Back to Journals » Patient Preference and Adherence » Volume 14
Preferences for Outcomes Among Adults with Type 1 Diabetes and Caregivers of Children with Type 1 Diabetes
Authors Marinac M, Sutphin J, Hutton C, Klein K, Sullivan S, Mansfield C
Received 14 May 2020
Accepted for publication 27 August 2020
Published 28 September 2020 Volume 2020:14 Pages 1719—1731
DOI https://doi.org/10.2147/PPA.S262358
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Video abstract presented by Marjana Marinac.
Views: 88
Marjana Marinac,1 Jessie Sutphin,2 Campbell Hutton,1 Kathleen Klein,2 Sean Sullivan,3 Carol Mansfield2
1JDRF, Washington, DC, USA; 2RTI Health Solutions, Research Triangle Park, NC, USA; 3The Leona M. and Harry B. Helmsley Charitable Trust, New York, NY 10169, USA
Correspondence: Carol Mansfield Email [email protected]
Purpose: Hemoglobin A1c (HbA1c) is the accepted measure of effectiveness for type 1 diabetes therapies. We investigated preferences for measures of diabetes control in addition to HbA1c among adults with type 1 diabetes and caregivers of children with type 1 diabetes.
Methods: Using discrete-choice experiment methodology, surveys for adults with type 1 diabetes and caregivers presented choices between hypothetical treatments described by six attributes with varying levels: HbA1c, time in optimal glucose range, weekly number and severity of hypoglycemic and hyperglycemic events, additional disease management time, and additional treatment cost. Choice data were analyzed using random-parameters logit.
Results: A total of 300 adults with type 1 diabetes and 400 caregivers completed the survey. Adults and caregivers placed the most importance on reducing hypoglycemic and hyperglycemic events. For adults, avoiding 1– 5 mild-to-moderate hypoglycemic events (glucose 54– 69 mg/dL)/week was five times more important than being a half-point above target HbA1c. Avoiding 1– 5 hyperglycemic events (glucose > 180 mg/dL)/week was seven times more important than being a half-point above target HbA1c. Additional time in optimal glucose range was as important as a reduction greater than a half-point in HbA1c. Avoiding hyperglycemic and hypoglycemic events was more important than all other outcomes for caregivers of younger children. Caregivers of children > 12 years placed relatively more weight on avoiding hypoglycemic events < 54 mg/dL than those with younger children and preferred avoiding additional costs.
Conclusion: Adults with type 1 diabetes and caregivers prioritize controlling hypoglycemic and hyperglycemic events, including mild-to-moderate events. These preferences should be considered in drug development and regulatory decisions.
Keywords: type 1 diabetes, discrete choice, adults, caregivers, stated preferences
Introduction
While frequent and consistent monitoring of blood glucose and dosing of insulin are required for disease management to prevent hypoglycemia, hyperglycemia, and the severe complications associated with them, they are time-consuming and burdensome for people living with type 1 diabetes and their caregivers.1 Despite diligent monitoring, many people with type 1 diabetes do not achieve recommended hemoglobin A1c (HbA1c) levels and experience frequent hyperglycemic and hypoglycemic events.2 In the United States (US), it is estimated that only 17% of children and adolescents and 21% of adults with type 1 diabetes meet HbA1c targets.3
HbA1c is a well-accepted measure of the efficacy of diabetes therapies and technologies and a surrogate for the risk of complications in diabetes. Nevertheless, HbA1c has limitations. Specifically, HbA1c does not capture short-term blood glucose variations or hypoglycemic or hyperglycemic events, which have a negative impact on disease management and functioning and increase health care utilization for individuals with type 1 diabetes.4–6
With recent advances in technologies for type 1 diabetes, it is possible to assess the benefits of its treatment with outcomes that are relevant to people with type 1 diabetes, such as hypoglycemia, hyperglycemia, and time in optimal blood glucose range. The leading diabetes clinician organizations, type 1 diabetes researchers, and funding organizations recently identified and standardized the definitions of clinically meaningful outcomes beyond HbA1c.1 Similarly, consensus statements issued by other diabetes research organizations have emphasized the importance of a broader range of measures than HbA1c alone when considering clinical outcomes that are relevant to people with type 1 diabetes.7–9
In recent years, there has been a growing recognition of the importance of incorporating patient preferences in health care decision-making. Many public and private sector organizations have developed formal processes for consulting with the patient community. Concurrently, the methodologies of measuring patient preferences quantitatively have become more widely accepted, and authorities such as the US Food and Drug Administration have encouraged the incorporation of such patient preference data in regulatory submissions and decisions. The objective of this study was to use discrete-choice experiment (DCE) methodology to quantify preferences among adults with type 1 diabetes and caregivers of children with type 1 diabetes for a set of treatment outcomes for type 1 diabetes beyond HbA1c.
Materials and Methods
The DCE, designed and conducted according to good research practices,10 was used to evaluate preferences for treatment outcomes of type 1 diabetes among adults with type 1 diabetes and caregivers of children with type 1 diabetes. DCEs provide quantitative measures of the relative importance of the features of multiattribute products (in this case, type 1 diabetes treatments). The study was reviewed and, because it was survey research and the survey procedures did not put respondents at risk of being identifiable, granted an exemption from full review by the RTI International Institutional Review Board and complied with the Declaration of Helsinki. The DCE was administered online to adults with type 1 diabetes and caregivers of children with type 1 diabetes in two separate survey instruments. Data collection occurred in two waves. For the adult survey, data collection occurred from 13 February 2018 to 23 March 2018 and from 27 August to 31 August 2018. For the caregiver survey, data collection occurred from 13 February 2018 to 4 April 2018 and from 27 August to 20 September 2018.
Survey Development
In the DCEs respondents were asked to choose between pairs of hypothetical treatments for type 1 diabetes (an example is in Figure 1). Each hypothetical treatment was defined by a set of six attributes with varying levels that represented different treatment outcomes that were of interest (Table 1). The attributes were a subset of the outcomes identified and defined by Agiostratidou et al.1 Treatment outcome measures were selected to focus on the day-to-day management of type 1 diabetes, whereas management time and personal cost were included to provide equivalence measures for the relative value of treatment outcomes. The attributes were presented in respondent-friendly language. The pattern of respondents’ choices among the hypothetical treatment profiles revealed their preferences among the treatment attributes.
 |
Table 1 Attributes and Levels |
 |
Figure 1 Example Choice Questions. (A) Patient Survey, Which treatment option would you choose? (B) Caregiver Survey, Which treatment option would you choose for your child? |
The combinations of attribute levels for the two hypothetical treatments presented in each DCE question were created by an experimental design, which was created in Sawtooth using a D-efficient algorithm to construct a fractional factorial experimental design.11–15 The design was evaluated for level balance and correlation. The design contained 36 DCE questions, split into four blocks of nine questions each, and respondents were randomly assigned to one of the four blocks. For each DCE question in the series, respondents were asked to assume the treatment levels described the outcome they or their child would experience over the next 3 months. Before the surveys were administered to the study samples, draft versions were cognitively pretested with adults with type 1 diabetes and with caregivers to ensure that respondents were willing to accept that outcomes would vary independently and were able to make tradeoffs between attribute levels in the treatment profile choice questions. In addition, the draft survey instruments were reviewed by clinical experts for clarity and effectiveness.
Study Populations
Survey Sampling International (SSI), a market research company with a specialty in health care, invited potential respondents from SSI’s opt-in panels and partner panels through a variety of channels to complete the survey. Eligible adults (aged ≥18 years) had a self-reported diagnosis of type 1 diabetes, were US residents, and had either used an insulin pump in the past or were currently injecting themselves with insulin ≥3 times/day. Eligible caregivers were aged ≥18 years; were the parent, guardian, or primary caregiver for a child aged <18 years with type 1 diabetes who lived with the respondent 7 days/week; and were US residents.
A quota-sampling approach was used to identify eligible respondents, such that data collection was discontinued once 300 adults with type 1 diabetes and 400 caregivers of children with type 1 diabetes had completed the survey. In addition, soft quotas were set for particular characteristics (eg, age <40 years, income <$50,000, race and ethnicity, health care provider type, and experience with an insulin pump and/or continuous glucose monitor [CGM]) to support analyses by subgroup.
Statistical Analyses
Responses to all survey questions were summarized using descriptive statistics. A random-parameters logit (RPL) model was used to analyze the DCE data, which relates the choices respondents make to the differences in the attribute levels across the alternatives in each choice question.16 The RPL model avoids the potential for estimation bias from respondents’ unobserved preference heterogeneity by estimating a distribution of preferences for each preference parameter.17,18 The cost variable was multiplied by the natural log of the respondent’s estimated income to account for differences in the marginal value of an additional dollar of income across income levels. Log-odds parameter estimates resulting from the RPL model are interpreted as relative preference weights, which indicate the relative strength of preferences for each attribute level, such that more preferred levels within a specific attribute have higher preference weights. The difference between the preference weights for the most- and least-preferred levels of each attribute is a measure of the attribute’s importance relative to the other attributes in the study given the range of levels of that attribute (ie, conditional relative importance).
Treatment choices for respondent subgroups also were analyzed using the same RPL model specification. For each mutually exclusive pair of subgroups, we created a variable that was equal to one if the respondent belonged to one of the two groups and interacted the variable with each of the explanatory variables in the equation. The parameter on each of these interaction terms can be interpreted as the difference between the two subgroups for the preference weights for the corresponding attribute level. Differences in preferences were tested through a log-likelihood 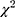 test of the joint statistical significance of all the interaction terms (P <0.05). For the adult survey, we tested for differences between respondents who currently use a CGM and those who do not, respondents who see an endocrinologist and those who do not, and those diagnosed more than 20 years ago compared to those diagnosed more recently. For caregivers, we tested for differences between respondents with children who currently use a CGM and those who do not, children who see an endocrinologist and those who do not, and children aged 11 years of age or less compared to children aged 12 to 17 years.
test of the joint statistical significance of all the interaction terms (P <0.05). For the adult survey, we tested for differences between respondents who currently use a CGM and those who do not, respondents who see an endocrinologist and those who do not, and those diagnosed more than 20 years ago compared to those diagnosed more recently. For caregivers, we tested for differences between respondents with children who currently use a CGM and those who do not, children who see an endocrinologist and those who do not, and children aged 11 years of age or less compared to children aged 12 to 17 years.
Monetary equivalents, also known as willingness to pay (WTP), were calculated using the RPL parameter estimates. WTP is the value the individuals in the sample place on an improvement in a medicine feature, expressed in monetary terms. WTP was calculated as the difference between the preference weights for two levels of an attribute divided by the preference weight for cost, multiplied by the natural log of median income.
The DCE results also were used to calculate preference shares, or the probability that the average respondent would select treatments with specific combinations of attribute levels.17 Preference shares were calculated for three pairs of treatment profiles. In the first profile pair, all attributes were held constant except HbA1c and time in optimal range. In the second and third profile pairs, hypoglycemic and hyperglycemic events were introduced.
Results
The target sample size of 300 adults with type 1 diabetes and 400 caregivers of children with type 1 diabetes who met the eligibility criteria, provided consent, and completed the survey were recruited for the survey through SSI’s opt-in panels and partner panels.
Respondent Characteristics
Tables S-1 and S-2 (Supplemental Appendix A), respectively, present the demographic characteristics and diabetes experience for adults with type 1 diabetes and caregivers of children with type 1 diabetes who completed the surveys.
The 300 adults with type 1 diabetes who completed the survey had a mean age of 47 years; 60% of the sample was female. Sixty percent were currently seeing an endocrinologist, 61.0% were currently using an insulin pump and/or a CGM, and 42.0% were currently using a CGM at the time of the survey. More than half (51.7%) had been diagnosed >20 years ago, whereas 33.7% had been diagnosed 6–20 years ago and 14.6% had been diagnosed ≤5 years ago. A majority had a most recent HbA1c result <7.0 (29.7%) or between 7.0 and 7.5 (27.7%); 15.3% were between 7.6 and 7.9, 13.0% were between 8.0 and 8.5, and 13.7% were >8.5. Most adults reported experiencing mild-to-moderate hypoglycemic events (54–69 mg/dL) at least once/week (48.0%) or at least once/day (14.7%) and reported experiencing serious hypoglycemic events (<54 mg/dL) at least once/month (34.7%) or at least once/week (20.3%). Most adults reported experiencing hyperglycemic events (181–250 mg/dL) at least once/week (39.7%) or at least once/day (36.3%) and reported experiencing hyperglycemic events with very elevated glucose (>250 mg/dL) at least once/month (28.7%) or at least once/week (36.0%).
The 400 caregivers who completed the survey had a mean age of 39 years; 59.3% of the sample were female. Caregivers’ children had a mean age of 10.7 years and received a type 1 diabetes diagnosis at a mean age of 6.7 years. Approximately half of the caregivers had children who were currently seeing an endocrinologist, 73.3% had children who were currently using an insulin pump and/or a CGM, and 59.3% had children who were currently using a CGM at the time of the survey. A minority of children had a most recent HbA1c result <7.0 (14.0%) or between 7.0 and 7.5 (27.3%); 27.0% were between 7.6 and 7.9, 12.0% were between 8.0 and 8.5, and 12.5% were >8.5. Most caregivers reported that their child experienced mild-to-moderate hypoglycemic events (54–69 mg/dL) at least once/month (32.0%), at least once/week (38.3%), or at least once/day (8.5%); nearly half reported that their children experienced serious hypoglycemic events (<54 mg/dL) at least once/month (28.3%) or at least once/week (19.8%). A majority of caregivers reported that their child experienced hyperglycemic events (181–250 mg/dL) at least once/month (22.8%), at least once/week (29.3%), or at least once/day (22.5%). A majority of caregivers reported that their child experienced hyperglycemic events with very elevated glucose (>250 mg/dL) at least once/month (23.8%) or at least once/week (27.5%).
Preference Weights and Attribute Relative Importance
Figure 2A and B present the normalized mean preference weight estimates for each attribute level among adults with type 1 diabetes and caregivers of children with type 1 diabetes. The preference weights indicate the ranking of levels within each attribute (ie, a higher preference weight indicates that a level is more preferred).
Among adults with type 1 diabetes, preferences for attribute levels were ordered as expected, with better levels within each attribute being preferred to worse levels. Respondents preferred experiencing fewer hypoglycemic events (glucose <69 mg/dL) and hyperglycemic events (glucose >180 mg/dL), having lower personal monthly treatment costs, achieving their HbA1c target at their next appointment compared with being more than a half-point above their target HbA1c, spending less time each day managing type 1 diabetes, and spending more time in optimal blood glucose range. Avoiding 1–5 mild-to-moderate hypoglycemic events (glucose 54–69 mg/dL) per week was five times more important to adults than being a half-point above their target HbA1c. Avoiding 1–5 hyperglycemic events (glucose >180 mg/dL) per week was seven times more important than being a half-point above their target HbA1c.
Among caregivers of children with type 1 diabetes, preferences for hypoglycemic events, hyperglycemic events, and cost were ordered as expected. Respondents preferred that their child experience fewer hyperglycemic and hypoglycemic events and preferred having lower personal monthly treatment costs. Attribute levels for additional time spent managing type 1 diabetes were not statistically significantly different, so respondents did not have a preference between spending any additional time, 30 extra minutes, or 60 extra minutes managing their child’s type 1 diabetes. Respondents preferred that their child spends more time in optimal blood glucose range, but their choices did not demonstrate a statistically significant difference in preferences between being in range almost all of the day (about 22 hours) and being in range more than half of the day (about 18 hours). The differences between all other attribute levels were statistically different from one another at the 5% level. For caregivers, avoiding 1–5 mild-to-moderate hypoglycemic events/week was three times more important than being more than a half-point above their target HbA1c. Avoiding 1–5 hyperglycemic events/week was six times more important than being more than a half-point above their target HbA1c.
For a single attribute, the vertical distance between the most-preferred level to the least-preferred level indicates the overall importance of that attribute for each sample. Figure 2A and B show the conditional relative importance weight estimates for each attribute. The most important change among adults with type 1 diabetes and among caregivers was a reduction in hypoglycemic events from a serious event and 5–7 mild-to-moderate events each week to 2–4 mild-to-moderate events each week, followed by a reduction in hyperglycemic events from a very high event and 5–7 high events each week to 2–4 high events each week, and a change in additional monthly treatment cost from $80 to $30.
Subgroup analyses revealed some differences in preferences across the samples (Figures S-1–S-6, Supplemental Appendix B). Specifically, adults with type 1 diabetes not currently using a CGM placed a higher relative importance on avoiding hypoglycemic events than those using a CGM. Adults with type 1 diabetes who were diagnosed >20 years ago also placed a higher relative importance on avoiding hypoglycemic events than those diagnosed more recently. Respondents whose children were not currently using a CGM placed higher relative importance on being in range almost all of the day (about 22 hours) compared with more than half of the day (about 18 hours) or half of the day (about 12 hours), whereas respondents whose children were currently using a CGM only placed higher relative importance on being in range more than half of the day compared with half of the day. While both groups indicated a strong relative preference for avoiding serious hypoglycemic events <54 mg/dL, respondents whose children were currently using a CGM placed statistically significantly more weight on reducing the number of hypoglycemic events from 5 to 7 mild-to-moderate events to 2 to 4 mild-to-moderate events (glucose 54–69 mg/dL) each week compared with those whose children do not use a CGM. Respondents who had a child who was currently seeing an endocrinologist placed higher relative importance on avoiding a serious hypoglycemic event (<54 mg/dL) compared with those who did not see an endocrinologist. For caregivers of younger children, avoiding hyperglycemic and hypoglycemic events was relatively more important than all other outcomes; however, caregivers of children older than 12 years placed relatively more weight than those with younger children on avoiding serious hypoglycemic events <54 mg/dL and indicated a relatively stronger preference for avoiding additional monthly personal treatment costs. There was no statistically significant difference in the preferences of adults who see an endocrinologist compared to those who do not using the test of joint significance.
Willingness to Pay Additional Out-of-Pocket Cost
Table 2 presents the WTP values/month for the different measures of improvement in type 1 diabetes control among adults with type 1 diabetes and caregivers of children with type 1 diabetes. The largest WTP values were for the most important changes, which were avoiding hypoglycemic and hyperglycemic events among both samples.
 |
Table 2 Willingness to Pay for Improvements in Treatment Outcomes |
Preference Shares
To calculate preference shares, we started with an initial pair of treatment profiles where all attributes were held constant except HbA1c test result at the next appointment and time in optimal range. In the first profile, time in optimal range was set to the most-preferred level and HbA1c test result at the next appointment was set to the least-preferred level. In the second profile, time in optimal range was set to the least-preferred level and HbA1c test result at next appointment was set to the most-preferred level. We then introduced the number of hypoglycemic and hyperglycemic events in an average week in two additional profile pairs. In the second pair, having fewer mild-to-moderate hypoglycemic events and fewer hyperglycemic events each week was paired with the most-preferred level of time in optimal range. In the third profile pair, fewer hypoglycemic and hyperglycemic events each week were paired with the most preferred level of HbA1c test result at the next appointment. Figure 3 presents the profiles used in and the results of the preference share predictions for adults with type 1 diabetes and caregivers.
 |
Figure 3 Preference Share Predictions. |
Among adults with type 1 diabetes, in the first comparison (profile pair number 1), the average respondent had approximately a 52% probability of choosing to achieve their target HbA1c and approximately a 48% likelihood of choosing to be in optimal blood glucose range almost all of the day (Figure 3). In the second comparison (profile pair number 2), when hypoglycemic and hyperglycemic events were no longer held constant, the average respondent had approximately an 80% probability of choosing Treatment A, which would result in fewer hypoglycemic and hyperglycemic events, being in optimal range almost all of the day, and being more than a half-point above their target HbA1c, when all other attributes were held constant (Figure 3). In the third comparison (profile pair number 3), when the fewest hypoglycemic and hyperglycemic events were paired with Treatment B instead of Treatment A, the average respondent had approximately an 83% probability of choosing Treatment B, which would result in fewer hypoglycemic and hyperglycemic events, being in optimal range half of the day, and achieving their target HbA1c, when all else was held constant. In both cases, when hypoglycemic and hyperglycemic events were no longer held constant, the model predicted that approximately 80% of the respondents in the sample would choose the treatment option resulting in fewer hypoglycemic and hyperglycemic events.
Among caregivers of children with type 1 diabetes, in the first comparison (profile pair number 1), when all else was held constant, the average respondent had approximately a 46% probability of choosing Treatment B, which would achieve their child’s target HbA1c, and a 54% probability of choosing Treatment A, which would keep their child in an optimal blood glucose range almost all of the day (Figure 3). In the second comparison (profile pair number 2), when hypoglycemic and hyperglycemic events were no longer held constant, the average respondent had approximately a 72% probability of choosing Treatment A, which would result in fewer hypoglycemic and hyperglycemic events, being in optimal range almost all of the day, and being more than a half-point above their target HbA1c, when all else was held constant. In the third comparison (profile pair number 3), when the fewest hypoglycemic and hyperglycemic events were paired with Treatment B instead of Treatment A, the average respondent had approximately a 65% probability of choosing Treatment B, which would result in fewer hypoglycemic and hyperglycemic events, being in optimal range half of the day, and achieving their target HbA1c, when all else was held constant (Figure 3). In both cases, the average respondent had a greater probability of choosing a treatment that would reduce the number of hypoglycemic and hyperglycemic events their child would experience each week.
Discussion
Recent research has sought to identify and standardize the definitions of clinically meaningful outcomes beyond HbA1c in type 1 diabetes.1,8,9 This study evaluated the preferences of people with type 1 diabetes and caregivers of children with type 1 diabetes over a set of these outcome measures, including target HbA1c, time in optimal glucose range, and number and severity of hypoglycemic and hyperglycemic events.
For the changes in outcomes presented in this study, adults with type 1 diabetes and caregivers of children with type 1 diabetes placed the greatest importance on reducing the frequency and severity of weekly hypoglycemic and hyperglycemic events relative to achieving their HbA1c target and more time in optimal range. Achieving the changes in HbA1c presented in the survey was not as important to respondents in both samples as reducing mild-to-moderate and serious hypoglycemic events and mild-to-moderate and very high hyperglycemic events each week. Generally, adults with type 1 diabetes and caregivers in the sample were willing to accept being more than a half-point above their HbA1c target to achieve better hyperglycemic and hypoglycemic outcomes in an average week. Respondents also valued reductions in weekly hyperglycemic and hypoglycemic events over increases in time in optimal range, given the ranges presented in the survey. Further, caregivers’ preferences did not demonstrate statistically significant differences between being in range almost all of the day and being in range more than half of the day for their children, but both levels were preferred to being in range half the day. This suggests caregivers value even moderate improvements in time in optimal range.
Taken together, this study’s results suggest that the survey respondents valued the benefit of reducing mild-to-moderate hyperglycemic and hypoglycemic events, as multiple daily insulin-dosing decisions are based in large part on blood glucose levels (and other factors). The respondents may well recognize that reductions in these events could lead to more time in range and, in the long run, to better HbA1c. However, given the choice between ranges presented in the survey, the respondents preferred reducing hyperglycemic and hypoglycemic events to achieving their HbA1c target.
Evidence suggests that people with type 1 diabetes—even those who receive quality care and have access to technology—struggle to achieve their HbA1c target and continue to experience burdensome hyperglycemic and hypoglycemic events. A need exists for more effective treatments to improve outcomes for patients with type 1 diabetes. Given the importance of hypoglycemic and hyperglycemic events, we cannot conclude with certainty that time in optimal range is unimportant to respondents. Time spent in optimal blood glucose range is difficult to know without technology such as CGMs and is a relatively new concept, albeit one that people with type 1 diabetes report has a considerable impact on their daily lives.19 Additionally, even if they do use technology, patients and caregivers may not be familiar with how to access this information or be comfortable with how to make broader dosing adjustments without input from their physicians.
Limitations
The results of the DCE survey should be interpreted in the context of limitations related to the survey instrument and sample. A limited number of outcomes can be included in a DCE. The outcome measures were selected from outcomes in the consensus report,1 and other outcome measures that people with type 1 diabetes care about were not included to keep the survey cognitively manageable and limited in length. Creating a DCE survey instrument requires balancing a thorough description of the treatment against the limits of respondent comprehension and burden. In the descriptions of the attributes and types of treatments, efforts were made to present neutral descriptions that provided an accurate, concise description of the benefits and risks. The survey text was reviewed by clinical experts and pretested with patients and caregivers of children with type 1 diabetes. The survey presents hypothetical scenarios to respondents, and a survey instrument does not replicate the experience of talking with a doctor about treatment options. Decisions made in the survey may not fully predict decisions made in a clinical setting, where other considerations may come into play. Moreover, the importance of the attributes relative to the other attributes included in the survey is conditional on the range presented for each attribute.
An additional limitation is that the clinical characteristics and experiences with type 1 diabetes collected in the survey were self-reported or reported by a parent or caregiver and not clinically confirmed. The samples were quota samples recruited through opt-in panels of individuals who choose to participate in research. Although the sample recruitment included quotas for age, race, income, and type 1 diabetes treatment experience, the study samples may not be representative of the broader population of adult patients with type 1 diabetes or caregivers of children with type 1 diabetes, potentially limiting the generalizability of the results. In particular, diabetes control as reported by the adult respondents and children of caregiver respondents may not be reflective of glycemic outcomes observed for the general type 1 diabetes population.3 Overall, there is the potential for the results to be biased toward individuals who are most interested in the management of type 1 diabetes, either because their diabetes is well managed through careful glycemic control or because they are concerned about their suboptimal glycemic outcomes. In addition, respondents’ experiences with hypoglycemic events may have influenced their preferences for avoiding hypoglycemia. Recent studies have shown that hypoglycemia, including severe and non-severe hypoglycemic episodes, is more common than previously thought,20,21 suggesting that understanding of hypoglycemic outcomes in this population is evolving.
Further, the final survey was administered online. Although research has shown that results from online stated-preference surveys are, in general, not statistically significantly different from those elicited through face-to-face interviews,22,23 the online setting of the survey may also have influenced the choices respondents made.
Four of the outcome features included in this study (HbA1c test result at next appointment, time in optimal range, and numbers of hypoglycemic and hyperglycemic events in an average week) are related. Pretests of the survey instruments confirmed that respondents could complete the DCE exercise despite the potential interconnection between the outcome measures and accepted the hypothetical scenarios where the outcome measures varied independently.
Conclusions
The primary measure of treatment effectiveness for type 1 diabetes therapies and technologies is currently HbA1c. This preference study sought to understand the importance of HbA1c and additional outcomes to patients and caregivers who live with the daily burden of managing type 1 diabetes. Overall, patients and caregivers prioritized control of weekly hypoglycemic events including mild-to-moderate events (54–69 mg/dL), and hyperglycemic events >180 mg/dL. These preference study results imply that outcomes used to measure the benefits of treatment in research, development, and reimbursement of therapies for type 1 diabetes should consider the importance that patients and caregivers place on reducing the frequency of hypoglycemic and hyperglycemic events.
Ethics
The study was reviewed and granted an exemption from full review by the RTI International Institutional Review Board (submission no. 14231). All participants provided informed consent electronically. The study followed the guidelines outlined in the Declaration of Helsinki.
Acknowledgments
Kimberly Moon of RTI Health Solutions provided overall project management for this study. Kate Lothman provided medical writing services, which were funded by JDRF and the Leona M. and Harry B. Helmsley Charitable Trust. This study was conducted under a research contract between RTI Health Solutions and JDRF and was funded by JDRF and the Leona M. and Harry B. Helmsley Charitable Trust.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was conducted under a research contract between RTI Health Solutions and JDRF and was funded by JDRF and the Leona M. and Harry B. Helmsley Charitable Trust.
Disclosure
KK, CM, and JS are salaried employees of RTI Health Solutions, which received research funding from JDRF and Leona M. and Harry B. Helmsley Charitable Trust for this study. CH and MM are salaried employees of JDRF. SS is a salaried employee of the Leona M. and Harry B. Helmsley Charitable Trust. The authors report no other conflicts of interest in this work.
References
1. Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40(12):1622–1630.
2. Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care. 2005;28:2361–2366. doi:10.2337/diacare.28.10.2361
3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66–72. doi:10.1089/dia.2018.0384
4. Brod M, Wolden M, Christensen T, Bushnell DM. A nine-country study of the burden of non-severe nocturnal hypoglycaemic events on diabetes management and daily function. Diabetes Obes Metab. 2013;15(6):546–557. doi:10.1111/dom.12070
5. Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. The burden of severe hypoglycemia in type 1 diabetes. Curr Med Res Opin. 2018;34(1):171–177. doi:10.1080/03007995.2017.1391079
6. Morales J, Schneider D. Hypoglycemia. Am J Med. 2014;127(10 Suppl):S17–S24. doi:10.1016/j.amjmed.2014.07.004
7. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care. 2017;40(1):155–157. doi:10.2337/dc16-2215
8. Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care. 2016;39(7):1175–1179. doi:10.2337/dc15-2716
9. Beyond A1C Writing Group. Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care. 2018;41(6):e92–e94. doi:10.2337/dci18-0010
10. Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413.
11. Chrzan K, Orme B An overview and comparison of design strategies for choice-based conjoint analysis. Technical paper series. Sawtooth Software Inc; 2000.
12. Kuhfeld W. Marketing Research Methods in SAS: Experimental Design, Choice, Conjoint, and Graphical Techniques. Cary (NC): SAS Institute Inc.; 2010.
13. Kuhfeld W Efficient experimental designs using computerized searches.
14. Kuhfeld W, Tobias F, Garratt M. Efficient experimental design with marketing research applications. J Mark Res. 1994;31:545–557. doi:10.1177/002224379403100408
15. Sawtooth Software, Inc. CBC User Manual. Sequim: Sawtooth Software; 1999.
16. Hauber AB, González JM, Groothuis-Oudshoorn C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
17. Train K. Discrete Choice Methods with Simulation.
18. Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, editors. Application of Simulation Methods in Environmental and Resource Economics. Dordrecht: Springer Publisher; 2005:117–134.
19. Runge AS, Kennedy L, Brown AS, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes. 2018;36:112–119. doi:10.2337/cd17-0094
20. Uzoigwe C, Hamersky CM, Arbit DI, Weng W, Radin MS. Assessing prevalence of hypoglycemia in a medical transcription database. Diabetes Metab Syndr Obes. 2020;13:2209–2216. doi:10.2147/DMSO.S235298
21. Ratzki-Leewing A, Harris SB, Mequanint S, et al. Real-world crude incidence of hypoglycemia in adults with diabetes: results of the InHypo-DM Study, Canada. BMJ Open Diabetes Res Care. 2018;6(1):e000503. doi:10.1136/bmjdrc-2017-000503
22. Nielsen JS. Use of the internet for willingness-to-pay survey: a comparison of face-to-face and web-based interviews. Res Energy Econ. 2011;33:119–129. doi:10.1016/j.reseneeco.2010.01.006
23. Marta-Pedroso C, Freitas H, Domingos T. Testing for the survey mode effect on contingent valuation data quality: a case study of web based versus in-person interviews. Ecol Econ. 2007;62(3–4):388–398. doi:10.1016/j.ecolecon.2007.02.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

