Back to Journals » International Journal of Women's Health » Volume 10
Pregnancy-induced growth of a spinal hemangioblastoma: presumed mechanisms and their implications for therapeutic approaches
Authors da Mota Silveira Rodrigues A, Simões Fernandes F , Farage L , Almeida Prado Franceschi LE, Brito Vogt MDF, Zaconeta AM
Received 24 February 2018
Accepted for publication 25 April 2018
Published 21 June 2018 Volume 2018:10 Pages 325—328
DOI https://doi.org/10.2147/IJWH.S166216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Amanda da Mota Silveira Rodrigues,1 Fábio Simões Fernandes,2 Luciano Farage,3 Luiz Eduardo Almeida Prado Franceschi,4 Maria de Fátima Brito Vogt,1 Alberto Moreno Zaconeta1
1Department of Gynecology and Obstetrics, University Hospital of Brasilia, University of Brasília, Brasília, Brazil; 2Department of Neurosurgery, University Hospital of Brasilia, Brasília, Brazil; 3Faculty of Medicine, University of Brasília, Brasília, Brazil; 4Diagnose Pathology and Cytology Laboratory, Brasília, Brazi
Abstract: Hemangioblastomas are benign tumors of the central nervous system (CNS) that may occur either sporadically or as part of von Hippel–Lindau (VHL) disease, in which they coexist with a series of other tumors outside the CNS. Because of their low mitosis rate, hemangioblastomas usually have slow-growing and late manifestations, but may cause sudden neurological symptoms if tumor hemorrhage occurs. Few studies have evaluated the impact of pregnancy on the evolution of hemangioblastomas. Some authors have reported tumor growth in women with VHL disease, but no such association was observed by others. The influence of pregnancy on sporadic hemangioblastomas remains largely unexplored. We report here the case of a pregnant woman whose first manifestation of sporadic spinal hemangioblastoma was life-threatening, rapidly progressive dysautonomia. In addition, we discuss the role of pregnancy in the triggering of symptoms, as well as the possibility of medically indicated delivery for the management of these tumors.
Keywords: spinal hemangioblastoma, syringomyelia, pregnancy tumors, symptomatic hemangioblastoma, bulbomedullary edema, pregnancy-related hemangioblastoma
Case presentation
A 28-year-old woman without a relevant clinical history sought medical care in the 36th week of gestation. She reported muscle spasms and reduced muscle tone of the lower limbs in the past 2 weeks, accompanied by episodes of falling. Profuse, continuous sweating in the upper trunk caught our attention on physical examination, as did the presence of myoclonic twitches in the lower limbs. Anterior neck flexion caused pain resembling an electric shock at the C4–C5 level (Lhermitte’s sign). No focal neurological signs or alterations in strength or sensitivity were observed. The patient was admitted for observation. Laboratory tests for metabolic, infectious, or autoimmune causes of myelopathy revealed no abnormality.
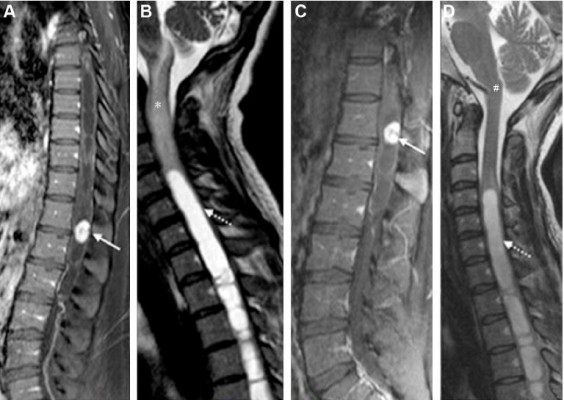
Four days later, there was rapid progression of dysautonomia characterized by tachycardia and sweating, in addition to hyperventilation leading to respiratory alkalosis and metabolic acidosis. Given the risks of fetal acidosis, an emergency cesarean section under general anesthesia was performed, with extraction of a healthy newborn. Complete reversal of the patient’s symptoms was observed on the day after delivery, although no specific treatment had been performed. Contrast-enhanced magnetic resonance imaging (MRI) performed on the fifth postpartum day showed a tumor suggestive of hemangioblastoma in the thoracic spine (T11–T12), accompanied by hydrosyringomyelia and edema from C4 to the bulbopontine transition (Figure 1A and B). In view of the significant improvement of symptoms, the patient was discharged to outpatient follow-up. Imaging control performed 55 days later showed a reduction in the size of the tumor and complete remission of bulbomedullary edema (Figure 1C and D). The clinical stability observed after delivery permitted breast-feeding and planning for elective surgery, which was performed without complications 8 months after the birth of the child. Histopathological analysis confirmed the diagnosis of hemangioblastoma (Figure 1E), and immunohistochemistry was negative for estrogen and progesterone receptors (Figure 1F).
Literature review
A survey was conducted in PubMed, SciELO, and LILACS databases. We searched for articles using the keywords “hemangioblastoma” AND “pregnancy”; “spinal tumor” AND “pregnancy”; ”von Hippel–Lindau” AND “pregnancy”; and “spinal cord hemangioblastoma” AND “pregnancy,” with no restrictions on language or date of publication. Articles that did not mention the histological examination of hemangioblastoma, that did not specify the spinal location of the hemangioblastoma, or not evaluate their effect on pregnancy were excluded. The reference lists of selected articles were hand-searched for other publications relevant to the review. Our research revealed eight articles directly related to the evolution of spinal hemangioblastomas during pregnancy, which were included in the discussion.
Discussion
Hemangioblastomas are rare tumors of the spinal cord that account for only 2% of all spinal tumors.1 In non-pregnant patients, spinal hemangioblastomas grow slowly, so small and asymptomatic tumors are managed only by serial observation. In symptomatic patients, surgery or radiotherapy is the main treatment and, recently, the use of antiangiogenic drugs has been considered. The case reported here is important because it suggests an alternative approach to the management of symptomatic spinal hemangioblastomas in pregnant women. If the increase in tumor size was secondary to the transient modifications imposed by pregnancy, medically indicated delivery may be an alternative in term pregnant women, avoiding or postponing surgery to a more convenient time.
The observation that some spinal tumors have their first manifestation in late pregnancy, followed by spontaneous resolution after delivery, is not new. In 1958, Newman2 focused on spinal angiomas and drew attention to sudden-onset neurological symptoms in the third trimester, with resolution after delivery “as if by a miracle,” followed by recurrence in subsequent pregnancies. The growth of these tumors would be explained by an increase in venous pressure caused by the enlarged uterus and by the effect of estrogen and progesterone on the walls of tumor blood vessels. In Newman’s view, regardless of the mechanism involved, the clear correlation between childbirth and clinical improvement, as well as recurrence in future pregnancies, made a causal rather than merely random relationship much more plausible.2
Hemangioblastomas are much less common spinal tumors than angiomas and little is known about their behavior during pregnancy. In a retrospective study, Frantzen et al3 observed significant growth of cerebellar hemangioblastomas during pregnancy in women with von Hippel–Lindau (VHL) disease, but the small number of cases in the sample did not permit conclusions to be drawn about the evolution of spinal-located tumors. In another study, Ye et al4 prospectively evaluated a series of women with a diagnosis of VHL disease and found no significant influence of pregnancy on the growth or appearance of new hemangioblastomas in the CNS.
Reviewing the literature, we found only two reports of an association of pregnancy with sporadic hemangioblastomas, ie, not associated with VHL disease.5,6 Ortega-Martínez et al5 reported the case of a woman with multiple filum terminale hemangioblastomas in the absence of any other evidence of VHL disease, in which pregnancy triggered neurological symptoms that disappeared after the interruption of pregnancy. This evolution is similar to that observed in the present report, suggesting that, at least in some cases, the hemodynamic and hormonal changes of pregnancy can cause an increase in tumor growth – or in the surrounding edema – triggering acute neurological symptoms that are reversible after the completion of pregnancy.
Several hypotheses have been proposed in an attempt to explain this phenomenon. According to the first hypothesis, rapid expansion or engorgement of the vascular beds during pregnancy could cause rapid enlargement of this highly vascular tumor. This is considered presumably to be the result of a generalized increase in circulating blood volume as well as the tendency to retain extracellular and intracellular fluid during pregnancy. Another hypothesis includes direct (pregnancy-related) hormonal effects on tumor growth rates, mediated by hormonal receptors. A third theory suggests involvement of proangiogenic growth factors7. In the present case, immunohistochemical evaluation of the tumor was negative for both estrogen and progesterone receptors, a finding that makes it unlikely that these hormones played a direct role in the increase in tumor size. The effect of placental growth factors cannot be ruled out in the case reported here. Laviv et al analyzed the involvement of placental growth factor (PlGF) and its receptor vascular endothelial growth factor receptor-1 (VEGFR-1) in processes that can lead to accelerated growth of hemangioblastoma during pregnancy. Their high specificity and their unique lack of any detrimental impact on other relevant physiological processes make them attractive objects for future study on therapeutic interventions since side effects are expected to be minimal.7 In the present case, considering the rapid reversal of symptoms after delivery, we believe that the main mechanism was hemodynamic. The pressure increase in the vena cava would be transmitted to the paravertebral veins, favoring the formation of peritumoral edema.8 This hypothesis is compatible with the finding of a moderate reduction in tumor size, but complete disappearance of bulbopontine edema, on the control MRI (Figure 1D). A limitation of our case report is that no MRI was performed before delivery, when the edema may have been larger than on the fifth postpartum day, a time when the patient had almost no symptoms. Gormeli et al6 described the case of a woman at 30 weeks of gestation with sudden-onset paraplegia due to bleeding from a spinal hemangioblastoma. These findings support the importance of immediate imaging evaluation of pregnant women with sudden-onset neurological symptoms.
Symptomatic spinal hemangioblastomas are extremely uncommon in pregnant women and the ideal treatment is not well established. In our opinion, serial follow-up by a multidisciplinary team is preferable for women with mild symptoms. On the other hand, severe or sudden-onset neurological symptoms remote from term require a neurosurgical approach that should consider the peculiarities of pregnancy, such as the limitation of ventral decubitus, tissue hypervascularization, and the increased risk of thromboembolism. Based on the arguments presented, we suggest that medically indicated delivery be considered in women with severe neurological symptoms at or near to term, postponing surgery to a time with less intraoperative and postoperative risk.
Acknowledgment
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
Na JH, Kim HS, Eoh W, Kim JH, Kim JS, Kim ES. Spinal cord hemangioblastoma: diagnosis and clinical outcome after surgical treatment. J Korean Neurosurg Soc. 2007;42(6):436–440. | ||
Newman MJ. Spinal angioma with symptoms in pregnancy. J Neurol Neurosurg Psychiatry. 1958;21(1):38–41. | ||
Frantzen C, Kruizinga RC, van Asselt SJ, et al. Pregnancy-related hemangioblastoma progression and complications in von Hippel-Lindau disease. Neurology. 2012;79(8):793–796. | ||
Ye DY, Bakhtian KD, Asthagiri AR, Lonser RR. Effect of pregnancy on hemangioblastoma development and progression in von Hippel-Lindau disease. J Neurosurg. 2012;117(5):818–824. | ||
Ortega-Martínez M, Cabezudo JM, Fernández-Portales I, Pineda-Palomo M, Rodríguez-Sánchez JA, Bernal-García LM. Multiple filum terminale hemangioblastomas symptomatic during pregnancy. Case report. J Neurosurg Spine. 2007;2:254–258. | ||
Gormeli CA, Sarac K, Ozdemir ZM, et al. Sudden-onset paraplegia during pregnancy caused by haemorrhage in a spinal cord haemangioblastoma: a case report. J Pak Med Assoc. 2016;66(9):1182–1184. | ||
Laviv Y, Wang JL, Anderson, MP Kasper EM. Accelerated growth of hemangioblastoma in pregnancy: the role of proangiogenic factors and upregulation of hypoxia-inducible factor (HIF) in a non-oxygen-dependent pathway. Neurosurg Rev. Epub 2017 Oct 13. | ||
Blecher R, Smorgick Y, Mirovsky Y. Symptomatic spinal hemangioma in pregnancy. Isr Med Assoc J. 2010;12(5):311–313. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.