Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 7
Preliminary open-label clinical evaluation of the soothing and reepithelialization properties of a novel topical formulation for rosacea
Authors Sparavigna A , Tenconi B , De Ponti I
Received 14 June 2014
Accepted for publication 24 July 2014
Published 24 October 2014 Volume 2014:7 Pages 275—283
DOI https://doi.org/10.2147/CCID.S69410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Video abstract presented by Adele Sparavigna.
Views: 409
Adele Sparavigna, Beatrice Tenconi, Ileana De Ponti
Derming Srl, Monza, Italy
Background: Rosacea is a common, incurable skin barrier disorder characterized by relapses and remissions.
Purpose: To evaluate the efficacy of Farmaka Rosacea Cream (FRC), a novel topical formulation for rosacea.
Methods: This single-center, open-label pilot study comprised a single-dose substudy in 20 healthy subjects and a long-term, repeat-dose substudy in 22 subjects with rosacea. The 2-hour, controlled, single-dose substudy assessed the soothing and reepithelialization properties of FRC after stripping-induced erythema based on the erythema index, transepidermal water loss, skin hydration, and clinical assessments of erythema. In the long-term substudy, subjects applied FRC twice daily for 8 weeks. Clinical assessments included vascular and pigmentary homogeneity and erythema and hemoglobin indices. Subjects completed questionnaires to assess FRC efficacy and cosmetic acceptability.
Results: Greater reductions were seen in FRC-treated areas compared with untreated areas for the erythema index (-16% versus -8%; P<0.001) and mean transepidermal water loss (-35.8% versus -10.1%; P<0.001) 30 minutes after stripping. Significant improvements over untreated areas were maintained 2 hours after stripping. Skin hydration and clinical erythema assessments also indicated that FRC soothed rosacea symptoms and promoted skin reepithelialization. Erythema and hemoglobin indices were significantly reduced from baseline after 4 and 8 weeks of treatment. Clinically assessed parameters were significantly improved following FRC application. Subjects assessed FRC positively.
Conclusion: Improvement of rosacea symptoms was noted with FRC application. The main film-forming ingredients of FRC (trehalose, cholesterol, ceramide, and fatty acids), combined with other soothing and calming ingredients and ultraviolet filters, could explain its efficacy.
Keywords: rosacea, erythema, skin hydration, re-epithelialization
Introduction
Rosacea is a chronic inflammatory skin disorder characterized by relapses and remissions. Predominantly affecting the face, rosacea often begins with flushing and progresses to persistent redness and the appearance of visible blood vessels. If untreated, bumps and pimples develop and, in severe cases, the nose becomes swollen and bumpy (rhinophyma). Four clinical subtypes, with different symptoms, have been characterized (subtypes I–IV).1
With a reported prevalence varying from <1% to >20% in US and European populations,2 rosacea is more common in those aged >30 years, in fair-skinned people of Northern European descent,3 and in women.4 The causes of rosacea are complex and multifactorial,5 but involve damage to the skin’s upper layer, the stratum corneum (SC), making it more susceptible to insult. Certain foods and environmental, chemical, and psychological factors aggravate rosacea, with ultraviolet radiation considered an important trigger.6 An elevated immune response and neurovascular dysregulation leads to heightened vasodilatory skin responses to various stimuli,7 and increased transepidermal water loss (TEWL), resulting from disruption of the SC permeability barrier, may contribute to skin dryness and sensitivity.8,9
Currently available treatments have limited efficacy and many can only be used for a short time. Topical prescription therapies include metronidazole, azelaic acid, and sulfacetamide–sulfur formulations. Rosacea can also be treated with oral antibiotics. In recalcitrant cases where antibiotics have failed, topical or oral treatment with retinoids may be indicated.10 Several reports have found light-based treatments to be effective in reducing erythema and telangiectasia, including long-pulsed dye, potassium titanyl phosphate, and diode lasers, which have been associated with little or no purpura. They also may be reduced by intense pulsed light therapy and electrocautery.11 In addition, basic skincare regimens, including the daily use of a sunscreen, moisturizers, and appropriate nonirritating cosmetics and non-soap cleanser, may offer significant benefits – enhancing skin properties and relieving skin discomfort.12
Farmaka Rosacea Cream (FRC; Farmaka srl, Milan, Italy) is a novel topical formulation containing a patented combination of ingredients that form a protective film on the skin. The combination of trehalose and key skin elements (cholesterol, ceramide, and fatty acids) moisturize the skin, restoring and hydrating the upper layer. Soothing and calming ingredients (Echinacea angustifolia extract, bisabolol, esculin, Boswellia serrata resin extract) and specific ultraviolet filters (ethylhexyl methoxycinnamate [PARSOL® MCX], butyl methoxydibenzoylmethane [PARSOL® 1789]) may also be effective against the main symptoms and triggers of rosacea. This pilot study evaluated the soothing and reepithelialization activity of a single application of FRC on experimentally induced erythema, as well as the anti-couperose efficacy of FRC after repeated application. Tolerability, antiaging/photoaging properties, and cosmetic acceptability were also assessed.
Materials and methods
Study design
This single-center, open-label study, conducted by Derming Srl (Monza, Italy), consisted of a short-term controlled substudy in healthy subjects and a long-term substudy in subjects with rosacea. Data were generated, recorded, and processed in accordance with the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines for good clinical practice.13 Informed consent was provided by all subjects prior to study entry. Since 2011, FRC has been launched in several European Union countries and has therefore been used extensively by subjects with rosacea following this study. The full protocol for this trial is available at Derming Srl.
Subjects
Healthy female subjects aged >18 years, who agreed not to receive treatment that would change their skin characteristics for the study duration or to use anti-rosacea or -couperose products for a month preceding the study, were recruited. Subjects in the long-term substudy had rosacea subtype I (erythematotelangiectatic; redness, flushing, and visible blood vessels)1 or subtype II (papulopustular; redness, swelling, papules/pustules, and acne-like breakouts).1
For both substudies, subjects with dermatitis or eczema were excluded, as were subjects with diabetes, endocrine disease, hepatic, renal or cardiac disorders, pulmonary disease, cancer, neurological or psychological disease, inflammatory/immunosuppressive disease, or drug allergy. Pregnant or lactating women, subjects who changed their normal lifestyle in the month preceding the investigation, and those with hypersensitivity to the product or its ingredients were also excluded. Subjects with recurrent facial/labial herpes were excluded from the long-term substudy.
Study treatment
For the short-term substudy, skin erythema was induced by positioning a freshly cyanoacrylate-coated microscope slide onto the skin of subjects’ backs for approximately 60 seconds. This “skin stripping” was performed three consecutive times on each control and test area. Immediately after stripping, FRC (2.00 mg/cm2) was applied to the test area by the investigator using light massage and left to absorb. The control area was left untreated.
Subjects in the long-term substudy applied FRC at home twice daily (morning and evening) on the face for 8 weeks, using light massage, until complete absorption.
In both substudies, subjects were randomly assigned in a 1:1 ratio to receive FRC on the left or right side, according to a previously generated randomization list. Use of systemic corticosteroids, aspirin, or nonsteroidal anti-inflammatory drugs, antihistamines, narcotics, antidepressants, immunosuppressive drugs, or drugs that could influence the test results was prohibited.
Study assessments and endpoints
Subjects could not smoke, drink coffee or alcohol, or use any product on the face for 3 hours before the visit. All measurements were performed under standard environmental conditions (temperature 22°C±2°C; relative humidity ≤60%), and subjects were acclimatized under relaxed conditions for at least 15 minutes prior to testing. Adverse or serious adverse events were recorded. In both substudies, the erythema index (EI) was evaluated using an optical densitometer (X-Rite® model 404; X-Rite, Inc., Grand Rapids, MI, USA) and was calculated as follows:
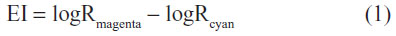
Short-term substudy
Evaluations were performed bilaterally on treated and untreated areas at baseline (T0; before product application), immediately after stripping (Ti), and 30 minutes (T30m) and 2 hours (T2h) after a single application of FRC. Erythema was scored by a dermatologist according to a five-point scale, with zero being “no erythema” and four being “severe erythema.”
Skin barrier function (TEWL) was assessed using an Evaporimeter EP2 (Servo Med AB, Kinna, Sweden). Electrical skin capacitance (hydration) was measured using a Corneometer® CM820 (Courage + Khazaka electronic GmbH, Cologne, Germany).
Long-term substudy
Evaluations were performed unilaterally on the face at baseline (T0; before product application), at Week 4 (T4) and at the final Week 8 visit (T8). Instrumental evaluations were performed at least 8 hours after the last application of FRC.
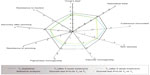
Wrinkles (at the nasolabial folds and area around the eyes), surface microrelief, skin tone/elasticity, skin brightness, dryness, and vascular and pigmentary homogeneity were scored according to visual scales. Facial clinical signs (excluding skin brightness) were correlated using a proprietary “spider web” graph (Spiderming®),14 which provides a fully comprehensive evaluation of aging skin.
Hemoglobin spectroscopic evaluation was performed using a SIAscope™ (Astron Clinica, Cambridge, UK), which visualizes skin structure, vascular composition, and reticular pigment networks and measures the concentration and distribution of skin melanin, collagen, and hemoglobin. Photographic recovery was performed using the FotoFinder® Dermoscope (FotoFinder Systems GmbH, Bad Birnbach, Germany) with a magnification of 20×.
At T8, the investigator interviewed each subject to determine the cosmetic acceptability of treatment. The tolerance and efficacy of FRC was evaluated using a subject questionnaire and investigator assessments, including any treatment-related adverse events or events that may have interfered with the results. At T4 and T8, compliance with the treatment regimen was verified by evaluation of personal diary cards in which subjects recorded the date and time of each FRC application.
Statistical analysis
Statistical analysis of short-term efficacy was performed for T0 and Ti using the Friedmann test for clinical evaluations and analysis of variance for instrumental evaluations. If the initial test was statistically significant, Tukey’s post hoc analysis was used. Comparisons of the treated and untreated (control) skin patches at T30m and T2h were performed using the Wilcoxon test for clinical evaluations and Student’s t-test for instrumental evaluations.
Long-term evaluation of efficacy at T4 and T8 versus T0 was performed using the Friedmann test for clinical evaluations and analysis of variance for instrumental evaluations. If the initial test was statistically significant, Dunnett’s post hoc analysis was used. Although no sample size calculations were performed, previous studies of the efficacy of treatments for rosacea have used similar numbers of patients.15,16
Results
Subjects
Forty-two of the 48 recruited subjects received treatment. In the short-term substudy, 20 subjects, with a mean age of 48 years (range 23–69 years), were treated. The long-term population comprised 22 subjects with rosacea (ten subjects [45%] subtype I; 12 subjects [55%] subtype II), with a mean age of 56 years (range 32–69 years). Half (50%) of the subjects in each substudy received treatment on the left side and half (50%) on the right side. In the long-term substudy, skin was classified as dry (eleven subjects [50%]), combination (six subjects [27%]), or normal (five subjects [23%]), with a skin phototype (Cesarini’s classification) of subtype II or IV (nine subjects each [41%]), subtype III (three subjects [13%]), or subtype V (one subject [5%]). Two subjects from the long-term population discontinued due to personal reasons unrelated to FRC. Thus, 20 subjects completed the long-term substudy (Figure 1).
  | Figure 1 Study flow diagram. |
Short-term evaluation of efficacy
Investigator assessments revealed no significant difference in erythema intensity between control and treated areas at Ti, confirming that skin stripping consistently induced erythema. At T30m, a statistically significant reduction in skin erythema was observed on FRC-treated skin (−43.8% at T30m versus T0; P<0.05), although the reduction was not significantly different to that of untreated skin (−27.1%). At T2h, however, erythema was reduced by a significantly greater extent on FRC-treated skin (−87.2%) compared with the control (−73.6%; P<0.01).
Skin stripping caused a statistically significant increase of the EI compared with T0 on treated and untreated areas (38.9% increase in both; P<0.05). After FRC application, there was a statistically significant reduction of the EI compared with the control (untreated) area at T30m (−16% versus −8%; P<0.001) and T2h (−28% versus −20%; P<0.01) (Figure 2).
A significant increase in TEWL compared with baseline (P<0.05) was observed at Ti for treated and untreated areas, suggesting the cutaneous barrier was damaged by skin stripping. After FRC application, TEWL was reduced by 35.8% at T30m compared with Ti (P<0.05), returning to a level comparable to baseline (T30m 5.25 g/m2/hour versus T0 4.74 g/m2/hour). This reduction was significantly greater than the reduction observed in the control area (−35.8% versus −10.1%; P<0.001). At T2h, TEWL remained low with no statistical difference from T0, whereas control areas had sustained barrier dysfunction (Figure 3).
At Ti, a statistically significant increase in electrical capacitance (hydration) was reported for the control skin compared with T0 (P<0.05), whereas the increase in areas to be treated with FRC was not significant. At T30m and T2h, statistically significant differences in capacitance were reported between treated and untreated skin (P<0.001). For untreated skin, capacitance at both time points remained similar to those at Ti (73.3 at Ti, 75.3 at T30m, and 75.0 at T2h). In FRC-treated skin, statistically significant increases in capacitance were observed at T30m and T2h compared with Ti (74.3 at Ti, 87.2 at T30m, and 81.9 at T2h; P<0.05).
Long-term evaluation of efficacy
Compared with T0, statistically significant reductions were reported for EI (−18%; P<0.05) and skin hemoglobin (−21%; P<0.05) at T4, which were maintained for the 8 week treatment period (EI −27% and hemoglobin −34% at T8 versus T0; P<0.05) (Figure 4). A statistically significant reduction in vascular homogeneity score was observed after 4 weeks (−55% at T4 versus T0; P<0.05) and 8 weeks (−75% at T8 versus T0; P<0.05) of treatment. Video dermoscopy revealed a reduction in capillary diameter (Figure 5).
  | Figure 5 Farmaka Rosacea Cream reduces capillary caliber after 4 weeks (T4) and 8 weeks (T8) of treatment compared with baseline (T0). |
The “spider web” graph (Figure 6) revealed statistically significant improvements in vascular and pigmentary homogeneity at T4 and T8. Furthermore, 60% and 70% of the subjects demonstrated an improvement in pigmentary homogeneity at T4 and T8, respectively, compared with T0 (P<0.05). All other parameters represented on the graph improved after treatment with FRC but did not reach statistical significance.
A high proportion of subjects (60%–100%) judged the effect of FRC to be medium, marked, or very marked for all efficacy parameters. Generally, the cosmetic acceptability of FRC was rated as good. The tolerability of FRC was viewed as good or excellent by 100% of subjects and investigators, with no adverse effects considered related or unrelated to the product.
Discussion
Rosacea is thought to be triggered by damage to the skin’s natural barrier (the SC) and increasing evidence indicates a role of sebaceous fatty acids in the maintenance of SC acidification and integrity.17 The combination of ingredients in FRC aims to reduce flare-ups, protect the skin from the main triggers of rosacea, and soothe and calm the skin. This study investigated both short- and long-term use of FRC.
After stripping-induced skin damage in subjects without rosacea, FRC elicited statistically significant improvements in the skin’s EI (soothing effect), barrier functionality (measured by TEWL), and hydration (measured by electrical capacitance), which occurred as soon as 30 minutes after application and were maintained at 2 hours posttreatment. In the long-term substudy, application of FRC twice daily for 8 weeks also resulted in statistically significant improvements in rosacea signs and symptoms, demonstrated by significant decreases in erythema and hemoglobin indices after 4 weeks of treatment. These reductions were maintained or increased after 8 weeks, suggesting that regular and prolonged treatment may be viable. At the end of the study, skin color was more homogeneous, with visible improvements in skin aging signs such as dryness and crow’s feet.
These preliminary results indicate a potential clinical benefit for patients with rosacea. However, although the efficacy of FRC compared to untreated areas was demonstrated in the short-term substudy, a control group was not included in the long-term substudy. The effects of FRC over placebo would be of interest, as repeated application of cream to the affected skin may have had a nonspecific soothing effect. These preliminary studies were also not blinded and while a number of objective, instrumental assessments were employed, several also included a subjective component. Nonetheless, these initial results suggest a beneficial effect of FRC for relief of rosacea symptoms and indicate that further blinded, placebo-controlled, and/or split-face assessment of FRC is warranted. Additionally, although rosacea most frequently occurs in females aged >30 years3,4 and the study population was representative of this group, assessment of FRC in men would be of interest.
Previous studies of rosacea treatments have predominantly focused on the efficacy of oral antibiotics (eg, doxycycline) or topical metronidazole and azelaic acid.1,18–22 However, unlike the current study, none assessed the subjects’ views and satisfaction with treatment.23 Importantly, most subjects and investigators regarded FRC’s efficacy and tolerability to be good or excellent in the current study.
To the best of the authors’ knowledge, no published clinical trials have directly studied the efficacy of FRC constituents on rosacea symptoms. However, data from a number of studies provide support for the contribution of individual constituents to the efficacy observed. De Paepe et al24 reported that lotioned handkerchiefs containing fatty alcohols and mineral oils helped prevent skin damage caused by the wiping on skin-stripped forearms of volunteers. Additionally, topical application of a combination nanoparticle product (trehalose, ceramide, and cholesterol) increased skin capacitance by up to 40% and decreased TEWL by 15% compared with placebo.25 These data suggest that the fatty acid and sugar components of FRC contribute to its efficacy in reducing the signs and symptoms of rosacea.
Other components of FRC include ceramides and fatty acids, which a number of studies have shown to be involved in maintenance of the SC. Ceramides represent the simplest sphingolipids,26 which are major, essential components of the epidermal water barrier. Inhibition of sphingolipid synthesis is accompanied by a significant delay in the rate of barrier recovery after acute disruption in murine epidermis.27 FRC also contains cholesterol, an extracellular and intracellular fatty acid and the main constituent of the cellular membrane.28 Inhibition of epidermal fatty acid synthesis in stripped skin significantly delayed barrier recovery in male hairless mice, while providing exogenous fatty acids by co-application of palmitate was found to improve barrier recovery.29 Topical provision of missing lipids, either cholesterol or sphingolipid, can also overcome inhibition of skin barrier repair.27,30,31 Together, the results of these studies have confirmed the significance of lipids for the construction and repair of the SC, which could partly explain the efficacy of FRC.
A key component of FRC is the naturally occurring disaccharide trehalose, which is widely used in the cosmetics, medical device, and pharmaceutical industries for its moisturizing properties.32
Conclusion
Short-term application of FRC reduced erythema in healthy subjects following skin stripping, while longer-term treatment in subjects with rosacea helped to counter the associated signs and symptoms. The fatty acid and sugar content of FRC could explain its efficacy in the treatment of rosacea.
Acknowledgments
This study was funded by Farmaka srl (Milan, Italy). The authors thank Insight Medical Writing (Kidlington, UK) for assistance in the preparation of this manuscript.
Disclosure
The study was funded by the manufacturer of the rosacea cream. The authors report no other conflicts of interest in this work.
References
Goldgar C, Keahey DJ, Houchins J. Treatment options for acne rosacea. Am Fam Physician. 2009;80(5):461–468. | |
Chosidow O, Cribier B. Epidemiology of rosacea: updated data. Ann Dermatol Venereol. 2011;138(Suppl 3):S179–S183. | |
Blount BW, Pelletier AL. Rosacea: a common, yet commonly overlooked, condition. Am Fam Physician. 2002;66(3):435–440. | |
Berg M, Liden S. An epidemiological study of rosacea. Acta Derm Venereol. 1989;69(5):419–423. | |
Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4(8):31–49. | |
Murphy G. Ultraviolet light and rosacea. Cutis. 2004;74(Suppl 3):13–16, 32–34. | |
Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5(3):16–25. | |
Dirschka T, Tronnier H, Folster-Holst R. Epithelial barrier function and atopic diathesis in rosacea and perioral dermatitis. Br J Dermatol. 2004;150(6):1136–1141. | |
Lonne-Rahm SB, Fischer T, Berg M. Stinging and rosacea. Acta Derm Venereol. 1999;79(6):460–461. | |
Cohen AF, Tiemstra JD. Diagnosis and treatment of rosacea. J Am Board Fam Pract. 2002;15(3):214–217. | |
Odom R, Dahl M, Dover J, et al. Standard management options for rosacea, part 2: options according to subtype. Cutis. 2009;84(2):97–104. | |
Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34(1):38–45. | |
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med. 2001;47(1):45–50. | |
Serri R, Romano MC, Sparavigna A. “Quitting smoking rejuvenates the skin”: results of a pilot project on smoking cessation conducted in Milan, Italy. Skinmed. 2010;8(1):23–29. | |
Tremaine AM, Ortiz A, Elkeeb L, Tran M, Weinstein G. Long-term efficacy and safety of topical PRK 124 (0.125%) lotion (Pyratine-XR) in the treatment of mild-to-moderate rosacea. J Drugs Dermatol. 2010;9(6):647–650. | |
Leyden JJ. Efficacy of a novel rosacea treatment system: an investigator-blind, randomized, parallel-group study. J Drugs Dermatol. 2011;10(10):1179–1185. | |
Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117(1):44–51. | |
Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized Phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. | |
Fowler JF Jr. Combined effect of anti-inflammatory dose doxycycline (40-mg doxycycline, USP monohydrate controlled-release capsules) and metronidazole topical gel 1% in the treatment of rosacea. J Drugs Dermatol. 2007;6(6):641–645. | |
Sanchez J, Somolinos AL, Almodovar PI, Webster G, Bradshaw M, Powala C. A randomized, double-blind, placebo-controlled trial of the combined effect of doxycycline hyclate 20-mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. J Am Acad Dermatol. 2005;53(5):791–797. | |
Elewski BE, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139(11):1444–1450. | |
Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999; 40(6 Pt 1):961–965. | |
van Zuuren EJ, Kramer S, Carter B, Graber MA, Fedorowicz Z. Interventions for rosacea [review]. Cochrane Database Syst Rev. 2011;3:CD003262. | |
De Paepe K, De Rop E, Houben E, Adam R, Rogiers V. Effects of lotioned disposable handkerchiefs on skin barrier recovery after tape stripping. Skin Res Technol. 2008;14(4):440–447. | |
Sinerga Spa. Tri-Solve: High Performance Modulator for Skin Barrier Recovery. Pero: Sinerga; 2008. Available from: http://www.sinerga.it/news/VOLANTINO%20TRI-SOLVE.pdf. Accessed September 15, 2014. | |
Schreiner V, Gooris GS, Pfeiffer S, et al. Barrier characteristics of different human skin types investigated with X-ray diffraction, lipid analysis, and electron microscopy imaging. J Invest Dermatol. 2000;114(4):654–660. | |
Holleran WM, Man MQ, Gao WN, Menon GK, Elias PM, Feingold KR. Sphingolipids are required for mammalian epidermal barrier function. Inhibition of sphingolipid synthesis delays barrier recovery after acute perturbation. J Clin Invest. 1991;88(4):1338–1345. | |
Sinerga SpA. Tri-Solve: high performance skin barrier recovery. Pero: Sinerga SpA; 2009. Available from: http://www.sinerga.it/schede/st_en_tri-solve.pdf. Accessed October 6, 2014. | |
Mao-Qiang M, Elias PM, Feingold KR. Fatty acids are required for epidermal permeability barrier function. J Clin Invest. 1993;92(2):791–798. | |
Feingold KR, Man MQ, Menon GK, Cho SS, Brown BE, Elias PM. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86(5):1738–1745. | |
Menon GK, Feingold KR, Mao-Qiang M, Schaude M, Elias PM. Structural basis for the barrier abnormality following inhibition of HMG CoA reductase in murine epidermis. J Invest Dermatol. 1992;98(2):209–219. | |
Teramoto N, Sachinvala ND, Shibata M. Trehalose and trehalose-based polymers for environmentally benign, biocompatible, and bioactive materials. Molecules. 2008;13(8):1773–1816. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.