoVue) was injected intradermally next to the areola. The enhancement patterns of SLNs were recorded and 125I seeds were deployed into the enhanced nodes. Then, all patients underwent standard sentinel lymph node biopsy (SLNB) and all 125I seeds were found out guided by a gamma detector in surgery. The localization was considered successful if 125I seeds were implanted in/beside the nodes.
Results: SLNs in 103 cases were successfully identified, the success rate was 89.6% (103/115), 118 SLNs were detected in total. 125I seeds were deployed successfully in 99 cases, and all of the 125I-labeled SLNs were then successfully detected by combined method (radionuclides and blue dye). The accuracy of 125I seeds localization was 96.1% (99/103). Based on the enhancement patterns recorded, 34 cases were predicted to have SLNs metastasis (metastasis in 27 cases and no metastasis in 7 cases confirmed by postoperative pathology) and 65 cases were predicted to have no SLNs metastasis (metastasis in 5 cases and no metastasis in 60 cases by pathology). The positive predictive value and negtive predictive value of CEUS in assessing axillary status were 79.4% (27/34) and 92.3% (60/65), respectively. The axillary metastasis rate in CEUS combined with 125I seeds localization was 27.3% (27/99), while the metastasis rate in the combined method of SLNB was 32.3% (32/99). The sensitivity of 125I seeds localization was 84.4% (27/32), the false-negative rate was 15.6% (5/32), and the consistency evaluation was excellent (Kappa value=0.880, P< 0.001).
Conclusion: CEUS combined with 125I seeds implantation can locate SLNs accurately and has excellent consistency with the combined method. The enhancement patterns can provide helpful predicting information of axillary status preoperatively. However, more studies are needed to be carried out to verify our outcomes and explore the feasibility of applying CEUS technology in clinical work.
Keywords: breast cancer, contrast-enhanced ultrasonography, CEUS, sentinel lymph node biopsy, SLNB
Introduction
Axillary lymph node status is an important staging factor for breast cancer.1 In recent years, sentinel lymph node biopsy (SLNB) has replaced Axillary lymph node dissection (ALND) as standard surgery for early-stage breast cancer, which can significantly reduce complications, such as upper limb lymphedema, paresthesia, and dyskinesia.2–6 The combined application of radionuclides and blue dye can improve the success rate and reduce the false-negative rate of SLNB, therefore it has been recommended in many domestic and foreign guidelines.4,7 Blue dye is relatively economical and easy to obtain, so that it has been accepted by surgeons worldwide. However, the radionuclide method that applies 99mTc labeled thiocolloid to detect sentinel lymph nodes (SLNs) is a rather complex process that has seen its clinical applications become limited.
Contrast-enhanced ultrasound (CEUS) is a new technique that has been developed rapidly in recent years. CEUS is currently widely used in clinical diagnostics for benign and malignant tumors in the abdominal and superficial organs.8,9 However, few studies have concentrated on applying CEUS techniques to breast cancer. In our study, we tried to detect the possibility of CEUS technology as an SLN tracer in early-stage breast cancer. The aim of this study was to evaluate the feasibility of CEUS in predicting axillary lymph node statuses preoperatively and to explore the accuracy of SLN localization guided by CEUS combined with 125I seed implantation.
Materials and Methods
Clinical Data
One hundred and fifteen patients suffering from early-stage breast cancer (cT1-3N0M0) from August 2017 to February 2019 were recruited into the study. Inclusion criteria: 1) older than 18, 2) pathologically confirmed primary breast cancer, 3) clinical axillary lymph nodes negative; 4) no distant metastasis revealed by imaging examination, 5) Tumor diameter ≤5 cm (ultrasound, molybdenum target, magnetic resonance as reference), 6) Informed consent of the patients. Exclusion criteria: 1) older than 75, 2) previous history of axillary surgery or radiotherapy, 3) previous history of neoadjuvant therapy, 4) inflammatory breast cancer (IBC). Patients aged above 75 were excluded because some of them had poor underlining health conditions, many of them might choose other therapies, such as hormonotherapy, instead of general anesthesia surgery. In addition, the lymph vessels pressure may decrease with age, which may result in failure in both CEUS and SLNB procedure. IBC is the most aggressive form of breast cancer. The failure rates for the surgical treatments alone are very high. Radiotherapy and chemotherapy are of greater importance than surgery, so patients with IBC were also excluded.
This study was conducted in accordance with the Declaration of Helsinki and obtained the approval of the Ethics Committee of the Shandong Cancer Hospital (No. SDTHEC20110324). One hundred and fifteen female patients aged 28–70 years (median age 47 years) were enrolled.
CEUS Procedure
The patients took the supine position with the upper extremity abducted. 0.6 mL SonoVue (25mg/bottle, Bracco) was injected into the intradermal layer next to the areola. After the injection, the areolar area was massaged for 10–30 s. Then, lymphatic channels were visualized on the ultrasound contrast pulse sequencing mode and were tracked into the lymph nodes. The first enhanced lymph node drained by the lymphatic vessel was defined as the CEUS sentinel lymph node (SLN). When multiple lymphatic vessels were enhanced, the first enhanced node drained by each lymphatic vessel was defined as the SLN. The enhancement patterns for all SLNs were recorded. When SLNs were identified, 125I seeds were then implanted into them guided by ultrasound. After implantation, all patients received a mammogram to determine if the 125I particles were accurately positioned into the SLNs (Figure 1). If no enhanced lymph nodes were identified after the injection, 0.6 mL Sonovue would be, respectively, injected into the inner, upper, and outer sides of the areola. If no lymph nodes were identified after two consecutive injections, the case was abandoned.
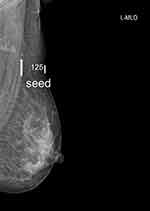 |
Figure 1 Post 125I seed implantation mammograms. The 125I seed was implanted inside the axillary lymph node successfully. Arrow: the implanted 125I seed. |
SLNB
The 99mTc-sulphocolloid was injected subcutaneously into the areola area 3–18 h before the operation with an injection dose of 1.0 mCi (0.5 mCi/mL). After anesthesia was administered, Methylthionine Chloride (1% MB injection, 2 mL: 20 mg, Jiangsu Jichuang Pharmaceutical Co. LTD.) was injected intradermally into the upper outer quadrant of the areola area (2 mL for breast-conservation surgery 2 mL and 4 mL for modified surgery). Fifteen minutes later, the SLNB procedure began. The gamma detector (Neoprobe Corporation) was switched to 27keV, then the axilla was carefully dissected and 125I particles were discovered, guided by the gamma detector. If 125I seeds were not implanted inside the SLNs, an area of 5 mm around the particles was carefully dissected. The localization was considered successful when the 125I particles were placed in/beside the nodes. If no lymph nodes were discovered in the 5 mm around, the location was considered a failure. All 125I seeds were then taken out and recycled. After that, the routine procedure of SLNB using nuclides combined with blue dye was administered.
Statistical Treatment
Statistical analysis was performed with SPSS 22.0. The differences between multiple sample rates were compared with the chi-square test, and the paired samples were compared with the Wilcoxon rank sum test. The paired chi-square test was used for the consistency test of dichotomous variables, and P<0.05 was considered statistically significant.
Results
Clinical Data
From August 2017 to February 2019, 115 patients with early-stage breast cancer (cT1-3N0M0) from the Breast Disease Center of the Shandong Cancer Hospital were included in this study. The median age was 47 years old (28–70 years old). Detailed information of the recruited patients is shown in Table 1.
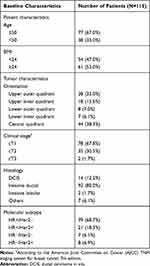 |
Table 1 Patients and Primary Tumor Characteristics |
Prediction of Axillary Status by CEUS Enhancement Patterns
Axillary lymph node enhancements were observed in 103 of 115 patients after the microbubble injection with a success rate of 89.6% (103/115). The lymph node enhancement process is shown in Figure 2. The comparison of the success rates and SLN number between CEUS and the combined SLNB method is shown in Table 2.
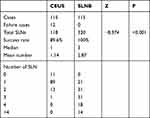 |
Table 2 Comparison of SLNs Detected by CEUS versus SLNB |
All 103 successful patients were implanted with at least one 125I seeds guided by ultrasound, for a total of 118 seeds implanted. In 99 cases, 125I seeds were successfully implanted in/around lymph nodes. The SLN enhancement patterns can be divided into three types: Type I, SLN presented significant homogenous enhancement; Type II, SLN showed significant enhancement but with low to no perfusion area. Type III, SLN showed weak enhancement (Figure 3).
Patients with enhancement patterns of type II and III were predicted to be SLN positive, and patients with type I were predicted to be SLN negative.10 If two or more SLNs were found in a patient, the SLN status would be considered positive if any SLN presented type II/III patterns. In our study, 34 of the 99 cases were predicted to have SLN metastasis, in which 27 patients were finally proved to be node-positive by postoperative pathology, while the other 7 were node-negative. Sixty-five patients were predicted to have no SLN metastasis by CEUS, in which 60 patients were confirmed to be node-negative, and the other 5 were node-positive pathologically. Enhancement patterns of type I accounted for the greatest proportion in non-metastatic cases (60/65), while type II was more common in metastatic cases (16/20) (Table 3). The positive predictive value and negtive predictive value of CEUS in assessing axillary lymph node status were 79.4% (27/34) and 92.3% (60/65).
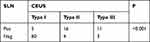 |
Table 3 Patterns of Enhancement and SLNs Status |
Exploration of CEUS Combined with 125I Seeds in SLNs Positioning
All 103 patients with successful enhancement were implanted with radioactive 125I seeds guided by ultrasonic. A total of 118 radioactive seeds were implanted. Post 125I Seed deployment mammograms showed that in 42 patients, 125I seeds were implanted in the SLN; in 25 patients, the seeds were implanted around SLN. A total of 67 patients were planted successfully according to the mammograms. In surgery, we certified that 99 patients had their 125I seeds implanted successfully. The 125I seeds implantation process is shown in Figure 4.
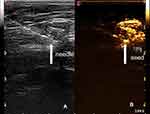 |
Figure 4 The 125I seed implantation process. (A) The needle (arrow) can be seen directly in ultrasound. (B) The 125I seed (arrow) was implanted in the enhanced SLN. |
In the successful 99 patients, all of the 125I-labeled lymph nodes were then detected by the combined method. The pathological results suggested that 27 metastatic cases were detected by 125I seeds, and the metastasis rate was 27.3% (27/99), while 32 metastatic cases were detected by the combined method with a metastasis rate of 32.3% (32/99). There were five patients who presented no SLN metastasis by CEUS but were proved to have at least one metastatic lymph node by the combined method. All of the five false-negative cases had positive SLNs other than the 125I-labeled SLNs. The sensitivity of SLN localization guided by CEUS technology was 84.4%, the false-negative rate was 15.6% with a specificity of 100%, a positive coincidence rate of 100%, and a negative coincidence rate of 93.1%. The consistency evaluation with the combined method showed that the Kappa value was 0.880 and P < 0.001, indicating an excellent consistency (Table 4).
 |
Table 4 The SLN Metastasis Rate of CEUS and the Combined Method |
Discussion
In recent years, SLNB has replaced ALND as the standard surgery for patients with early-stage breast cancer, which has significantly reduced the incidences of upper limb lymphedema, paresthesia, and dyskinesia.2–5 Domestic and foreign guidelines recommend that the combined administration of radionuclide and blue dye can improve the success rate as well as reduce the false-negative rate of SLNB.6,7 The purpose of this study was to investigate the CEUS clinical value in evaluating axillary lymph node status before surgery as well as the possibility of SLN localization guided by CEUS combined with 125I implantation. With CEUS technology, we expected to explore a new method for detecting SLNs in patients with early-stage breast cancer.
Previous studies11–13 showed that CEUS might be a valuable technology for evaluating SLN statuses of breast cancer. Compared with other tracers, CEUS is superior due to being non-invasive, radiation-free, economical, and effective. In this study, the success rate of CEUS guided by SLN enhancement was 89.6% (103/115), which was consistent with the relevant studies in this field.11 Among the 12 failed cases, 5 only showed lymphatic vessels enhancements, but no lymph node was enhanced, in which 2 patients had already had their primary tumors resected before, and the tumors were all located in the outer upper quadrant. It might lead to enhancing failures due to the contrast agent being unable to drain to the lymph nodes because of the removed lymphatic vessels. The other seven failed cases neither had lymphatic vessels nor lymph node enhancement. In three of those, vessel carcinoma embolus was reported in postoperative pathology, which might block the lymphatic vessels, and the contrast agent could not pass through them, resulting in failure enhancement. In addition, 8 of 12 failed cases occurred in the first 50% of the study, indicating there a learning curve might exist. Other factors, such as BMI, molecular typing was analyzed, and we found that none of these factors are related to enhancement failure (P>0.05).
One significant advantage of CEUS technology lies in its ability to enable us to observe enhanced lymphatic vessels and lymph nodes on the ultrasound screen in real time. According to the observed enhancement pattern, SLNs could be divided into 3 types.10 Type I: SLN significant homogenous enhancement. The contrast agents were uniformly distributed in the lymph nodes, which might suggest no tumor cell metastasis in the node. Type II: SLN significant homogenous enhancement with low to no perfusion area. The filling defect area of the SLN might be due to partial invasion of a tumor cell. Type III: SLN weak enhancement. In this type, tumor cells might have invaded the entire node and replaced all normal cells, or obstructed major lymphatic vessels, leading to weak enhancement. Based on the type of enhancement pattern, we explored the CEUS value by detecting SLN metastasis preoperatively. Thirty-four cases were predicted to have SLN metastasis (metastasis in 27 cases and no metastasis in 7 cases confirmed by postoperative pathology), and 65 cases were predicted to have no SLN metastasis (metastasis in 5 cases and no metastasis in 60 cases by pathology). The sensitivity and accuracy of our study were comparable to previous studies.13
SLN localization was another vital problem that needed to be solved with CEUS technology. Previous studies had attempted to locate SLN through body surface markers or puncturing needle routes; however, factors such as changing body positions easily affected localization accuracy.11 Guidewires had been used by some studies to improve the aforementioned deficiencies, but patients generally reported discomfort with long guidewire placed inside their bodies.14 Considering these factors, we used 125I particles to locate SLNs with sizes so tiny that we expected it to reduce the foreign body sensation as well as guarantee accuracy. In our study, the accuracy of 125I localization was 96.1% (99/103), higher than the results reported in other papers.15 The reason might be if the seeds were not implanted in the SLNs, we would scrupulously dissect the tissue around the seeds. Localization was also considered successful if lymph nodes could be found within a radius of 5 mm.
After 125I seed implantation, all patients received a routine mammogram to observe if the seeds were accurately placed into the SLNs. Compared with the successful implantation rate in surgery (96.1%), the accuracy of preoperative mammogram was lower (65.0%). The reason might be that the mammogram is not competent enough to show the entirety of the SLN clearly, and it is hard to determine the relative position through 2-dimensional images. A Tomography X-ray scan or MRI may be a better way to observe the position of 125I seeds in the axilla.
There are also some concerns with this technology. 125I seeds themselves are radioactive and may raise safety concerns. In fact, the average diameter of the radiation emitted by 125I particles is 1.7 cm.16 After being implanted in the body, the radiation energy on the body’s surface is almost negligible. To further reduce radioactivity, half-dosed 125I seeds were used in this study. Another notable concern lies in that 125I seeds preoperatively implanted may cause local hematoma that interfere with the normal SLNB process. However, in our study, there have been no failure cases reported in the SLNB process. In addition, the CEUS process may increase hospitalization costs, and many patients reported that the intradermal injections of contrast are painful.
In our study, the sensitivity, false-negative rate, specificity, positive coincidence rate, and negative coincidence rate of CEUS combined with 125I localization were 84.4%, 15.6%, 100%, 100%, and 93.1%, respectively. Besides, it achieved excellent consistency with the combined method, with a Kappa value of 0.880 and P < 0.001, indicating that our study may be pertinent in clinical applications. There were five false-negative cases, all of which were derived from SLN metastasis other than the 125I-labeled SLNs. In other words, it may be that we missed the SLNs that were connected to another lymphatic vessel, which exhibited no enhancements in CEUS.17 However, the number of SLNs discovered with CEUS, as we noticed, was generally less than that discovered by the combined method. Among the 103 patients with successful enhancements, 118 SLNs were detected by CEUS, while 299 were discovered by the combined method. The numbers detected by these two methods showed significant statistical disparities (P<0.05). Although numerically deficient, CEUS combined with 125I seed localization exhibited excellent consistency with the combined method, with a Kappa value of 0.880 and P < 0.001. That means, with a guarantee of total accuracy, CEUS combined with 125I seed localization might have an advantage in less surgical trauma, which would be beneficial for patients.
As a new technology, CEUS can accurately identify and locate SLNs in patients with early-stage breast cancer. CEUS provides a possible method for preoperatively evaluating axillary lymph nodes statuses, which may have an essential guiding significance for surgery options and follow-up treatment plans. However, related research on this topic is insufficient, more patient data and multi-center cohort trials, as well as long-term follow-up data on safety and treatment efficacy, are needed to verify these conclusions.
Acknowledgement
These authors contributed equally to this work and should be considered co-first authors: Pengpeng Zhou and Weizhen Zheng.
Funding
This work was funded by the National Natural Science Foundation of China (81672638, 81672104) and Shandong Provincial Key Research and Development Program (2017CXGC1207, 2019GSF108179, 2019GSF108104).
Disclosure
The authors have stated that they have no conflicts of interest in this work.
References
1. Benson JR, Della Rovere GQ; Axilla Management Consensus Group. Management of the axilla in women with breast cancer. Lancet Oncol. 2007;8(4):331–348. doi:10.1016/S1470-2045(07)70103-1
2. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi:10.1001/jama.2017.11470
3. Galimberi V, Cole BF, Viale G, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled Phase 3 trial. Lancet Oncol. 2018;19(10):1385–1393. doi:10.1016/S1470-2045(18)30380-2
4. Gradishar WJ, Anderson BO, Balassanian R, et al. Breast Cancer, Version 4. 2017, NCCN Clinical Practice Guidelines in Oncology[EB/OL]. J Natl Compr Canc Netw. 2018;16(3):310–320. doi:10.6004/jnccn.2018.0012
5. Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. doi:10.1200/JCO.2006.07.4062
6. Motomura K. Sentinel node biopsy for breast cancer: past, present, and future. Breast Cancer. 2015;22(3):212–220. doi:10.1007/s12282-012-0421-7
7. Shen S, Xu Q, Zhou Y, et al. Comparison of sentinel lymph node biopsy guided by blue dye with or without indocyanine green in early breast cancer. J Surg Oncol. 2018;117(8):1841–1847. doi:10.1002/jso.25058
8. Arai J, Shimozuma Y, Otoyama Y, et al. Three cases of histologically proven hepatic epithelioid hemangioendothelioma evaluated using a second-generation microbubble contrast medium in ultrasonography: case reports. BMC Gastroenterol. 2019;19(1):187. doi:10.1186/s12876-019-1113-y
9. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394. doi:10.3322/caac.21492
10. Xie F, Zhang D, Cheng L, et al. Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J Surg Oncol. 2015;13:319. doi:10.1186/s12957-015-0736-x
11. Nielsen Moody A, Bull J, Culpan AM, et al. Preoperative sentinel lymph node identification, biopsy and localisation using contrast enhanced ultrasound (CEUS) in patients with breast cancer: a systematic review and meta-analysis. Clin Radiol. 2017;72:959–971. doi:10.1016/j.crad.2017.06.121
12. Gkegkes ID, Iavazzo C. Contrast enhanced ultrasound (CEU) using microbubbles for sentinel lymph node biopsy in breast cancer: a systematic review. Acta Chir Belg. 2015;115(3):212–218. doi:10.1080/00015458.2015.11681099
13. Zhao J, Zhang J, Zhu QL, et al. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: A prospective study. Eur Radiol. 2018;28(4):1654–1661. doi:10.1007/s00330-017-5089-0
14. Sever AR, Mills P, Jones SE, et al. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol. 2011;196(2):251–256. doi:10.2214/AJR.10.4865
15. Barentsz MW, Verkooijen HM, Pijnappel RM, et al. Sentinel lymph node localization with contrast-enhanced ultrasound and an I-125 seed: an ideal prospective development study. Int J Surg. 2015;14:1–6. doi:10.1016/j.ijsu.2014.12.019
16. Cardoso RM, Souza CDD, Araki K. Highly efficient method for production of radioactive silver seed cores for brachytherapy. Appl Radiat Isot. 2017;120:76–81. doi:10.1016/j.apradiso.2016.11.023
17. Wang Y, Zhou W, Li C, et al. Variation of sentinel lymphatic channels (SLCs) and sentinel lymph nodes (SLNs) assessed by contrast-enhanced ultrasound (CEUS) in breast cancer patients. World J Surg Oncol. 2017;15(1):127. doi:10.1186/s12957-017-1195-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


