Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Preliminary Research of Main Components of Dll4/ Notch-VEGF Signaling Pathway Under High-Glucose Stimulation in vitro
Authors Gao N, Xiao L, Tao Z, Zheng Y, Wang W, Huang H
Received 28 December 2021
Accepted for publication 10 April 2022
Published 18 April 2022 Volume 2022:15 Pages 1165—1171
DOI https://doi.org/10.2147/DMSO.S355004
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Na Gao,1,* Linghui Xiao,1,* Zheng Tao,2 Yanlin Zheng,1 Wanjie Wang,1 Hui Huang1
1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan Province, People’s Republic of China; 2Eye College, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Huang, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-er-qiao Road, Jinniu District, Chengdu, 610072, Sichuan Province, People’s Republic of China, Tel +86-18782917219, Fax +86-28-87732407, Email [email protected]
Purpose: To establish a high-glucose (HG) stressed cell model and study the expression of main components of the Dll4/Notch-VEGF signaling pathway under high-glucose stimulation.
Methods: A model of HG-conditioned cells (human umbilical vein endothelial cells, HUVECs) was first established, and then the expression of Dll4, Notch1, Notch4 and VEGF in HG-stressed cells with or without Notch pathway blockage was analyzed by RT-PCR and Western blot. To observe cell migration, we also evaluated the Transwell assay.
Results: HUVECs stimulated with 30mmol/L HG was selected as a cell model. RT-PCR and Western blot results showed that HG stimulation induced the expression of Dll4, Notch1 and VEGF and downregulated Notch4. The expressions were reversed after Notch pathway blockage; meanwhile, the blockage of Notch pathway inhibited cell migration under HG condition.
Conclusion: The function of Notch4 in responses to HG stimulation deserves further researching. Combination therapy by blocking Dll4/Notch and VEGF pathways may provide us with a new way for anti-neovascularization.
Keywords: Dll4, Notch1, Notch4, VEGF, endothelial cells
Introduction
Vascular endothelial growth factor (VEGF) and its related cytokines signaling pathway play a critical role in neovascularization in diabetic retinopathy (DR).1,2 Despite the significant efficacy of anti-VEGF in clinical trials, the recurrence of neovascularization and non-sensitivity of anti-VEGF in some cases suggests a challenge for searching other drug targets on the pathogenesis of angiogenesis.3,4
The Notch pathway is considered vital for vascular morphogenesis and remodeling.5,6 The Notch family consists of four Notch receptors–Notch1, Notch2, Notch3 and Notch4, and five ligands–Jagged1, Jagged2, Delta-like ligand Dll1, Dll3, Dll4. Among them, the Dll4 ligand, Notch1 and Notch4 are specifically expressed in the vascular system. Dll4 is mainly restricted to the endothelial cells and a key ligand for Notch signaling pathway activation,7–9 which may control vascular branching and vessel density through participating in the differentiation, induction and selection of endothelial tip cells.10,11 Blocking the Dll4/Notch signaling pathway can increase non-productive angiogenesis of nutrient transport and significantly slow tumor growth. Maybe blocking the pathway of ocular delivery nanosystems with some kind of pharmacological tool is a good option to block the Dll4/Notch signaling pathway. Such as, some of the lipoplexes loaded with siRNA silencing HuR expression can significantly dump retinal HuR and VEGF levels in STZ-rats.12 Besides, DAPT (a kind of γ-secretase inhibitor with molecular formula C23H26F2N2O4) is a known specific inhibitor of Notch pathway and has been validated in numerous clinical trials.13,14 VEGF and Dll4/Notch pathways play distinct but complementary roles in physiological angiogenesis. VEGF induces the expression of Dll4/Notch signaling, which in turn regulates the VEGF pathway. Related literatures have revealed the role of both pathways in ocular angiogenesis.15 Various ligands, receptors and downstream molecules of Notch signaling are expressed in the retina and play key roles in retinal vasculature. Dong Xiao and etc. have found that regulated by HIF-1α and VEGF, DII4 participates in hypoxia signal transduction during CNV angiogenesis and promotes RF/6A cell proliferation.16
DR is closely related to endothelial dysfunction and the activity and migration of endothelial cells change in high glucose environment, which is a key step of neovascularization. In particular, the migration of tip cells (the vascular endothelial cells pointing to the tip of the vascular bud) is considered to be the initial step of neovascularization.17 Dextrose-induced hyperglycaemia causes the impairment of human umbilical vein endothelial cells (HUVECs) proliferation and migration.18 The source of human retinal materials is limited, and primary culture of human retinal endothelial cells is difficult, and cell traits are prone to change during the passage process. Compared with retinal endothelial cells, HUVECs are more stable, and the experimental results are better repeatability. To date, the expression of Dll4/Notch-VEGF signaling and its mechanism of angiogenesis under HG condition remain to be elucidated. Here, we hypothesized that the Delta-Notch signaling pathway has potential specificity in neovascularization as the VEGF pathway. In this preliminary study, we used HG-stressed HUVECs as an experimental model in vitro, which simulated DR microenvironment, and further highlighted that the main components in Dll4/Notch-VEGF pathway under HG stimulation modulate the expression of each other and may provide us some clues to the anti-angiogenic mechanism. In addition, this study examined a potential therapeutic drug DAPT, providing a new approach for the treatment of DR.
Materials and Methods
HG-Stressed Cell Modeling
HUVECs (from Shanghai cell bank of Chinese academy of sciences, China) were cultured in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (SH30021.01, Hyclone, USA) containing 10% fetal bovine serum (FBS) (FB15011, Clark, USA) at 37°C under humidified conditions with 5% CO2. After initial attachment, all cell mediums were replaced by fresh medium. Cells were then incubated with increasing concentration of glucose (10,20,30,40mmol/L) at 370C and 5% CO2 for the 24h, 48h and the 72h, respectively. Both the MTT assay (M-2128, Sigma, USA) and the concentration of glucose in the cell medium at different time points were analyzed according to kit instructions. Each sample was performed in sextuplicate from three independent experiments.
RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR)
Cells from the normal cultured group (control group), HG-treated group (HG group) and DAPT+HG co-cultured group (DAPT+HG group) were harvested at the 48th hour. The concentration of N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenyl- glycine t-butyl ester (DAPT) (D5942, Sigma, USA) used in this study was 100ug/mL, the concentration of HG was determined by the result of HG cell modeling. Total RNA was prepared with Trizol Reagent (15596–026, Invitrogen, USA) according to kit instructions, and reverse transcribed to cDNA using PrimeScript RT reagent Kit (RR047A, TAKARA, China). The cDNA was amplified by the RT-PCR system (PIKORed 96, ThermoFisher, USA) with the SYBR Premix Ex Taq II Kit (RR820A, TAKARA, China). The specific primer pairs targeting Dll4, Notch1, Notch4, VEGF and β-actin genes were generated using the Primer Premier software. Sequences of the primers are shown in Table 1. The conditions were 95oC for 10s, followed by 40 cycles of 5s at 95 oC,30s at 55 oC and 30s at 72 oC. CT (cycle threshold) values were calculated through the Thermo Scientific PikoReal software (Thermo, USA) and relative gene expression was recorded through 2- ΔΔCT method.
 |
Table 1 Sequences of Primers for RT-PCR |
Western Blot Analysis
Proteins were extracted from cells cultured in RIPA lysis buffer (RIPA, China) as described in the RT-PCR protocol. One SDS-PAGE gel was loaded with 50μg of protein per well. After the proteins were transferred on nitrocellulose membranes, antibodies specific for Dll4 (1:200, SC365429, Santa Cruz, CA), Notch1 (1:200, SC376403, Santa Cruz, CA), Notch4 (1:200, SC393893, Santa Cruz, CA) and the VEGF (1:200, SC365578, Santa Cruz, CA) were used for incubation. Band intensity was analyzed with a gel imaging analysis system (Image Lab, Bio-Rad, USA).
Transwell Assay (Cell Migration)
100μL of 1×105 Cells from control group (5.5mmol/L glucose), HG group (30mmol/L glucose) and DAPT+HG group (30mmol/L glucose, 100ug/mL DAPT) were seeded in each transwell chamber with 1% FBS in 24-well plates (Corning, USA), cell medium with 10% FBS was added in the bottom chamber. The medium in the chamber was removed after 8h incubation. Photos were taken after cell fixation and dyeing. The cell numbers were counted manually and represented graphically.
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism 8 software (GraphPad Software, USA). Data were expressed as the mean ± standard deviation (SD) or the mean from at least three independent experiments. Unpaired t-test was used for continuous variables of mRNA expression and band intensity. The two-way analysis of variance (ANOVA) followed by a Dunnett correction and was applied for multiple comparisons. P values less than 0.05 were considered significant.
Results
HUVECs Stimulated with 30mmol/L HG Was Chosen as Cell Model
To simulate the optimal microenvironment of diabetic retinopathy, the cell model should remain a certain level of viability and proliferation as well as the detrimental effects of hyperglycemia. HUVECs under different HG stimulations showed the decreased cell viability. As illustrated in Figure 1A, the OD values in MTT assay decreased in a concentration-dependent manner. Compared with normal control cells, in which 5.5mmol/L glucose was contained in a cell medium for basal cell growth, cells cultured with 30mmol/L and 40mmol/L HG showed significant decrease of OD values from the 24th hour. All the HG-stressed groups at the 72nd hour showed significant decrease of the OD values.
Meanwhile, we measured glucose concentration in cell mediums with or without HG at different time points (the 24th h,48th h,72nd h). The glucose concentrations in HG-treated groups reduced with time, and the reduction scores were different (Figure 1B), among them, the reduction trend was more gradually in 30mmol/L group than that in 40mmol/L group.
Both 30mmol/L HG group and 40mmol/L group had significant difference in cell viability and metabolism ability compared with normal cultured cell, but the variation tendency changed more gradually in 30mmol/L group, which is benefited to control the experimental conditions in the future. Here, we chose the 30mmol/L HG-stressed group as the cell model in this study.
The HG Stimulation Induced Dll4, Notch1 and VEGF Expression and Down-Regulated Notch4
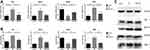
According to the first part of this study (HG-stressed cell model), the cell culture medium in the HG-stressed HUVECs group contained 30 mmol/L glucose. The normal cell cultured with 5.5mmol/L glucose was set as a control group. We illustrated the expression of the main signals in the Notch-VEGF pathway, which specifically related to vascularization. The results were illustrated by both RT-PCR and Western blot assay. Compared with the control group, the expression of Dll4, Notch1 and VEGF were significantly increased under HG condition, but another Notch receptor, Notch 4 was inhibited in HG-treated cells (Figure 2A).
Blocking Notch Signaling Down-Regulated Dll4, Notch1 and VEGF
DAPT is a γ-secretase inhibitor for blocking Notch signaling pathway. To evaluate the blocking effect of DAPT under HG condition, the expression of Dll4, Notch 1, Notch4 and VEGF in DAPT+HG co-cultured cells were analyzed. Compared with the HG group, the expression of Dll4, Notch1 and VEGF was decreased in DAPT+HG-treated cells, while the Notch4 was observed to be upregulated. No difference was found between the control group and the DAPT+HG group (Figure 2B and C).
DAPT Can Inhibit Cell Migration Under HG Condition
We counted the number of cells in the transwell chamber. Compared with the control group, it has fewer cells in the HG group and DAPT+HG group, indicating that the HG induced HUVECs migration. Meanwhile, DAPT could partially inhibit the cell migration induced by HG stimulation (Figure 3).
Discussion
DR is one of the microvascular complications of diabetes, it is based on the pathological changes of vascular endothelial cells due to hyperglycemia. The research strategies are generally limited to animal models, which are costly and relatively inefficient for screening targets. The use of cells in vitro allows easier manipulation of microenvironmental conditions for experimental studies and is particularly beneficial for the study of potential drug targets. The ideal cell model should maximally mimic the in vivo growth state. When it is under certain pathological conditions, it should not only have disturbed cellular metabolism but also maintain a certain level of viability. In this study, we took two parallel steps to assess it, ie, assaying live cells with MTT, and assessing cell metabolism with glucose consumption, which is actually based on cell viability. Here, we chose cells in an “intermediate state” as a cell model. Cells stimulated at 30 mmol/L HG met the above requirements.
Neovascularization in DR is a multifactorial complex pathogenesis, including oxidative stress and inflammation secondary to hyperglycemia.19,20 Recently, clinical pharmacological approaches are focusing on anti-VEGF. However, it remains insensitive and even drug-resistant. The VEGF pathway involved in neovascularization in DR is closely related to the Delta/Notch pathway. Molecular alterations in the Delta/Notch-VEGF pathway may provide clues to the novel drug targets, whereas studies on this signal pathway under hyperglycemia have not yet clarified the mechanism.
This study is a preliminary research of Dll4/Notch-VEGF pathway under HG stimulation in vitro. It researched the main molecules, which critically regulate angiogenesis in health and disease. The objective parameters barely contained Dll4, Notch1, Notch4, and VEGF, and meanwhile, the methods applied in this study were quite simple. Nevertheless, this study has explored the optimal cell model under HG stress, and highlighted some results of Notch pathway blockage in HG cell model. The result showed that Dll4, Notch1 and VEGF signaling were activated in HG conditions, while Notch4 was inhibited. Dll4 may control angiogenesis through the endothelial tip cells.7 Filopodia from tip cells leads the path of new vessel formation.21 Both Notch and VEGF pathways are vital for tip cell differentiation.22 Activation of Notch-1 coincides with increased VEGF expression was observed in HG-treated in diabetic animal model,23 which is consistent with our study. Since VEGF pathway can upregulate Notch pathway, we assume that increased VEGF in HG environment induced the expression of Dll4 and Notch1. Interestingly, Notch4, which binds to Dll4 during angiogenesis, expressed less in HG-stressed cells. Furthermore, through blocking the Notch pathway by DAPT, Notch4 was overexpressed while Dll4, Notch1 and VEGF were decreased.
The function of Notch4 is less reported, and its relationship with Notch1 and Notch4 involved in angiogenesis remains undefined. Previous studies have suggested that Notch1 and Notch 4 may have similar functions in angiogenesis,24,25 whereas the findings in this study bring different opinions to us. We suggested that Notch1 and Notch4 are activated through different molecules, and perform dissimilar functions under HG condition.
However, Notch signaling has a specific role in the angiogenesis process and in the maintenance of vascular homeostasis, and the VEGF and Notch signaling pathways regulate each other through multiple signaling modalities in vasculogenesis and angiogenesis. Constitutive activation of Notch signaling stabilizes network formation of endothelial cells on Matrigel and enhances formation of vessel-like structures in a three-dimensional angiogenesis mode.26 It is the normal physiological function of Notch signaling. Notch4, as a member of the Notch family, is specifically expressed in vascular endothelial cells, and it plays an important role in the development of blood vessels during embryonic life. However, overexpression of Notch4 in endothelial cells leads to increased vascular diameter.27 It is suggested that all cytokine levels should be in a dynamic homeostasis. For the results in this study, whether this is due to different cell growth environments, ie, differential expression of cytokines involved in the mechanism of endothelial cell injury under high glucose conditions, or an imbalance in cytokine expression, requires further validation.
Notably, cell migration induced by HG stimulation could be partially alleviated through Notch pathway blockage, the findings confirmed that Dll4/Notch pathway is involved in angiogenesis in HG environment. Combined therapy by blocking Dll4/Notch and VEGF pathways may provide us a new way for anti-neovascularization.
As mentioned above, this paper is a preliminary study of Dll4/Notch-VEGF pathway under HG condition. It is known that the interaction between Notch and VEGF pathway contains various gene regulation and crosstalk of molecules, so the future study would focus on the pathway targeting genes and downstream molecules; furthermore, the role of Notch4 under HG condition will be put at the first place.
Conclusion
Our study has established a HG-stressed cell model which may be utilized in pharmaceutical field for drug target screening. The function of Notch4 in Notch-VEGF pathway under HG background, especially the extent of its involvement in angiogenesis will be our next focus. However, this study does have some limitation such as the parameters and method design, which will be improved in our future studies. In the future, it is necessary to prove the function of Notch4 in Notch-VEGF pathway through primary endothelial cells or in vivo experiments.
Acknowledgments
We thank the ophthalmic laboratory of Chengdu University of Traditional Chinese Medicine for technical assistance. Na Gao and Linghui Xiao are co-first authors for this study.
Funding
This work was supported by the research program of Health and Family Planning Commission of Sichuan Province, China (17PJ534).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Paques M, Massin P, Gaudric A. Growth factors and diabetic retinopathy. Diabetes Metab. 1997;23(2):125–130.
2. Thomson SE, McLennan SV, Twigg SM. Growth factors in diabetic complications. Expert Rev Clin Immunol. 2006;2(3):403–418.
3. Cheung N, Wong IY, Wong TY. Ocular anti-VEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care. 2014;37(4):900–905.
4. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27(7):787–794.
5. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689.
6. Leslie JD, Ariza-Mcnaughton L, Bermange AL, et al. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134(5):839–844.
7. Patel NS, Li JL, Generali D, et al. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res. 2005;65(19):8690–8697.
8. Scehnet JS, Jiang W, Kumar SR, et al. Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007;109(11):4753–4760.
9. Blanco R. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3(1):a006569.
10. Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;16:1–8.
11. Liu Z, Fan F, Wang A, et al. Dll4-Notch signaling in regulation of tumor angiogenesis. J Cancer Res Clin Oncol. 2014;140(4):525–536.
12. Amadio M, Pascale A, Cupri S, Pignatello R. Nanosystems based on siRNA silencing HuRexpression counteract diabetic retinopathy in rat. Pharmacol Res. 2016;111:713–720.
13. Dai G, Deng S, Guo W, et al. Notch pathway inhibition using DAPT, a γ-secretase inhibitor (GSI), enhances the antitumor effect of cisplatin in resistant osteosarcoma. Mol Carcinog. 2019;58:3–18.
14. Hans CP, Sharma N, Dev R, Blain JM, Tonniges J, Agarwal G. DAPT, a potent Notch inhibitor regresses actively growing abdominal aortic aneurysm via divergent pathways. Clin Sci. 2020;134:1555–1572.
15. Nakamura K, Chiba C. Evidence for Notch signaling involvement in retinal regeneration of adult newt. Brain Res. 2007;1136(1):28–42.
16. Dong X, Wang YS, Dou GR, et al. Influence of Dll4 via HIF-1α-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS One. 2011;6(4):e18481.
17. Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569.
18. Chen X, Duong MN, Psaltis PJ, Bursill CA, Nicholls SJ. High-density lipoproteins attenuate high glucose-impaired endothelial cell signaling and functions: potential implications for improved vascular repair in diabetes. Cardiovasc Diabetol. 2017;16:121.
19. Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546–551.
20. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887.
21. Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22(5):617–625.
22. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–478.
23. Lin CL, Wang FS, Hsu YC, et al. Modulation of Notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes. 2010;59(8):1915–1925.
24. Zhang JF, Chen Y, Qiu XX, et al. The vascular delta-like ligand-4 (DLL4)-Notch4 signaling correlates with angiogenesis in primary glioblastoma: an immunohistochemical study. Tumour Biol. 2016;37(3):3797–3805.
25. James AC, Szot JO, Lyer K, et al. Notch4 reveals a novel mechanism regulating Notch signal transduction. Biochim Biophys Acta. 2014;843(7):1272–1284.
26. Liu ZJ, Shirakawa T, Li Y, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23(1):14–15.
27. Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci USA. 2001;98(10):5643.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.