Back to Journals » Cancer Management and Research » Volume 11
Preoperative Albumin Level Is Superior To Albumin-Globulin Ratio As A Predicting Indicator In Gastric Cancer Patients Who Underwent Curative Resection
Authors Xiao S, Feng F, Liu N, Liu Z, Guo Y, Lian X, Zhang H
Received 11 September 2019
Accepted for publication 18 October 2019
Published 25 November 2019 Volume 2019:11 Pages 9931—9938
DOI https://doi.org/10.2147/CMAR.S230741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Shuao Xiao,1,* Fan Feng,1,* Ni Liu,2,* Zhen Liu,1 Yinghao Guo,3 Xiao Lian,1 Hongwei Zhang1
1Division of Digestive Surgery, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an 710032, Shaanxi, People’s Republic of China; 2Department of Anesthesiology, Weinan Central Hospital, Weinan 714000, Shaanxi, People’s Republic of China; 3Health Company, 92667 Army of PLA, Qingdao 266100, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongwei Zhang; Fan Feng
Division of Digestive Surgery, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, 127 West Changle Road, Xi’an 710032, Shaanxi, People’s Republic of China
Tel/Fax +86-029-84771531
Email [email protected]; [email protected]
Objective: The preoperative value of albumin level and albumin/globulin ratio (AGR) has been discovered to be a possibility for predicting gastric cancer. However, their predictive accuracy remains unknown. This study’s objective is to evaluate the predictive value of albumin, globulin and AGR in gastric cancer.
Methods: A total of 3266 gastric cancer patients in our institution who underwent radical gastrectomy during the period from September 2008 to April 2015 were retrospectively analyzed. Levels of preoperative serum albumin and globulin were recorded. The optimal cut off points of albumin, globulin and AGR were calculated using X-tile software. The association of albumin and AGR with clinicopathological features and eventual prognosis was analyzed. The survival predictive accuracy and prognostic discriminatory ability among different variables were analyzed.
Results: This study consisted of 2531 males (77.5%) and 735 females (22.5%). Ages ranged from 20 to 90, with a median age of 58.0 years. The optimal cut off values of albumin, globulin and AGR were set at 42.0, 28.2 and 1.80, respectively. Patients in the high albumin group and high AGR group were both associated with younger age, smaller tumor size, as well as earlier T and N stages. Univariate and multivariate analysis demonstrated that albumin level and AGR value were both significant prognostic factors, while globulin level was not. Furthermore, albumin level displayed a prognostic discriminatory ability and a predictive accuracy superior to that of AGR. The multivariate model based on albumin also revealed a superior predictive accuracy than that based on AGR.
Conclusion: Preoperative albumin level is superior to AGR value in the prediction of prognosis of gastric cancer.
Keywords: albumin-globulin ratio, albumin, gastric cancer, prognosis
Introduction
As one of the most prevalent health-threatening malignant tumors globally, gastric cancer is the third most fatal type of cancer in China.1 Radical gastrectomy still remains the dominant option for medical fit patients without distant metastasis. Although there have been considerable improvements in the management of gastric cancer, prognosis still remains unsatisfactory.2 The generally accepted classification for gastric cancer patients is the tumor, lymph node, metastasis (TNM) category. However, it appears to be inadequate in the era of precision medicine.3
In recent years, numerous studies have been carried out to explore possible prognostic factors for gastric cancer with promising results,4–7 among which albumin and globulin have aroused much interest for ease of detection before surgery. Albumin reflects nutrition status of patients, and a previous meta-analysis has demonstrated that low serum albumin is related to poor outcomes for gastrointestinal tract tumors.8 Globulin plays a crucial role in inflammation and immunity, and elevated serum globulin is reported to be associated with poor outcomes in ovarian and prostate cancers.9,10 However, the results remain inconsistent in gastric cancer.7 Moreover, the albumin/globulin ratio (AGR) is supposed to be a novel predictive factor in gastric cancer.
Therefore, this study seeks to explore the predictive role of preoperative serum albumin, globulin as well as AGR in gastric cancer patients.
Materials And Methods
Patients
The study was conducted in the Xijing Hospital of Digestive Diseases affiliated to the Fourth Military Medical university. A total of 3266 gastric cancer patients were studied who received curative resection from September 2008 to April 2015. All patients met the following criteria: 1) diagnosed as gastric cancer; 2) without distant metastasis; 3) did not receive neoadjuvant chemoradiotherapy and immunotherapy; 4) without chronic inflammatory disease; 5) with normal hepatic and renal function; 6) underwent radical D2 gastrectomy; 7) with complete clinicopathological and follow-up data. The protocol for the research project has been approved by the Ethics Committee of Xijing Hospital and that it conforms to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Written informed consent was obtained from all patients before surgery.
Clinicopathological Data
All patients received proximal, distal or total gastrectomy with R0 resection and D2 lymphadenectomy. The resection procedure was performed on the basis of the Japanese Gastric Cancer Treatment Guidelines11 Preoperative data such as serum albumin level, age, gender and serum globulin level were recorded. Lymph node metastasis, tumor depth, differentiation status, tumor size and tumor location were collected on the basis of pathological examination. The UICC/AJCC TNM staging system, 7th edition, was used to identify the tumor stage. Patients received follow until November 2017.
The serum levels of albumin and globulin were analyzed using an automatic biochemical analyzer (Hitachi 7600, Japan), and the equation AGR= albumin/globulin was used to calculate the value of AGR. The optimal cut off values for the levels of albumin, globulin and AGR were calculated using the X-tile software (Version 3.6.1, Yale University School of Medicine, New Haven, CT, USA)12
Statistical Analysis
SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA) was employed for data analysis. Chi-squared test or Fisher exact test was used to analyze discrete variables and differences among groups. Overall survival time was defined as the duration from the date when the surgery was performed to the date of death or the last follow-up. The log-rank test was used to compare the survival curves obtained through the method of Kaplan–Meier. Univariate analysis was used to identify prognostic risk factors and multivariate analysis was conducted using a Cox’s proportional hazards regression model. Then, two multivariable models were built. The multivariable selection was based on goodness of fit as represented by the Akaike information criterion (AIC) with a backward procedure. The prognostic predictive accuracy of the variables or multivariable models was compared using the values of the concordance index (C-index) and the AIC was computed using the package Harrell Miscellaneous (http://CRAN.R-project.org/package=Hmisc) in R software (version 3.6.0, http://www.R-project.org/). A smaller AIC value and/or a larger C-index represented a greater predictive accuracy.13,14 A P value of 0.05 was considered as the threshold for statistical significance.
Results
Patients’ clinicopathological characterizations are summarized in Table 1. There were 2531 males (77.5%) and 735 females (22.5%), with ages ranging from 20 to 90, and a median age of 58.0 years. The median levels of serum albumin and globulin were 42.8 g/L (range 20.8–55.0 g/L) and 24.5 g/L (range 10.7–40.2 g/L), respectively. The median value of AGR was 1.74 (range 0.79–3.62). The optimal cut off values calculated by X-tile software for albumin, globulin and AGR were 42.0, 28.2 and 1.80, respectively (Figure 1).
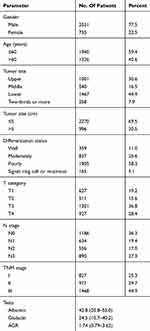 |
Table 1 Clinicopathological Features Of Gastric Cancer Patients |
 |
Figure 1 Calculation of cut off value of albumin, globulin and AGR by X-tile software. |
The associations of albumin and AGR with gastric cancer patients’ clinicopathological characterizations are summarized in Table 2. Results showed that individuals within the high albumin group presented younger age, smaller tumor sizes and earlier T and N stages in comparison to the low albumin group. Additionally, patients in the high AGR group presented younger age, male gender, smaller tumor sizes and earlier T and N stages.
 |
Table 2 Clinicopathological Features Of Patients Stratified By Preoperative Albumin And AGR Levels |
Then, the prognostic predictive ability of variables was analyzed. Univariate analysis displayed that both albumin level and AGR value were significantly associated with survival, but globulin level was not (Table 3). In addition, the albumin level represented a higher prognostic predictive accuracy than AGR (C-index: 0.54089 vs 0.52747; AIC: 18,409.45 vs 18,426.49, P<0.001). The overall survival time of patients stratified by different levels of albumin and AGR was exhibited in Figures 2 and 3.
 |
Table 3 Univariate Analysis Of Overall Survival In Gastric Cancer |
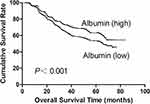 |
Figure 2 Overall survival of gastric cancer patients stratified by different values of albumin. |
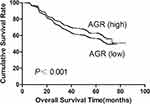 |
Figure 3 Overall survival of gastric cancer patients stratified by different values of AGR. |
To eliminate bias, the study utilized two multivariable models (Table 4). Model albumin was built on the basis of differentiation status, tumor size, age, N stage, T category and albumin level. Model AGR was built on the basis of age, tumor size, differentiation status, T category, N stage and AGR value. The albumin level and AGR value were independent prognostic risk factor in each model, respectively. The results showed that the model albumin revealed a superior predictive value than the model AGR (C-index: 0.76369 vs 0.76158; AIC: 17,423.14 vs 17,437.16, P<0.001).
 |
Table 4 Multivariate Models For Predicting Overall Survival In Gastric Cancer |
Discussion
As is known that chronic inflammation plays decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion and metastasis.15 Therefore, the inflammation-related markers such as the modified Glasgow Prognostic Score4 neutrophil–lymphocytes ratio6 and platelet–lymphocytes ratio5 have been wildly explored in GC. Recently, the preoperative AGR was proposed as a useful predictive factor in GC based on its reflection of both nutrition status and system inflammation. Previous studies associated with GC have always focused on the prognostic value of AGR; however, the predictive accuracy of which has not been evaluated. Within this investigation, we did find that preoperative AGR value was an independent prognostic factor in gastric cancer. But further analysis showed that AGR did not show any superiority to albumin in predictive accuracy and prognostic discriminatory ability.
As a major element of serum protein produced by the liver, albumin plays a significant part in the maintenance of the plasma osmotic pressure and material transport.16 It is not only a window into the nutrition condition of the body but also a mirror of the level of inflammation, both of which could form a vicious cycle and contribute to tumor development17 This is so far a number of reasons. Firstly, compared with patients at early stages, advanced gastric cancer patients are at a higher risk of malnutrition and cachexia.18 Secondly, cytokines induced by the tumor microenvironment (such as tumor necrosis factor-alpha, interleukin-6 and interleukin-1) could suppress the synthesis of albumin in the liver and increase vascular permeability, which leads to an increased albumin level in interstitial fluid and a decreased albumin level in serum.19 Thirdly, serum albumin has an antioxidant function against carcinogenesis and helps to maintain the stabilization of DNA replication and cell growth.20 What is more, low serum albumin level could impair the body’s natural defense mechanisms, including homeostasis of calcium and steroid hormone, anatomic barriers, as well as the distribution and pharmacological activities of anti-cancer drugs.21 A series of studies have shown that the lower level of serum albumin was related to worse survival in gastric cancer,17,22,23 which is consistent with this study’s findings.
The predictive role of globulin level in gastric cancer has been under debate. Globulin is produced by immune system organs and presents complex components, such as acute phase proteins, complement components, interleukins and immunoglobulins.24 Changes in serum globulins are reflective of both immunity and inflammation status,25 which have opposing effects on tumor progression. Thus, it is hard to determine which components prevail or change more when the level of serum globulin changes. As a result, it is hard to evaluate the predictive role of globulin level in gastric cancer. Up till now, no association has been found between the level of serum globulin and prognosis of gastric cancer,7,12,26,27 including in the present study.
The predictive value of preoperative AGR has been widely explored in gastric cancer. In a study consisting of 862 gastric cancer patients at all stages (stage I–IV), Mao et al found that low AGR value was correlated with worse survival.28 In four additional studies on gastric cancer patients with stage I–III who received curative surgery, it was also demonstrated that preoperative AGR was a significant prognostic indicator for gastric cancer patients.12,25–27 Additionally, Bozkaya et al discovered that AGR was a significant prognostic factor in metastatic gastric cancer patients.29 But the optimal cut off points of AGR vary a lot, ranging from 1.20 to 1.93. Our study obtained the similar result with the best cut off value of AGR being 1.80. More significantly, for the first time, this study assessed the predictive accuracy among albumin, globulin and AGR, and demonstrated that the combination of albumin and globulin did not show any superiority to solely albumin in prognostic discriminatory ability and predictive accuracy. Moreover, since there is no unified optimal cut off value for AGR, it is also impractical in clinical practice. Consequently, we think that it is unnecessary to utilize AGR to predict the prognosis of gastric cancer.
Many limitations constrain this study. Firstly, only retrospective analysis was applied focusing on a single center. Secondly, there are currently still no unified methods to determine the cut off value of AGR. Although many techniques have been proven effective to evaluate the cut off value, including the receiver operating characteristic curve, median value and the software X-tile, different statistical techniques might obtain different cut off points.
In conclusion, although the level of preoperative albumin and AGR value were both significantly related to the prognosis of gastric cancer patients, albumin level is superior to AGR in the prediction of outcomes of gastric cancer.
Abbreviations
AGR, the ratio of albumin to globulin; OS, overall survival; AIC, Akaike information criterion; C-index, Harrell’s concordance index; HR, hazard ratio; CI, confidence interval.
Disclosure
The authors report no conflicts of interest for this study.
References
1. Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40(3):250–260. doi:10.1111/apt.2014.40.issue-3
2. Jung H, Lee HH, Song KY, Jeon HM, Park CH. Validation of the seventh edition of the American Joint Committee on Cancer TNM staging system for gastric cancer. Cancer. 2011;117(11):2371–2378. doi:10.1002/cncr.25778
3. Marrelli D, Morgagni P, de Manzoni G, et al. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255(3):486–491. doi:10.1097/SLA.0b013e3182389b1a
4. McMillan DC. The systemic inflammation-based glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi:10.1016/j.ctrv.2012.08.003
5. Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi:10.1186/1471-2407-13-350
6. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73(3–4):215–220. doi:10.1159/000127412
7. Chen J, Zhou Y, Xu Y, Zhu HY, Shi YQ. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients. Tumour Biol. 2016;37(3):3905–3911. doi:10.1007/s13277-015-3778-3
8. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi:10.1186/1475-2891-9-69
9. Huang R, Ma Y, Holm R, Trope CG, Nesland JM, Suo Z. Sex hormone-binding globulin (SHBG) expression in ovarian carcinomas and its clinicopathological associations. PLoS One. 2013;8(12):e83238. doi:10.1371/journal.pone.0083238
10. Kristal AR, Schenk JM, Song Y, et al. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;168(12):1416–1424. doi:10.1093/aje/kwn272
11. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14(2):113–123. doi:10.1007/s10120-011-0042-4
12. Liu J, Chen S, Geng Q, et al. Prognostic value of pretreatment albumin-globulin ratio in predicting long-term mortality in gastric cancer patients who underwent D2 resection. Onco Targets Ther. 2017;10:2155–2162. doi:10.2147/OTT.S99282
13. Deng J, Zhang R, Wu L, et al. Superiority of the ratio between negative and positive lymph nodes for predicting the prognosis for patients with gastric cancer. Ann Surg Oncol. 2015;22(4):1258–1266. doi:10.1245/s10434-014-4121-8
14. Huitzil-Melendez FD, Capanu M, O’Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28(17):2889–2895. doi:10.1200/JCO.2009.25.9895
15. Singh R, Mishra MK, Aggarwal H. Inflammation, immunity, and cancer. Mediators Inflamm. 2017;2017:6027305. doi:10.1155/2017/6027305
16. Sonnenschein C, Soto AM, Michaelson CL. Human serum albumin shares the properties of estrocolyone-I, the inhibitor of the proliferation of estrogen-target cells. J Steroid Biochem Mol Biol. 1996;59(2):147–154. doi:10.1016/S0960-0760(96)00112-4
17. Lien YC, Hsieh CC, Wu YC, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8(8):1041–1048. doi:10.1016/j.gassur.2004.09.033
18. Alkan A, Koksoy EB, Utkan G. Albumin to globulin ratio, a predictor or a misleader? Ann Oncol. 2015;26(2):443–444. doi:10.1093/annonc/mdu554
19. Pfensig C, Dominik A, Borufka L, Hinz M, Stange J, Eggert M. A new application for albumin dialysis in extracorporeal organ support: characterization of a putative interaction between human albumin and proinflammatory cytokines IL-6 and TNFalpha. Artif Organs. 2016;40(4):397–402. doi:10.1111/aor.12557
20. Anraku M, Shintomo R, Taguchi K, et al. Amino acids of importance for the antioxidant activity of human serum albumin as revealed by recombinant mutants and genetic variants. Life Sci. 2015;134:36–41. doi:10.1016/j.lfs.2015.05.010
21. Rehman MT, Khan AU. Understanding the interaction between human serum albumin and anti-bacterial/anti-cancer compounds. Curr Pharm Des. 2015;21(14):1785–1799. doi:10.2174/1381612821666150304161201
22. Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–389. doi:10.1245/s10434-006-9093-x
23. Alici S, Kaya S, Izmirli M, et al. Analysis of survival factors in patients with advanced-stage gastric adenocarcinoma. Med Sci Monit. 2006;12(5):Cr221–Cr229.
24. Chang WJ, Du Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol. 2014;20(16):4586–4596. doi:10.3748/wjg.v20.i16.4586
25. Toiyama Y, Yasuda H, Ohi M, et al. Clinical impact of preoperative albumin to globulin ratio in gastric cancer patients with curative intent. Am J Surg. 2017;213(1):120–126. doi:10.1016/j.amjsurg.2016.05.012
26. Xue F, Lin F, Yin M, et al. Preoperative albumin/globulin ratio is a potential prognosis predicting biomarker in patients with resectable gastric cancer. Turk J Gastroenterol. 2017;28(6):439–445. doi:10.5152/tjg
27. Shimizu T, Ishizuka M, Shibuya N, et al. Preoperative globulin-to-albumin ratio predicts outcome after curative resection in patients with gastric cancer. Ann Gastroenterol Surg. 2018;2(5):367–375. doi:10.1002/ags3.2018.2.issue-5
28. Mao MJ, Wei XL, Sheng H, et al. Clinical significance of preoperative albumin and globulin ratio in patients with gastric cancer undergoing treatment. Biomed Res Int. 2017;2017:3083267. doi:10.1155/2017/3083267
29. Bozkaya Y, Erdem GU, Demirci NS, et al. Prognostic importance of the albumin to globulin ratio in metastatic gastric cancer patients. Curr Med Res Opin. 2019;35(2):275–282. doi:10.1080/03007995.2018.1479683
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
