Back to Journals » Journal of Pain Research » Volume 16
Prenatal Origins of Endometriosis Pathology and Pain: Reviewing the Evidence of a Role for Low Testosterone
Received 8 September 2022
Accepted for publication 4 January 2023
Published 3 February 2023 Volume 2023:16 Pages 307—316
DOI https://doi.org/10.2147/JPR.S389166
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ellen M Soffin
Bernard J Crespi,1 Susan F Evans2
1Department of Biological Sciences, Simon Fraser University, Burnaby, British Columbia, Canada; 2Adelaide Medical School, School of Medicine, University of Adelaide, Adelaide, South Australia, Australia
Correspondence: Bernard J Crespi, Email [email protected]
Abstract: Endometriosis is a polygenic, estrogen-dependent, inflammatory disorder of uncertain aetiology associated with pain, infertility and reduced quality of life. While the positive association between endometriosis and estrogen is established, a suite of recent studies has demonstrated an inverse association between the presence of endometriosis lesions and levels of testosterone both prenatally and postnatally. The following narrative review provides new insights into the roles of testosterone in the aetiology, diagnosis, and management of endometriosis and associated symptoms, especially pain. A relatively short anogenital distance (AGD) is indicative of lower levels of testosterone during fetal development. A shorter AGD has recently been correlated with both a higher risk of developing endometriosis in adult life, and with known correlates of endometriosis including earlier onset of reproductive cycling, lower ovarian follicle number, lower postnatal testosterone, and premature ovarian insufficiency. During adult life, lower levels of testosterone are positively associated with key comorbidities of endometriosis, including days per month of pelvic pain and increased pain sensitivity. Biochemically, lower levels of testosterone are associated with higher levels of pro-inflammatory IL-1β and lower levels of β-endorphin. In rodents, prenatal administration of testosterone to females reduces their pain sensitivity in adulthood. The emerging convergent links of endometriosis with low prenatal and postnatal testosterone provide evidence of a centrally mediated effect beginning in early prenatal development, and persisting through adult life, with notable effects on pain sensitivity. They generate a novel conceptual framework for understanding, studying and treating this disorder, whereby endometriosis is mediated by a combination of high estrogen in endometrial tissue with low systemic and ovarian testosterone.
Keywords: endometriosis, pain, testosterone, anogenital distance, fetal development
Lay Summary
Endometriosis is a painful reproductive disorder, associated with reduced fertility, that affects 5–15% of women of reproductive age. Its causes are largely unknown, although the important roles of hormones, inflammation and cytokines are recognized. Recent studies indicate that a person’s lifetime risk of developing endometriosis is associated with low levels of testosterone during fetal development. Low levels of testosterone before birth are associated with lower levels of testosterone in adult life, and lower testosterone as an adult is associated with increased days per month of pelvic pain. Optimal levels of testosterone are important for many aspects of female reproduction, including egg development, ovulation, fertilization, and embryo implantation. The finding that low testosterone is associated with an increased risk for endometriosis in adults has important implications. New therapies that normalize testosterone activity without undue side effects may offer ways to reduce the incidence and symptoms of endometriosis in women.
Introduction
Endometriosis is a gynaecological disorder affecting about 10% of women globally that is associated with inflammation and activation of the immune system.1–3 Endometriosis is characterized by the presence of endometrial glands and stroma outside the uterus, most commonly involving the ovaries, pelvic peritoneum, or rectovaginal area.2 It is associated with the presence of pelvic pain and a diminished quality of life in those affected.4 The altered immune environment present within the peritoneal cavity of women with endometriosis includes macrophage activation with increased release of proinflammatory cytokines including interleukin 1, nuclear factor kappa B (NF-κB) and tumor necrosis factor alpha (TNFα).3,5 In most cases, a definitive diagnosis of endometriosis requires visualization of lesions during a laparoscopic surgical procedure, with its associated surgical risks and health economic burden.6
Traditionally, endometriosis has been considered as a disorder predominantly driven by estrogen, while androgens have been regarded as hormones mainly relevant to the physiology of males. However, androgen receptors are also widely distributed in female tissues including the uterus, breasts, endometrium, ovary, brain, bone, and muscle. As such, androgens play essential roles in female reproduction. In addition, androgen levels modulate symptoms of mental and physical well-being including cognitive performance, cardiovascular health and sexual function.7–10 This is established for males but relatively under-researched in females.10
A suite of recent studies has provided novel evidence pointing to low prenatal and postnatal levels of testosterone as contributing factors in the development of endometriosis lesions and many of the diverse traits and symptoms linked to the condition, including its cardinal symptom, pain. In this article, we review and describe this new evidence, explain how it provides a novel and productive framework for understanding the etiology of endometriosis, and discuss implications of these findings for treatment.
Methods
This article provides a hypothesis-driven narrative review describing and evaluating the theory that low testosterone plays key roles in endometriosis risks and symptoms, especially pain. Literature for the review was collected through comprehensive searches that focused on the diverse roles of prenatal and postnatal testosterone in endometriosis and its correlates. Articles were included if they provided insight into the hypothesis that testosterone mediates endometriosis risks and symptoms.
AGD as a Marker for Prenatal Testosterone Effects on Endometriosis Risk
Testosterone regulates sex differentiation and divergence in early human prenatal development.11 The anogenital distance (AGD), measured either from the anus to the posterior fourchette (AGD-AF) or the anus to the clitoral surface (AGD-AC) (Figure 1),12 provides a convenient, easily measurable proxy for androgen levels present during the early “programming window” of fetal development in both sexes. Perineal growth at this time is caused by androgen-mediated caudal movement of the genital tubercle.12 Higher androgen levels result in greater AGD length, and lower levels result in shorter AGD length. This finding is consistent with the approximately twofold longer AGD in males compared to females.13
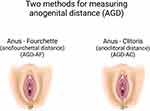 |
Figure 1 Depiction of measurements used for the two metrics of anogenital distance, in women. |
Increasing evidence over recent years, from both animal and human studies, demonstrates that a relatively short AGD is strongly associated with the presence of endometriosis and its clinical correlates. In humans across several study populations, AGD-AC, AGD-AF, or both were significantly shorter among women with endometriosis than among matched controls.14–19 These AGD differences are substantial and in some circumstances predictive; for example, Mendiola et al14 computed an odds ratio of 41.6 (p = 0.002) for deep infiltrating endometriosis in women with AGD below the mean, compared to women with AGD above the mean. Crestani et al18 reported, for endometriosis as a whole, a diagnostic specificity of 0.98 and a positive predictive value of 0.97, for an MRI-measured AGD-AF length of less than 20-mm. By contrast, a recent study20 found a lack of significant difference, perhaps in part because sample sizes were relatively small. These studies are summarized in the systemic review of AGD and its relationship to endometriosis and polycystic ovarian syndrome by Pan et al.21
A shorter AGD has also been associated with several correlates of endometriosis, including low relative fertility. These correlates include lower levels of anti‐Müllerian hormone (AMH) in subjects without endometriosis undergoing in vitro fertility treatment22 and the presence of premature ovarian insufficiency.23 Moreover, among 100 typical, college-aged premenopausal women, shorter AGD has been associated with lower serum testosterone,24 lower ovarian follicle number,25 and more-regular menstrual cycles in their mothers.26 These studies raise the possibility of phenotypic consequences of low fetal testosterone beyond the presence or absence of endometriosis lesions.
Although the process of menstruation is mainly restricted to primates, the link between a low-androgen fetal environment, a short AGD, and reproductive effects is also supported by non-primate animal models. While in utero, the exposure of a female fetus to testosterone is affected by the presence or absence of adjacent male siblings and their higher levels of testosterone production. Female laboratory mice with no adjacent male fetuses, and thus with lower exposure to prenatal testosterone, exhibit shorter AGDs, lower postnatal serum testosterone, earlier vaginal opening, and shorter and more regular cycles, when compared with females flanked by one or two males in utero (reviewed in Crespi and Dinsdale27). Similarly, female rats subject to lower testosterone in utero exhibit shorter AGDs, earlier vaginal opening, and shorter estrus cycles. In Mongolian gerbils, female fetuses flanked by either one or no males while in utero had lower postnatal adult serum testosterone, earlier onset of estrus and shorter cycles than female fetuses that were flanked by two males in utero.27 These results parallel many of the patterns observed clinically among women with endometriosis, who, in addition to exhibiting shorter AGDs, also show evidence of earlier menarche, lower postnatal serum testosterone, and shorter, more regular menstrual cycles than controls.21,28–30 With regard to obstetric outcomes, Berlanda et al found a higher rate of preterm delivery in women with endometriosis that conceived naturally, when compared to women without endometriosis.31
In contrast to these results for endometriosis, women with polycystic ovary syndrome (PCOS), a disorder characterized by multifollicular ovaries, oligomenorrhea, and a relatively higher serum testosterone in adult life than controls, manifest multiple clinical features that are the opposite to those experienced by women with endometriosis.21,27,32 Women with PCOS exhibit longer AGDs, increased AMH, lower sex hormone binding globulin (SHBG), elevated luteinizing hormone (LH), increased waist to hip ratios (WHR), and increased body mass index (BMI) when compared to controls.21,32 Interpretation of these comparisons is complicated by the presence of both endometriosis and PCOS within some individuals.33 However, as the prevalence of both conditions is multifactorial, additional factors may be present. For example, prolonged medical management of PCOS using hormonal therapies to induce more regular menstruation may increase exposure of the pelvic peritoneum to menstrual fluid and the enhanced development of endometriosis lesions. Systemic hormonal therapies may reduce androgen effect through the induction of SHBG in the liver and increased androgen protein binding. In addition, the development of endometriosis lesions may be facilitated by the chronic low-grade inflammation present in PCOS women with increased BMI.
Reduced Androgen Levels as Outcomes of Genetic and Epigenetic Factors in Women with Endometriosis
Genetic factors mediate approximately 50% of the risk of developing endometriosis.34 Genetic risk is mediated by the effects of many alleles each of small effect. Recent GWAS meta-analysis of 17,045 women with endometriosis and 191,596 controls has documented five novel genome-wide significant SNPs, that are enriched for a set of genes (including FN1, CCDC170, ESR1, SYNE1 and FSHB) involved in steroid hormone levels and activities.35 Of these genes, the FSHB gene is of particular interest, because the haplotype (genetic region) conferring higher endometriosis risk has also been significantly associated with lower serum testosterone, lower luteinizing hormone, heavier menstruations, shorter menstrual cycles, earlier menarche and earlier menopause, all of which characterize the endometriosis phenotype as noted above.30,36 This haplotype also confers a lower risk of PCOS.37
More broadly, Mendelian Randomization analyses using endometriosis GWAS data can be used to help uncover the causal, genetic bases of the clinical correlates of endometriosis. These analyses have shown that endometriosis shares causal genetic risk factors with early menarche, shorter menstrual cycles, lower WHR, lower BMI, and lower levels of AMH.38 As described above, lower AMH is independently associated with a shorter AGD among women (without endometriosis or PCOS) undergoing in vitro fertilization22; AMH levels are also positively correlated with serum testosterone among reproductive-age women,39,40 and BMI is positively associated with serum testosterone among premenopausal women without reproductive disorders.41,42 Taken together with the data on AGD, these findings provide evidence that low testosterone and its correlates are involved in the genetic and developmental basis of endometriosis.
Low Androgens as a Consequence of Prenatal Endocrine Disruption
Endocrine disrupting substances, including organochlorines, with anti-androgenic or pro-estrogenic properties are ubiquitous in modern life and influence the risk of many disorders, including endometriosis.43,44 However, endometriosis has existed throughout history, prior to the development of these chemicals, and not all women with endometriosis have been exposed to high levels of organochlorines during their lifetime. Prenatal factors or factors present during the menstrual years of her mother may also not be present during the life of the woman affected by endometriosis. For example, prenatal exposure to the potent synthetic estrogen diethylstilbestrol (DES) notably increases endometriosis risk in offspring.45
Prenatal exposure to the anti-androgenic chemical bisphenol A (BPA), results in shorter AGDs among female rats,46 and, in mice, it has been linked with earlier first estrus and the production of endometriosis-like lesions.47 In humans, prenatal BPA exposure in the first trimester leads to shorter AGD in daughters.48
Prenatal exposure prenatally to phthalates, a set of mainly anti-androgenic agents, has been associated with shorter AGD in both mice and humans.49 In contrast, a recent meta-analysis demonstrated only low-level associations between elevated phthalates and the presence of endometriosis in adult women,50 although this research did not consider prenatal phthalate exposures. These findings support the relative importance of prenatal rather than postnatal exposure in the genesis of endometriosis lesions.
The Relationship Between Low Androgens, Pain and Pain-Related Symptoms in Women
Pain sensitivity is higher among women with endometriosis than in healthy controls.51,52 However, while endometriosis lesions are associated with pain and pain-related symptoms in the majority of affected women, there is no consistent relationship between the severity of endometriosis lesions and the severity of pain.53 Additional factors across the biopsychosocial spectrum influence the pain experience, and multiple lines of evidence across both preclinical and human studies support increased chronic pain symptoms with reduced levels of testosterone.
For example, pain sensitivity is higher among women than men.51,52 Female sensitivity to pain varies with the menstrual cycle and correlates more closely with levels of the anti-nociceptive testosterone than levels of the pro-nociceptive estradiol.54 Women with dysmenorrhea-related pelvic pain demonstrate strong inverse correlations between measures of chronic pain and androgen levels, especially for the correlation between days per month of pelvic pain and the free androgen index which measures the unbound fraction of testosterone within blood.55,56
Experimental and observational studies in humans and animal models have consistently shown that treatment with testosterone can ameliorate chronic pain in a proportion of individuals. Female-to-male transsexuals report lower levels of chronic pain following hormonal transition using testosterone therapy, while male-to-female transsexuals report increased pain.57 Testosterone therapy has been used to reduce pain in opioid-induced chronic pain58 and fibromyalgia,59 both centrally mediated pain conditions. Preclinically, an experimental study of female rats exposed prenatally to testosterone showed reduced pain responses in adult life that were similar to those of males.60 This latter study is especially important because it establishes the key importance of prenatal, organizational effects of testosterone on adult pain sensitivity, and leads to the prediction that shorter anogenital distance in humans, as a metric of lower prenatal testosterone, should be associated with higher sensitivity to pain.
A potential mechanism for the pain-modulating effect of androgens on chronic pain is via their inverse association with levels of systemic immune-based inflammation.61 Androgens are considered to be generally immunosuppressive, resulting in decreased T- and B-cell proliferation, and decreased immunoglobulin and cytokine production.62 A higher prevalence of autoimmune disorders is found in females when compared to males,63 in males or females with low androgen levels64–66 and in women with endometriosis.67–69 Testosterone has been shown to reduce inflammation, as measured by levels of the pro-inflammatory cytokine IL-1β and pain symptoms in patients with rheumatoid arthritis.
An additional mechanism for the effects of testosterone on pain may be through their links with β-endorphin levels within the central nervous system. Pluchino et al70 showed that testosterone administration increased β-endorphin levels in the brains and plasma of ovariectomized rats. In human studies, pain sensitivity in women with endometriosis has been associated with lower levels of β-endorphins;53 whereas PCOS is associated with higher levels of β-endorphins.71–74 Although some studies have been inconclusive,75,76 the opioid antagonist naltrexone is effective in reducing endocrine symptoms in women with PCOS,77–79 also suggesting that high β-endorphin levels are a feature of this condition. Taken together, the studies described above provide diverse evidence suggesting that the pain symptoms of endometriosis may derive, at least in part, from low testosterone, through both prenatal and postnatal effects.
Androgens and Fertility in Women with Endometriosis
Optimal levels of serum and ovarian androgens, especially testosterone, play key roles in additional aspects of female reproductive development, functions and disease.8,9 Testosterone is essential for folliculogenesis,80 and low levels of testosterone are associated with both apoptosis of follicular granulosa cells,81 and reduced implantation success of embryos.82
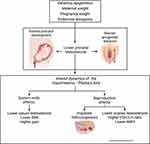
A shorter AGD, representing low androgen levels during fetal development, is directly related to a set of fertility correlates including higher risk of premature ovarian insufficiency,23,83 and poor ovarian response to controlled ovarian stimulation,22 consistent with roles in folliculogenesis. Moreover, by a recent meta-analysis, testosterone therapy increased IVF success in women with a poor response to ovarian stimulation.84 These convergent results suggest that low prenatal testosterone, with sequelae that include altered programming of the hypothalamic-pituitary-ovarian axis during fetal development, and consequent reduced post-natal testosterone levels and higher levels of pain, results in a broad set of sexually dimorphic traits that may partially underlie the reduced fertility found in women with endometriosis (Figure 2).
Currently, the best-known correlates of low amniotic fluid testosterone among female fetuses are young maternal age, low weight gain during pregnancy, and low amniotic fluid cortisol; these factors together account for 64% of variation in testosterone level.85 However, the relationship between amniotic fluid testosterone and fetal AGD remains unknown.
Translational Implications for Research and Treatment
Probably the most important long-term implication of the recent findings that link endometriosis with low prenatal testosterone is that they begin to establish endometriosis as a developmental condition, within the general paradigm of DoHAD, the developmental origins of health and adult disease.86 A DoHAD framework for studying and treating endometriosis compels a focus on early-developmental programming of the HPO axis by testosterone, in the context of KNDy neuron activity and pulsatile secretion patterns of GnRH (eg, Cernea et al87). This paradigm is relatively well developed with regard to PCOS, especially by the development of animal models that recapitulate major features of the disease in humans through experimental increases in prenatal testosterone.88 In principle, comparable animal models can be developed for the analysis of endometriosis, built around the downstream effects of reduced testosterone during early prenatal development. Importantly, the DoHAD paradigm has yet to address prenatal programming of pain sensitivity, despite its importance in disease and well-being.
Risal et al89 and Parker et al90 recently published on the transgenerational inheritance of PCOS, a condition with genetic pre-disposition but potential for amplification by the developmental environment: in utero exposure to elevated testosterone levels. The risk of endometriosis, a condition also showing a strong genetic predisposition, appears, in contrast, to be augmented by exposure to relatively low testosterone levels in utero. These findings provide the potential for alleviation of endometriosis among high-risk individuals, through modulation of factors that mediate levels of testosterone and other androgens during prenatal as well as postnatal development.
The potential to influence a woman’s androgen levels offers the possibility of reduced pain symptoms, improved fertility and reduced lesions. In 1971, the synthetic androgen danazol was the first drug approved for treating endometriosis.91 This treatment was effective in reducing pain among about 90% of the women with endometriosis.92 However, as a proportion of women experienced androgenic adverse effects, and with the advent of GnRH analogue drugs, the focus of treatment moved from administration of androgens to suppression of estrogen. The recent resurgence of interest in roles for testosterone as treatment for chronic pain and infertility, with its relatively advantageous hormonal profile when compared with danazol, suggests that a new, more nuanced role for androgen therapy in a proportion of women with endometriosis or associated pain-related symptoms may be possible.55,56 Navigating a path forward with menstrual suppression, reduced estrogen effects, enhanced androgen effects, and minimization of intralesional conversion of testosterone to estradiol via the enzyme aromatase will require innovative developments in hormonal therapeutics.
Conclusions and Future Questions
The links described here between a shorter anogenital distance and an individual woman’s risk of endometriosis immediately raise a number of key questions. Do women with endometriosis with shorter AGDs consistently show lower serum or ovarian testosterone? Do they experience higher levels of pain? Is lower testosterone in mothers a risk factor for endometriosis and a shorter AGD in their daughters, in the same general way that longer AGD, and higher prenatal testosterone, have been shown to mediate transgenerational effects on risk for PCOS?89 Could modification of lifestyle-related causes of variation in prenatal testosterone levels reduce risk of developing endometriosis lesions during adult life? And might high levels of pain in many autoimmune conditions,93 which are strongly female biased in prevalence and have been linked with low adult testosterone (eg, Tomassini et al94), also be linked with prenatal testosterone?
Additional studies using rodent models of endometriosis might usefully analyze the endocrine correlates of an especially low AGD in more detail. Do short-AGD females of such species show increased pain and inflammation, and more-pronounced development of ectopic endometrial implants than those with higher AGD? Do they show alterations to KNDy neurons, and GnRH-system regulation, that could provide new insights into the developmental predispositions and neuroendocrine origins of this disorder?95 The new perspectives described here are highly predictive and should help guide both laboratory research and clinical studies in developing better ways to alleviate endometriosis.
Acknowledgments
We thank Miriam Dodd for artistic assistance, and Natalie Dinsdale for her role in the development of this work.
Author Contributions
All authors made significant contributions to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
We thank the Natural Sciences and Engineering Council of Canada for support (NSERC Discovery Grant 2019-02408).
Disclosure
Dr Susan Evans reports that she is a minor shareholder of Havah therapeutics which is commercialising a testosterone product for use in breast cancer treatment. The authors report no conflicts of interest in this work.
References
1. Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev. 2019;40(4):1048–1079. doi:10.1210/er.2018-00242
2. Saunders PT, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184(11):2807–2824. doi:10.1016/j.cell.2021.04.041
3. Donnez J, Cacciottola L. Endometriosis: an inflammatory disease that requires new therapeutic options. Int J Mol Sci. 2022;23(3):1518. doi:10.3390/ijms23031518
4. Facchin F, Barbara G, Saita E, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol. 2015;36(4):135–141. doi:10.3109/0167482X.2015.1074173
5. Hogg C, Horne AW, Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol. 2020;11:7. doi:10.3389/fendo.2020.00007
6. D’Alterio MN, Saponara S, D’Ancona G, et al. Role of surgical treatment in endometriosis. Minerva Obstet Gynecol. 2021;73(3):317–332. doi:10.23736/S2724-606X.21.04737-7
7. Davis SR, Wahlin-Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. 2015;3(12):980–992. doi:10.1016/S2213-8587(15)00284-3
8. Walters KA, Paris VR, Aflatounian A, Handelsman DJ. Androgens and ovarian function: translation from basic discovery research to clinical impact. J Endocrinol. 2019;242(2):R23–R50. doi:10.1530/JOE-19-0096
9. Gibson DA, Simitsidellis I, Collins F, Saunders PT. Androgens, oestrogens and endometrium: a fine balance between perfection and pathology. J Endocrinol. 2020;246(3):R75–R93. doi:10.1530/JOE-20-0106
10. Bianchi VE, Bresciani E, Meanti R, Rizzi L, Omeljaniuk RJ, Torsello A. The role of androgens in women’s health and wellbeing. Pharmacol Res. 2021;171:105758. doi:10.1016/j.phrs.2021.105758
11. Rey R, Josso N, Racine C. Sexual differentiation. In: Feingold KR, Anawalt B, Boyce A, editors. Endotext [Internet]. MDText.com, Inc.; 2020. Available from:: https://www.ncbi.nlm.nih.gov/books/NBK279001/.
12. Thankamony A, Pasterski V, Ong KK, Acerini CL, Hughes IA. Anogenital distance as a marker of androgen exposure in humans. Andrology. 2016;4(4):616–625. doi:10.1111/andr.12156
13. Singal AK, Jain VG. Maternal and infant characteristics influencing the anogenital distance and penile length in newborns. Andrologia. 2016;48(6):708–713. doi:10.1111/and.12507
14. Mendiola J, Sánchez-Ferrer ML, Jiménez-Velázquez R, et al. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum Reprod. 2016;31(10):2377–2383. doi:10.1093/humrep/dew163
15. Sánchez-Ferrer ML, Mendiola J, Jiménez-Velázquez R, et al. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod BioMed Online. 2017;34(4):375–382. doi:10.1016/j.rbmo.2017.01.002
16. Sánchez‐Ferrer ML, Jiménez‐Velázquez R, Mendiola J, et al. Accuracy of anogenital distance and anti‐Müllerian hormone in the diagnosis of endometriosis without surgery. Int J Gynecol Obstet. 2019;144(1):90–96. doi:10.1002/ijgo.12691
17. Peters HE, Laeven CH, Trimbos CJ, et al. Anthropometric biomarkers for abnormal prenatal reproductive hormone exposure in women with Mayer-Rokitanksy-Küster-Hauser syndrome, polycystic ovary syndrome, and endometriosis. Fertil Steril. 2020;114(6):1297–1305. doi:10.1016/j.fertnstert.2020.06.029
18. Crestani A, Arfi A, Ploteau S, et al. Anogenital distance in adult women is a strong marker of endometriosis: results of a prospective study with laparoscopic and histological findings. Hum Reprod Open. 2020;2020(3):hoaa023. doi:10.1093/hropen/hoaa023
19. Crestani A, Abdel Wahab C, Arfi A, et al. A short anogenital distance on MRI is a marker of endometriosis. Hum Reprod Open. 2021;2021(1):hoab003. doi:10.1093/hropen/hoab003
20. Buggio L, Somigliana E, Sergenti G, Ottolini F, Dridi D, Vercellini P. Anogenital distance and endometriosis: results of a case–control study. Reprod Sci. 2022;1–8. doi:10.1007/s43032-022-01009-7
21. Pan Z, Zhu F, Zhou K. A systematic review of anogenital distance and gynecological disorders: endometriosis and polycystic ovary syndrome. Front Endocrinol. 2021;12. doi:10.3389/fendo.2021.696879
22. Fabregues F, González-Foruria I, Peñarrubia J, Carmona F. Ovarian response is associated with anogenital distance in patients undergoing controlled ovarian stimulation for IVF. Hum Reprod. 2018;33(9):1696–1704. doi:10.1093/humrep/dey244
23. Dural O, Kurbanova T, Yasa C, et al. Idiopathic primary ovarian insufficiency is associated with anogenital distance, a marker for prenatal environment. Eur J Obstet Gynecol Reprod Biol. 2021;258:304–308. doi:10.1016/j.ejogrb.2021.01.017
24. Mira-Escolano MP, Mendiola J, Mínguez-Alarcón L, et al. Anogenital distance of women in relation to their mother’s gynaecological characteristics before or during pregnancy. Reprod BioMed Online. 2014a;28(2):209–215. doi:10.1016/j.rbmo.2013.09.026
25. Mendiola J, Roca M, Mínguez-Alarcón L, et al. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environ Health. 2012;11(1):1–8. doi:10.1186/1476-069X-11-90
26. Mira‐Escolano MP, Mendiola J, Mínguez‐Alarcón L, et al. Longer anogenital distance is associated with higher testosterone levels in women: a cross‐sectional study. BJOG. 2014b;121(11):1359–1364. doi:10.1111/1471-0528.12627
27. Crespi B, Dinsdale NL. The sexual selection of endometriosis. Evol Behav Sci. 2021. doi:10.1037/ebs0000275
28. Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–5. doi:10.1016/j.bpobgyn.2018.06.001
29. Ponomarenko I, Reshetnikov E, Polonikov A, et al. Candidate genes for age at menarche are associated with endometriosis. Reprod BioMed Online. 2020;41(5):943–956. doi:10.1016/j.rbmo.2020.04.016
30. Dinsdale N, Nepomnaschy P, Crespi B. The evolutionary biology of endometriosis. Evol Med Public Health. 2021;9(1):174–191. doi:10.1093/emph/eoab008
31. Berlanda N, Alio W, Angioni S, et al. Impact of endometriosis on obstetric outcome after natural conception: a multicenter Italian study. Arch Gynecol Obstet. 2022;305(1):149–157. doi:10.1007/s00404-021-06243-z
32. Dinsdale NL, Crespi BJ. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol Appl. 2021;14(7):1693–1715. doi:10.1111/eva.13244
33. Holoch KJ, Moorhead A, Miller PB, Higdon HL, Likes CE, Lessey BA. Endometriosis in women with polycystic ovary syndrome (PCOS) and its role in poor reproductive outcomes. Fertil Steril. 2011;96(3):S133. doi:10.1016/j.fertnstert.2011.07.518
34. Zondervan KT, Rahmioglu N, Morris AP, et al. Beyond endometriosis genome-wide association study: from genomics to phenomics to the patient. Semin Reprod Med. 2016;34(04):242–254. doi:10.1055/s-0036-1585408
35. Sapkota Y, Steinthorsdottir V, Morris AP, et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8(1):1–12. doi:10.1038/ncomms15539
36. McGrath IM, Mortlock S, Montgomery GW. Genetic regulation of physiological reproductive lifespan and female fertility. Int J Mol Sci. 2021;22(5):2556. doi:10.3390/ijms22052556
37. Hong SH, Hong YS, Jeong K, Chung H, Lee H, Sung YA. Relationship between the characteristic traits of polycystic ovary syndrome and susceptibility genes. Sci Rep. 2020;10(1):1–8. doi:10.1038/s41598-020-66633-2
38. Garitazelaia A, Rueda-Martínez A, Arauzo R, et al. A systematic two-sample Mendelian randomization analysis identifies shared genetic origin of endometriosis and associated phenotypes. Life. 2021;11(1):24. doi:10.3390/life11010024
39. Pinola P, Morin-Papunen LC, Bloigu A, et al. Anti-Müllerian hormone: correlation with testosterone and oligo-or amenorrhoea in female adolescence in a population-based cohort study. Hum Reprod. 2014;29(10):2317–2325. doi:10.1093/humrep/deu182
40. Islam RM, Bell RJ, Skiba MA, Davis SR. Testosterone and androstenedione are positively associated with anti‐Müllerian hormone in premenopausal women. Clin Endocrinol. 2021;95(5):752–759. doi:10.1111/cen.14592
41. Sidhu S, Parikh T, Burman KD. Endocrine changes in obesity. In: Feingold KR, Anawalt B, Boyce A, editors. Endotext [Internet]. MDText.com, Inc.; 2000. Available from:: https://www.ncbi.nlm.nih.gov/books/NBK279053/.
42. Stanikova D, Zsido RG, Luck T, et al. Testosterone imbalance may link depression and increased body weight in premenopausal women. Transl Psychiatry. 2019;9(1):1–2. doi:10.1038/s41398-019-0487-5
43. Heilier J-F, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case–control study. Environ Res. 2007;103(1):121–129. doi:10.1016/j.envres.2006.04.004
44. Porpora MG, Medda E, Abballe A, et al. Endometriosis and organochlorinated environmental pollutants: a case-control study on Italian women of reproductive age. Environ Health Perspect. 2009;117(7):1070–1075. doi:10.1289/ehp.0800273
45. Ottolina J, Schimberni M, Makieva S, et al. Early-life factors, in-utero exposures and endometriosis risk: a meta-analysis. Reprod Bio Med. 2020;41(2):279–289. doi:10.1016/j.rbmo.2020.04.005
46. Christiansen S, Axelstad M, Boberg J, Vinggaard AM, Pedersen GA, Hass U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction. 2014;147(4):477–487. doi:10.1530/REP-13-0377
47. Pivonello C, Muscogiuri G, Nardone A, et al. Bisphenol A: an emerging threat to female fertility. Reprod Biol Endocrinol. 2020;18(1):1–33. doi:10.1186/s12958-019-0558-8
48. Barrett ES, Sathyanarayana S, Mbowe O, et al. First-trimester urinary bisphenol A concentration in relation to anogenital distance, an androgen-sensitive measure of reproductive development, in infant girls. Environ Health Perspect. 2017;125(7):077008. doi:10.1289/EHP875
49. Swan SH, Kristensen DM. Anogenital distance: a marker of steroidal endocrine disruption. Andrology. 2018;30:963b72. doi:10.1016/B978-0-12-801238-3.64379-9
50. Cai W, Yang J, Liu Y, Bi Y, Wang H. Association between phthalate metabolites and risk of endometriosis: a meta-analysis. Int J Environ Res Public Health. 2019;16(19):3678. doi:10.3390/ijerph16193678
51. van Aken M, Oosterman J, van Rijn T, et al. Experimental pain tolerance is decreased and independent of clinical pain intensity in patients with endometriosis. Fertil Steril. 2018;110(6):1118–1128. doi:10.1016/j.fertnstert.2018.06.040
52. Casale R, Atzeni F, Bazzichi L, et al. Pain in women: a perspective review on a relevant clinical issue that deserves prioritization. Pain Ther. 2021;10(1):287–314. doi:10.1007/s40122-021-00244-1
53. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266–271. doi:10.1093/humrep/del339
54. Bartley EJ, Palit S, Kuhn BL, et al. Natural variation in testosterone is associated with hypoalgesia in healthy women. Clin J Pain. 2015;31(8):730–739. doi:10.1097/AJP.0000000000000153
55. Evans SF, Hull ML, Hutchinson MR, Rolan PE. Androgens, endometriosis and pain. Front Reprod Health. 2021a;3:792920. doi:10.3389/frph.2021.792920
56. Evans SF, Kwok Y, Solterbeck A, et al. The relationship between androgens and days per month of period pain, pelvic pain, headache, and TLR4 responsiveness of peripheral blood mononuclear cells in young women with dysmenorrhoea. J Pain Res. 2021b;14:585. doi:10.2147/JPR.S279253
57. Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007;132(Supplement 1):S60–S67. doi:10.1016/j.pain.2007.02.006
58. Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Physician. 2012;15(3S):ES145.
59. White HD, Robinson TD. A novel use for testosterone to treat central sensitization of chronic pain in fibromyalgia patients. Int Immunopharmacol. 2015;27(2):244–248. doi:10.1016/j.intimp.2015.05.020
60. Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300(2):695–701. doi:10.1124/jpet.300.2.695
61. García-Gómez E, Vázquez-Martínez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbón M. Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front Endocrinol. 2020;10:935. doi:10.3389/fendo.2019.00935
62. Ben-Batalla I, Vargas-Delgado ME, von Amsberg G, Janning M, Loges S. Influence of androgens on immunity to self and foreign: effects on immunity and cancer. Front Immunol. 2020;11:1184. doi:10.3389/fimmu.2020.01184
63. Klein S, Flanagan K. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi:10.1038/nri.2016.90
64. Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann NY Acad Sci. 2002;966(1):131–142. doi:10.1111/j.1749-6632.2002.tb04210.x
65. Dane Ş, Timur H. Sex-related differences in tuberculin reaction, free and total testosterone concentrations in patients with autoimmune disorders and controls. Int J Neurosci. 2005;115(6):911–916. doi:10.1080/00207450590882190
66. Benagiano M, Bianchi P, D’Elios MM, Brosens I, Benagiano G. Autoimmune diseases: role of steroid hormones. Best Pract Res Clin Obstet Gynaecol. 2019;60:24–34. doi:10.1016/j.bpobgyn.2019.03.001
67. Zhang T, De Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. 2018;17(10):945–955. doi:10.1016/j.autrev.2018.03.017
68. Shigesi N, Kvaskoff M, Kirtley S, et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(4):486–503. doi:10.1093/humupd/dmz014
69. Porpora MG, Scaramuzzino S, Sangiuliano C, et al. High prevalence of autoimmune diseases in women with endometriosis: a case-control study. Gynecol Endocrinol. 2020;36(4):356–359. doi:10.1080/09513590.2019.1655727
70. Pluchino N, Ninni F, Casarosa E, et al. Sex differences in brain and plasma β-endorphin content following testosterone, dihydrotestosterone and estradiol administration to gonadectomized rats. Neuroendocrinology. 2009;89(4):411–423. doi:10.1159/000209506
71. Wortsman JA, Wehrenberg WB, Gavin JR, Allen JP. Elevated levels of plasma beta-endorphin and gamma 3-melanocyte stimulating hormone in the polycystic ovary syndrome. Obstet Gynecol. 1984;63(5):630–634.
72. Aleem FA, Eltabbakh GH, Omar RA, Southren AL. Ovarian follicular fluid β-endorphin levels in normal and polycystic ovaries. Am J Obstet Gynecol. 1987;156(5):1197–1200. doi:10.1016/0002-9378(87)90143-8
73. Carmina E, Ditkoff EC, Malizia G, Vijod AG, Janni A, Lobo RA. Increased circulating levels of immunoreactive β-endorphin in polycystic ovary syndrome is not caused by increased pituitary secretion. Am J Obstet Gynecol. 1992;167(6):1819–1824. doi:10.1016/0002-9378(92)91781-5
74. Kialka M, Milewicz T, Mrozinska S, Sztefko K, Rogatko I, Majewska R. Pressure pain threshold and [beta]-endorphins plasma level are higher in lean polycystic ovary syndrome women. Endocr Abstr. 2016;41. doi:10.1530/endoabs.41.EP751
75. Martinez-Guisasola J, Ferrer J, Guerrero M, et al. Circulating levels of immunoreactive β-endorphin in polycystic ovary syndrome. Gynecol Endocrinol. 1999;13(1):26–35. doi:10.1080/09513599909167528
76. Jaschke N, Lunger F, Wildt L, Seeber B. Beta endorphin in serum and follicular fluid of PCOS-and non-PCOS women. Arch Gynecol Obstet. 2018;298(1):217–222. doi:10.1007/s00404-018-4793-6
77. Fruzzetti F, Bersi C, Parrini D, Ricci C, Genazzani AR. Effect of long-term naltrexone treatment on endocrine profile, clinical features, and insulin sensitivity in obese women with polycystic ovary syndrome. Fertil Steril. 2002;77(5):936–944. doi:10.1016/S0015-0282(02)02955-2
78. Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod. 2008;23(11):2564–2569. doi:10.1093/humrep/den273
79. Boettcher B, Seeber B, Leyendecker G, Wildt L. Impact of the opioid system on the reproductive axis. Fertil Steril. 2017;108(2):207–213. doi:10.1016/j.fertnstert.2017.06.009
80. Duan H, Ge W, Yang S, et al. Dihydrotestosterone regulates oestrogen secretion, oestrogen receptor expression, and apoptosis in granulosa cells during antral follicle development. J Steroid Biochem Mol Biol. 2021;207:105819. doi:10.1016/j.jsbmb.2021.105819
81. Ono YJ, Tanabe A, Nakamura Y, et al. A low-testosterone state associated with endometrioma leads to the apoptosis of granulosa cells. PLoS One. 2014;9(12):e115618. doi:10.1371/journal.pone.0115618
82. Huang L, Chen M, Long L, et al. Low basal serum testosterone level is detrimental to the embryo implantation in the patients with severe endometriosis. J Obstet Gynaecol Res. 2021;47(6):2166–2174. doi:10.1111/jog.14791
83. Soman M, Huang LC, Cai WH, et al. Serum androgen profiles in women with premature ovarian insufficiency: a systematic review and meta-analysis. Menopause. 2019;26(1):78. doi:10.1097/GME.0000000000001161
84. Noventa M, Vitagliano A, Andrisani A, et al. Testosterone therapy for women with poor ovarian response undergoing IVF: a meta-analysis of randomized controlled trials. J Assist Reprod Genet. 2019;36(4):673–683. doi:10.1007/s10815-018-1383-2
85. Kallak TK, Hellgren C, Skalkidou A, et al. Maternal and female fetal testosterone levels are associated with maternal age and gestational weight gain. Eur J Endocrinol. 2017;177(4):379–388. doi:10.1530/EJE-17-0207
86. Ho SM, Cheong A, Adgent MA, et al. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod Toxicol. 2017;68:85–104. doi:10.1016/j.reprotox.2016.07.011
87. Cernea M, Padmanabhan V, Goodman RL, Coolen LM, Lehman MN. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156(9):3277–3291. doi:10.1210/en.2014-1609
88. Stener-Victorin E, Padmanabhan V, Walters KA, et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):bnaa010. doi:10.1210/endrev/bnaa010
89. Risal S, Pei Y, Lu H, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894–1904. doi:10.1038/s41591-019-0666-1
90. Parker J, O’Brien C, Gersh FL. Developmental origins and transgenerational inheritance of polycystic ovary syndrome. Aust N Z J Obstet Gynaecol. 2021;61(6):922–926. doi:10.1111/ajo.13420
91. Ashfaq S, Can AS. Danazol. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564344/.
92. Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short-term and long-term effectiveness. Am J Obstet Gynecol. 1981;139(6):645–654. doi:10.1016/0002-9378(81)90478-6
93. Mifflin KA, Kerr BJ. Pain in autoimmune disorders. J Neurosci Res. 2017;95(6):1282–1294. doi:10.1002/jnr.23844
94. Tomassini V, Onesti E, Mainero C, et al. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry. 2005;76(2):272–275. doi:10.1136/jnnp.2003.033324
95. Nagae M, Uenoyama Y, Okamoto S, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci USA. 2021;118(5):e2009156118. doi:10.1073/pnas.2009156118
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.