Back to Journals » Patient Preference and Adherence » Volume 13
Preferences of patients with rheumatoid arthritis regarding disease-modifying antirheumatic drugs: a discrete choice experiment
Authors van Heuckelum M , Mathijssen EGE, Vervloet M, Boonen A, Hebing RCF , Pasma A, Vonkeman HE , Wenink MH, van den Bemt BJF, van Dijk L
Received 11 February 2019
Accepted for publication 14 May 2019
Published 22 July 2019 Volume 2019:13 Pages 1199—1211
DOI https://doi.org/10.2147/PPA.S204111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Milou van Heuckelum,1,2 Elke GE Mathijssen,2 Marcia Vervloet,3 Annelies Boonen,4,5 Renske CF Hebing,6 Annelieke Pasma,7 Harald E Vonkeman,8,9 Mark H Wenink,2,10 Bart JF van den Bemt,1,11–12 Liset van Dijk3,13
1Department of Pharmacy, Sint Maartenskliniek, Nijmegen, the Netherlands; 2Department of Rheumatology, Sint Maartenskliniek, Nijmegen, the Netherlands; 3Nivel (Netherlands Institute for Health Services Research), Utrecht, the Netherlands; 4Care and Public Health Research Institute (CAPHRI), Maastricht University, Maastricht, the Netherlands; 5Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Centre+, Maastricht, the Netherlands; 6Department of Rheumatology, Reade, Amsterdam, the Netherlands; 7Department of Rheumatology, Erasmus Medical Centre, Rotterdam, the Netherlands; 8Department of Rheumatology and Clinical Immunology, Medisch Spectrum Twente, Enschede, the Netherlands; 9Department of Psychology, Health and Technology, Arthritis Center Twente, University of Twente, Enschede, the Netherlands; 10Department of Rheumatology, Radboud University Medical Center, Nijmegen, the Netherlands; 11Department of Pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands; 12Department of Clinical Pharmacy and Toxicology, Maastricht University Medical Centre+, Maastricht, the Netherlands; 13Department of PharmacoTherapy, Epidemiology & Economics (PTEE), Groningen Research Institute of Pharmacy, Faculty of Mathematics and Natural Sciences, University of Groningen, Groningen, the Netherlands
Background: Although patients have different treatment preferences, these individual preferences could often be grouped in subgroups with shared preferences. Knowledge of these subgroups as well as factors associated with subgroup membership supports health care professionals in the understanding of what matters to patients in clinical decision-making.
Objectives: To identify subgroups of patients with rheumatoid arthritis (RA) based on their shared preferences toward disease-modifying antirheumatic drugs (DMARDs), and to identify factors associated with subgroup membership.
Methods: A discrete choice experiment to determine DMARD preferences of adult patients with RA was designed based on a literature review, expert recommendations, and focus groups. In this multicenter study, patients were asked to state their preferred choice between two different hypothetical treatment options, described by seven DMARD characteristics with three levels within each characteristic. Latent class analyses and multinomial logistic regressions were used to identify subgroups and the characteristics (patient characteristics, disease-related variables, and beliefs about medicines) associated with subgroup membership.
Results: Among 325 participating patients with RA, three subgroups were identified: an administration-driven subgroup (45.6%), a benefit-driven subgroup (29.7%), and a balanced subgroup (24.7%). Patients who were currently using biologic DMARDs were significantly more likely to belong to the balanced subgroup than the administration-driven subgroup (relative risk ratio (RRR): 0.50, 95% CI: 0.28–0.89). Highly educated patients were significantly more likely to belong to the benefit-driven subgroup than the balanced subgroup (RRR: 11.4, 95% CI: 0.97–133.6). Patients’ medication-related concerns did not contribute significantly to subgroup membership, whereas a near-significant association was found between patients’ beliefs about medication necessity and their membership of the benefit-driven subgroup (RRR: 1.12, 95% CI: 1.00–1.23).
Conclusion: Three subgroups with shared preferences were identified. Only biologic DMARD use and educational level were associated with subgroup membership. Integrating patient’s medication preferences in pharmacotherapy decisions may improve the quality of decisions and possibly medication adherence.
Keywords: rheumatoid arthritis, disease-modifying antirheumatic drugs, discrete choice experiment, treatment preferences
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovial inflammation, which can lead to irreversible articular damage, a decrease in physical functioning, and radiologic progression.1–3 Patients are recommended to use disease-modifying antirheumatic drugs (DMARDs) to suppress the inflammatory response and to improve clinical outcomes.1,3 Nevertheless, non-adherence to these drugs is a major issue. Depending on the measurement method used, adherence rates to DMARDs vary from 58% to 92%.4–6 Low adherence rates contribute to increased disease activity, the impairment of physical functioning and quality of life, structural damage to cartilage and bone, and high individual and societal costs.1,4 In recent years, tailoring treatment to patients’ medication preferences has gained increased attention as a promising strategy to improve medication adherence.1,7,8
Conventional and targeted DMARDs have different characteristics, providing the opportunity to fit treatment options to patient’s medication preferences. Each DMARD consists of a set of characteristics (ie, attributes, for instance route of administration) with multiple levels (eg, oral, subcutaneous, and intravenous within the attribute “route of administration”). These attributes and levels enable patients to make trade-offs regarding treatment benefits and drawbacks.9 Integrating patient’s medication preferences in treatment decisions is essential, since patient preferences are not always in line with treatment protocols or the preferences of rheumatologists.10 Misalignment between patient’s and rheumatologist’s preferences or treatment protocols might result in non-adherence to medication. Thus, prescribers should take patient preferences into account to increase decision quality and possibly medication adherence.
It has previously been shown that patient’s beliefs about a specific medicine (ie, necessity beliefs and concern beliefs about the prescribed treatment) are associated with treatment preferences and adherence to medication.11–13 The association between subgroups with shared DMARD preferences and patient-related characteristics (including necessity and concern beliefs) in rheumatic diseases is, however, understudied. Only one previous study investigated the contribution of beliefs about medicines to treatment preferences in patients with RA, but the researchers disregarded the possible existence of subgroups with similar preference patterns within patients with RA.14 Insight in meaningful subgroups as well as patient characteristics (eg, demographic, clinical, and psychological determinants) associated with these subgroups might support health care professionals in the understanding of what matters to patients in clinical decision-making. These insights allow further optimization of patient-tailored decisions regarding DMARD treatment by applying these insights in, eg, communication strategies, communication styles, and patient education.
The primary objective of this study is therefore to elicit the preferences of patients with RA regarding DMARD characteristics, and to identify subgroups with shared preferences. The secondary objective is to study different patient characteristics, including beliefs about medicines, which may be associated with these subgroups.
Patients and methods
Study design and setting
This cross-sectional multicenter study was performed in collaboration with five rheumatology departments across the Netherlands: Sint Maartenskliniek, Reade, Erasmus MC, Medisch Spectrum Twente, and Maastricht UMC+. A discrete choice experiment (DCE), implemented in an online survey, was used to elicit patient preferences toward DMARDs. The survey was conducted between April 12, 2017 and November 30, 2017. The STROBE statement for cross-sectional studies provided guidance for the adequate reporting of this study.15
Eligibility criteria and patient recruitment
The eligibility criteria for patients participating in this study were: 1) clinical diagnosis of RA by a rheumatologist, 2) current user of at least one DMARD according to their medical file, 3) aged ≥18 years, and 4) proficiency of the Dutch language. All patients were approached in collaboration with their treating clinician and the medical head of the department. One to three weeks before their regular outpatient visit, an information letter and informed consent form were sent to all eligible patients. After receiving a patient’s written informed consent, the researcher contacted the patient by email or telephone to send a web link to complete the online survey at home or to schedule a research appointment at the study site, respectively.
Procedures of data collection
If patients chose the option to complete the survey at home but did not complete it within one week, a reminder was sent by email. If patients completed the survey more than once, they were contacted by telephone to ask which set of answers best represented their preferences, and to explore the reasons for duplicates.
As part of the online survey, patients were asked about their preferences toward DMARDs, demographics (age, gender, nationality, employment status, educational level, and marital status), clinical characteristics (disease duration, current and previous DMARD use), and medication beliefs based on the 10-item Beliefs about Medicines Questionnaire (BMQ)-Specific. Data were extracted from the online database using Lighthouse Studio (Sawtooth Software version 9.5.2.).
Measurement instruments
Discrete choice experiment (DCE)
The checklist developed by the ISPOR Good Research Practices for Conjoint Analysis Task Force was used to report the steps involved in conducting this DCE.16,17
Identification and selection of relevant attributes and levels
The identification and selection process of attributes and levels is described briefly in this article. For a more detailed description of this process, see Mathijssen et al.18
A literature search in PubMed, CINAHL, and Embase was performed on September 27, 2016, to identify DMARD attributes and levels from previous research involving patients with RA. The following search terms, both MeSH terms and free text words, were included in the literature search: rheumatoid arthritis, DMARDs, preferences, and attributes. Titles and abstracts were screened independently by two researchers (pairs formed between EM, MH, LvD, and MV), followed by screening the full texts. Full texts were included if they were: 1) studies on adult (≥18 years) patients with RA; and 2) studies on attributes of DMARDs, preferences for DMARDs, or experiences with DMARDs. Subsequently, a group of experts was asked to individually complement this list of attributes and levels from the literature search. This group of experts consisted of two rheumatologists, two rheumatology-specialized nurses, two researchers (not members of the research team), two pharmacists, and two patients with RA. The recommendations from this expert panel were used to complement the list of attributes and levels before the focus group discussions. Three focus groups involving a total of 23 patients with RA were held to obtain further in-depth information and to individually rank the 22 identified attributes and levels. Participants were also asked to complement the list with missing attributes and their corresponding levels. The identified attributes and levels were discussed during a consensus meeting (attendees: BB, EM, LvD, MH, and MV), which resulted in a final subset of seven attributes (each with three levels) included in the online survey (see Table 1).
DCE design
A choice-based conjoint design, including 12 random choice tasks and two fixed dominant choice tasks, was used for discrete choice modeling (Lighthouse Studio: CBC, Sawtooth Software). The random tasks were used to elicit patient preferences, whereas the fixed dominant tasks were used to measure internal validity. Examples of a random choice task and a fixed dominant choice task are presented in Supplementary materials (Table S1 and S2 respectively). A traditional full-profile choice-based conjoint design with complete enumeration was used, providing orthogonality (all attribute levels varied independently across attributes), minimal overlap, and equally balanced level combinations (each level was presented an equal number of times within an attribute).17 A forced choice-elicitation format without an “opt-out” or “no-treatment” option was used due to the decision context of the experiment. The complexity of the DCE choice tasks was tested on a scale from 0 to 10 with higher scores indicating a higher level of complexity. The online survey was pretested in five patients with RA to assess response efficiency, including the complexity of the DCE choice tasks, respondent fatigue, and the comprehensibility of the online survey.17 Data collection started after pretesting.
Beliefs about medicines questionnaire specific (BMQ-Specific)
The BMQ-Specific was used to measure patient’s necessity beliefs and concerns about DMARDs. Each item of the BMQ-Specific was scored from 1 (strongly disagree) to 5 (strongly agree), resulting in a summated score from 5 to 25 for each subscale (necessity and concerns). Low necessity or concern beliefs were defined as summated scale scores <15.19 High necessity or concern beliefs were defined as summated scale scores ≥15.19 Patients were further classified into four profiles according to the necessity-concerns framework developed by Horne et al: accepting (high necessity, low concerns), ambivalent (high necessity, high concerns), indifferent (low necessity, low concerns), and skeptical (low necessity, high concerns).11,19,20
Study size
The sample size was calculated based on the rule of thumb (N>500c/(t×a)) proposed by Orme et al, which considers the number of choice tasks (t), the number of alternatives (a), and the number of analysis cells (c).21 The number of random tasks was estimated to be 12, the number of alternatives per task (not including the “none” alternative) was two, and the number of analysis cells (based on the main effects) was three, as each attribute consisted of three levels.21 Based on these estimates, the minimum sample size for the choice-based conjoint analysis was 63. Considering the research question regarding the identification of different subgroups and the hypothesis that respondents would be divided into at least three subgroups, a sample size of at least 200 patients was considered appropriate for this study.21,22
Statistical methods
Statistical analyses were performed using STATA version 13.1. Descriptive statistics were used to describe the patient and disease characteristics. Educational level was classified in low, moderate, or high educational level. Low educational level was defined as no education, (extended) primary education or pre-vocational education, moderate educational level was defined as vocational education or selective secondary education, and high educational level was defined as education provided by universities of applied sciences and research universities. Data were presented as means and standard deviations or percentages. Incomplete surveys were excluded from the data analysis.
Part-worth utilities were the numerical data obtained from discrete choice modeling. Higher part-worth utilities represented stronger preferences for levels within an attribute, whereas negative utility scores were considered less attractive. The DCE data were analyzed in a latent class analysis to identify subgroups within the total study sample (Analysis Manager version 9.5.2; Lighthouse Studio, Sawtooth Software). Model fit tests (Consistent Akaike Information Criterion and Bayesian Information Criterion) were performed to identify the number of segments in our data. The highest probability for subgroup membership was decisive in assigning respondents to subgroups. Each subgroup was characterized by their shared part-worth utilities.23 In other words, patients who displayed similar part-worth utilities (ie, preferences toward DMARD characteristics) were categorized in the same subgroup, whereas patients who displayed conflicting part-worth utilities were categorized in different subgroups. Part-worth utilities represent the strength and direction of preferences for DMARD characteristics within an attribute, whereas the relative importance of an attribute represents the importance of this attribute relative to other attributes. The relative importance of an individual attribute in the overall choice for a DMARD was calculated by dividing the range of part-worth utilities within an attribute by the sum of ranges across attributes, multiplied by 100.
Multinomial logistic regression models (with one subgroup as base scenario) were used to determine whether patient characteristics, disease-related characteristics, and beliefs about medicines were related to subgroup membership. Bivariate analyses were performed to select the most important predictors to prevent overfitting of the model due to the large number of variables measured in this study. Determinants with P-values <0.2 were entered in the final model. In the final model, P-values ≤0.05 were considered statistically significant.
Ethical approval
The study protocol was reviewed by the Institutional Review Board of the Sint Maartenskliniek Nijmegen and ethical approval was obtained from the local Medical Ethics Committees. This study was conducted according to the ethical principles for medical research as stated in the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, 2013).
Results
Sample characteristics
Initially, 1,317 patients were invited to participate in this study. Of these patients, 24.7% (N=325; range for hospitals: 18.8–28.6%) completed the survey. Figure 1 shows the flowchart of the survey response. Patient characteristics are shown in Table 2. On average, patients were 63.3 (SD=11.9) years old and had a mean disease duration of 14.7 (SD=11.2) years. Of the patients who completed the survey, 69.2% were female, 39.7% had a low educational level, 40.0% were (early) retired, and 20.0% were living alone. Regarding beliefs about medicines, 52.9% of the patients were categorized in the “accepting” quadrant and 39.1% comprised the “ambivalent” quadrant. The “indifferent” and “skeptical” quadrants were both represented by 4.0% of the patients.
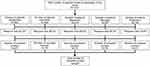 |
Figure 1 Flow chart of survey response across study sites. AStudy site was unknown for one completed survey. |
 |
Table 2 Sample characteristics. |
Internal validity
Mean score for the complexity of the online survey was 5.3 (SD=2.2). Of all respondents, 1.5% and 2.2% gave an irrational response (ie, stated a preference for the worst-case scenario, eg, “Medicine 1” in Table S2) to the first and second fixed choice task, respectively. Overall, those respondents did not influence the results on DMARD preferences and the identification of subgroups (data not shown); therefore, all respondents were included for analysis.
Identification of subgroups with similar preferences
The latent class analysis identified three subgroups with the following segment sizes: a benefit-driven subgroup (29.7%), a balanced subgroup (24.7%), and an administration-driven subgroup (45.6%). See Tables S3 and S4 for the results of the identification process of subgroups with latent class analyses in this study. The groups were identified with a name that characterized the aspects they consider most important. Within the benefit-driven subgroup, the relative importance of the attribute chance of efficacy (43.6%) was highest, whereas the relative importance of route of administration (38.2%) was highest in the administration-driven subgroup. In contrast with the other subgroups, the choice for a DMARD in the balanced subgroup was more equally influenced by multiple attributes (Figure 2); however, risk of cancer (17.0%) and onset of action (14.5%) were relatively more important for the balanced subgroup than for the other subgroups. Between-group differences were smallest for the attributes frequency of administration and risk of serious infections. The largest between-group differences were sequentially found for the attributes chance of efficacy, route of administration, risk of cancer, and onset of action (Figure 2).
 |
Figure 2 Relative importance of attributes for each subgroup. |
Part-worth utilities of levels between and within subgroups
The part-worth utilities of attribute levels differed between the subgroups, revealing that members of each subgroup made different trade-offs between DMARD characteristics (see Figure 3). Although the direction of most part-worth utilities within each attribute was similar for the three subgroups, the level part-worth utilities within the attribute route of administration were remarkably different. The administration-driven subgroup had a strong preference for tablets or capsules, and was less likely to prefer an intravenously administered DMARD. The benefit-driven subgroup also preferred tablets or capsules over subcutaneous injections; however, these part-worth utilities were not significantly different. The balanced subgroup had a strong preference for subcutaneous injections, followed by an intravenously administered DMARD. Members of this subgroup were less likely to prefer tablets or capsules for DMARD administration.
Factors associated with subgroup membership
Multinomial logistic regression (see Table S5) revealed that patients who were currently using bDMARDs were significantly less likely to belong to the administration-driven subgroup (with a strong preference for the oral route of administration) than the balanced subgroup (RRR: 0.50, 95% CI: 0.28–0.89). Highly educated patients were significantly more likely to belong to the benefit-driven subgroup than the balanced subgroup (RRR: 11.4, 95% CI: 0.97–133.7). The following patient characteristics were not significantly associated with subgroup membership: age, sex, employment status, study site, disease duration, current use of corticosteroids, and bDMARD and corticosteroid use in the past.
Patient preferences and beliefs about medicines
Since the “indifferent” and “skeptical” quadrants are represented to a limited extent in this study, summated scale scores for necessity and concern beliefs were used instead of the BMQ profiles in the statistical analyses. Multinomial logistic regression analyses (see Table S5) revealed that patients’ medication-related concerns did not contribute significantly to subgroup membership, whereas a near-significant association was found between patients’ beliefs about medication necessity and their membership of the benefit-driven subgroup (RRR: 1.12, 95% CI: 1.00–1.23).
Discussion
In this study, we identified three subgroups with the following segment sizes based on their shared preferences regarding DMARDs: a benefit-driven subgroup (29.7%), a balanced subgroup (24.7%), and an administration-driven subgroup (45.6%). Patients who were currently using bDMARDs were significantly less likely to belong to the administration-driven subgroup than the balanced subgroup, which could be explained by patients’ attitude toward or experience with subcutaneously administered DMARDs. A high educational level was significantly associated with membership of the benefit-driven subgroup (base scenario: balanced subgroup). However, significant results with wide confidence intervals should be interpreted with care.
A latent class analysis to identify subgroups within the population of patients with RA was previously reported by Fraenkel et al, who used a five-group solution to determine the following mutually exclusive categories/subgroups of patients: cost-driven (38.4%), bothersome side effects (25.8%), onset and infection (18.0%), rare side effects (11.2%), and administration-driven (6.6%).24 The attributes and levels included in their survey substantially differed from the attributes and levels included in our DCE, and the segment size of the administration-driven subgroup was much smaller (6.6% versus 45.6% in the present study).24 These contrary results might be explained by the differences in national health care systems (eg, out-of-pocket costs are different from patient to patient in the United States and are all covered in the Netherlands).24 Based on this, it can be concluded that, if the cost perspective is less important for patients in other settings, it is assumed that our DCE results are more generalizable to these settings than those of Fraenkel et al.24
To our knowledge, the association between patient preferences and beliefs about medicines was only previously described by Alten et al.14 These authors reported conflicting results on the association between patient’s beliefs about medicines and their preferences, which may be due to the use of a modified version of the validated BMQ (ie, adjustments in the number of items and item values).14 Alten et al reported that all four BMQ profiles significantly contributed to the best-worst pairs chosen in their DCE, whereas in our study only necessity beliefs were slightly, but not significantly, associated with membership of the benefit-driven subgroup.14 Differences in study setting (including health care systems and reimbursement), study population, DCE design, and statistical analyses of the current and previous studies may explain the differences in results, and also make it difficult to compare the results of both studies.10,14,24–31 For this reason, the extrapolation of DCE results to other settings or (sub)populations is not always justified. It is, however, assumed that our results can be generalized to other settings and populations if sample characteristics, health care systems, reimbursement, and access to medication largely correspond to those in our study.
The strength of our work relies on the extensive pre-study, which used a three-step mixed-methods approach to identify, refine, and select attributes and levels for our DCE.18 This contributed to a more accurate DCE design for investigating the preferences of this patient population.18 Together with the multicenter study design and our broad inclusion criteria, which had no restrictions in terms of disease duration, disease activity, and current or previous DMARD use, our results are assumed to be more representative of the diversity of patients visiting a rheumatologist than the strictly selected samples recruited in previous studies.10,14,24–31 Differences in patient recruitment (ie, nationwide panels/networks) and study settings were also considered to limit the generalizability of previous work.10,14,24–31 Also, in contrast with previous research, we avoided the use of ordinal-scaled levels due to their higher risk of heterogeneous interpretation and subjectivity. Instead, a sufficient, but realistic, contrast in levels was incorporated into the study, to avoid discouraging patients from completing the online survey.
Our work has also some limitations, one of which is the possible unintended selection bias or bias due to non-response to the information letter or questionnaire. However, it can be assumed that the low response rate (24.7%) will not affect the number and type of subgroups identified in this research, since no significant differences in sex, age, and proportion of bDMARD use between participants and the general RA population in the Sint Maartenskliniek were found. Due to the limited access of data on non-participating patients across study sites, the general RA population in the Sint Maartenskliniek was chosen as reference as this center is one of the largest rheumatology specialized centers in the Netherlands. Regarding beliefs about medicines, indifferent and skeptical profiles were underrepresented, however similar distributions were found in previous studies.19,32 Patients with low health literacy skills may also be underrepresented in our study sample, since our DCE was implemented in an online survey and health literacy was not measured. The absence of clinical data (ie, due to limited access to patients’ medical files at the different study sites and differences in measuring disease activity scores) and adherence data is another limitation, since clinical- and adherence data may be associated with subgroup membership and be relevant in clinical practice. Also, remarkable were the patients who were using a DMARD according to their medical file, but reported no current DMARD use (5.2%) when explicitly asked in the online survey. Most common reason for this finding was a (temporary) discontinuation of DMARD therapy (eg, due to the initiation of chemotherapy or side effects of DMARDs) between patient selection and patient inclusion. Additionally, decision-making in real-life settings may differ from the choices made in our study, in which respondents evaluated different treatment options within the same choice context and in a study setting. External factors may also influence actual choice behavior, such as the skills of rheumatologists to motivate patients to undergo DMARD treatment, disease flares, the involvement of significant others, hospital policies, and health insurance companies. Nevertheless, insights into intentional choice behavior can form the backbone for predicting actual behavior,33 and can support an effective communication strategy between patients and providers by integrating patient’s preferences into treatment decisions. This may eventually improve medication adherence in clinical practice.
In conclusion, three subgroups with shared DMARD preferences were identified in patients with RA. However, distinguishing patients based on their beliefs about medicines, and patient and clinical characteristics is complex. Rheumatologists should be aware of the existence of these subgroups and ask patients with RA about their preferences toward DMARD characteristics to increase decision quality, anticipate on their beliefs about medicines, and possibly increase medication adherence. Future research should focus on effective strategies to support rheumatologists and other clinicians in revealing DMARD preferences in real-life settings, which could eventually support and optimize patient-tailored decisions regarding DMARD treatment. From the patient’s perspective, the value of a decision aid, eliciting their preferences, beliefs about medicines, and likely medication adherence, should be further explored.
Acknowledgments
The authors would like to thank all patients who participated in this study, as well as the patient research partners involved in the design phase and pilot test of the DCE. Special thanks are extended to C. Delnoy-Meijers (MUMC+), J. Geusen (MUMC+), M. Hegeman (MST), and M. Hermus (Erasmus MC) for their assistance in patient recruitment and data collection on site. This research was supported financially by a grant from Pfizer. The abstract of this manuscript was presented at the EULAR 2018 (13–16 June, Amsterdam) and at the 47th European Symposium on Clinical Pharmacy (ESCP 2018, 24–26 October, Belfast). This abstract was published in the Annals of the Rheumatic Diseases 2018;77:188–189.
Disclosure
Dr van Heuckelum reports grants from Pfizer, during the conduct of the study. Dr Vervloet reports grants from Pfizer, during the conduct of the study; grants from Pfizer, grants from AbbVie and grants from AstraZenica, outside the submitted work. Prof. Dr Boonen reports grants from Abbvie, grants from Celgene, personal fees from UCB Pharma, personal fees from Lilly, personal fees from Sandoz, personal fees from Novartis and personal fees from Janssen Pharma, outside the submitted work. Prof. Dr van Dijk reports grants from Pfizer, during the conduct of the study; grants from Pfizer, grants from Abbvie and grants from Astra Zeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977.
2. Lindqvist E, Jonsson K, Saxne T, Eberhardt K. Course of radiographic damage over 10 years in a cohort with early rheumatoid arthritis. Ann Rheum Dis. 2003;62:611–616. doi:10.1136/ard.62.7.611
3. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi:10.1016/S0140-6736(01)06075-5
4. Waimann C, Marengo M, Achaval de S, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis. Consequences of low adherence. Arthritis Rheum. 2013;65(6):1421–1429. doi:10.1002/art.37917
5. van den Bemt BJF, van den Hoogen FH, Benraad B, Hekster Y, van Riel PL, van Lankveld W. Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol. 2009;36(10). doi:10.3899/jrheum.080430
6. Pasma A, den Boer E, van Spijker A, et al. Nonadherence to disease modifying antirheumatic drugs in the first year after diagnosis: comparing three adherence measures in early arthritis patients. Rheumatology(Oxford). 2016;55(10):1812–1819. doi:10.1093/rheumatology/kew247
7. Barton JL. Patient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapy. Patient Prefer Adherence. 2009;3:335–344.
8. Stacey D, Légaré F, Col N, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431.
9. Mühlbacher A, Johnson F. Choice experiments to quantify preferences for health and healthcare: state of the practice. Appl Heal Econ Heal Policy. 2016;14(3):253–266. doi:10.1007/s40258-016-0232-7
10. Nolla JM, Rodríguez M, Martin-Mola E, et al. Patients’ and rheumatologists’ preferences for the attributes of biological agents used in the treatment of rheumatic diseases in Spain. Patient Prefer Adherence. 2016;10:1101–1113. doi:10.2147/PPA.S106311
11. Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8(12):e80633. doi:10.1371/journal.pone.0080633
12. Uebelacker LA, Bailey G, Herman D, Anderson B, Stein M. Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. J Subst Abus Treat. 2016;66:48–53. doi:10.1016/j.jsat.2016.02.009
13. Chapman S, Dale P, Svedsater H, et al. Modelling the effect of beliefs about asthma medication and treatment intrusiveness on adherence and preference for once-daily vs. twice-daily medication. NPJ Prim Care Respir Med. 2017;27(61). doi:10.1038/s41533-017-0061-7.
14. Alten R, Krüger K, Rellecke J, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence. 2016;10:2217–2228. doi:10.2147/PPA.S117774
15. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi:10.1016/j.jclinepi.2007.11.008
16. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health - a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Heal. 2011;4:403–413. doi:10.1016/j.jval.2010.11.013
17. Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Heal. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223
18. Mathijssen EG, van Heuckelum M, van Dijk L, et al. A discrete choice experiment on preferences of patients with rheumatoid arthritis regarding disease-modifying antirheumatic drugs: the identification, refinement and selection of attributes and levels. Patient Prefer Adherence. 2018;12:1537–1555. doi:10.2147/PPA.S170721
19. Zwikker HE, van Dulmen S, van den Bemt BJ, van den Ende C. Perceived need to take medication is associated with medication non-adherence in patients with rheumatoid arthritis. Patient Prefer Adherence. 2014;8:1635–1645. doi:10.2147/PPA.S66849
20. Menckeberg TT, Bouvy ML, Bracke M, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64(1):47–54. doi:10.1016/j.jpsychores.2007.07.016
21. Orme B. Sample size issues for conjoint analysis. In: Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. Madison: Research Publishers LLC; 2010:57–66.
22. Kongsted A, Nielsen A. Latent class analysis in health research. J Physiother. 2017;63(1):55–58. doi:10.1016/j.jphys.2016.05.018
23. Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Heal. 2016;4:300–315. doi:10.1016/j.jval.2016.04.004
24. Fraenkel L, Nowell WB, Michel G, Wiedmeyer C. Preference phenotypes to facilitate shared decision-making in rheumatoid arthritis. Ann Rheum Dis. 2018;77(5):678–683. doi:10.1136/annrheumdis-2017-212407
25. Husni ME, Betts KA, Griffith J, Song Y, Ganguli A. Benefit-risk trade-offs for treatment decisions in moderate-to-severe rheumatoid arthritis: focus on the patient perspective. Rheumatol Int. 2017;37(9):1423–1434. doi:10.1007/s00296-017-3760-z
26. Poulos C, Hauber AB, González JM, Turpcu A. Patients’ willingness to trade off between the duration and frequency of rheumatoid arthritis treatments. Arthritis Care Res. 2014;66(7):1008–1015. doi:10.1002/acr.22265
27. Scarpato S, Antivalle M, Favalli EG, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology(Oxford). 2010;49(2):289–294. doi:10.1093/rheumatology/kep354
28. Louder AM, Singh A, Saverno K, et al. Patient preferences regarding rheumatoid arthritis therapies: a conjoint analysis. Am Heal Drug Benefits. 2016;9(2):84–93.
29. Huynh TK, Østergaard A, Egsmose C, Madsen OR. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence. 2014;8:93–99. doi:10.2147/PPA.S55156
30. Hazlewood G, Bombardier C, Tomlinson G, et al. Treatment preferences of patients with early rheumatoid arthritis: a discrete-choice experiment. Rheumatology(Oxford). 2016;55(11):1959–1968. doi:10.1093/rheumatology/kew280
31. Augustovski F, Beratarrechea A, Irazola V, et al. Patient preferences for biologic agents in rheumatoid arthritis: a discrete-choice experiment. Value Heal. 2013;16(2):385–393. doi:10.1016/j.jval.2012.11.007
32. Horne R, Parham R, Driscoll R, Robinson A. Patients’ attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(6):837–844. doi:10.1002/ibd.20846
33. Salampessy BH, Veldwijk J, Schuit AJ, de Wit GA, Lambooij MS. The predictive value of discrete choice experiments in public health: an exploratory application. Patient. 2015;8(6):521–529. doi:10.1007/s40271-015-0115-2
Supplementary material
 |
Table S1 Example of a random choice task. Twelve random choice tasks without an “opt-out” or “no-treatment” option were included in the discrete choice experiment. |
 |
Table S2 Example of a dominant fixed choice task. Two dominant fixed choice tasks without an “opt-out” or “no-treatment” option were included in the discrete choice experiment. |
 |
Table S3 Final settings latent class analysis (Lighthouse Studio: CBC, Sawtooth Software) |
 |
Table S4 Results of the identification process of latent classes in this study |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



