Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Predictors of Metabolic Syndrome Among People Living with HIV in Gedeo-Zone, Southern-Ethiopia: A Case–Control Study
Authors Bune GT, Yalew AW, Kumie A
Received 11 August 2020
Accepted for publication 10 September 2020
Published 6 October 2020 Volume 2020:12 Pages 535—549
DOI https://doi.org/10.2147/HIV.S275283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Girma Tenkolu Bune,1 Alemayehu Worku Yalew,2 Abera Kumie3
1School of Public Health, Dilla University, Dilla, Ethiopia; 2Schools of Public Health, Addis Ababa University/AAU, Addis Ababa, Ethiopia; 3Schools of Public Health, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Girma Tenkolu Bune
School of Public Health, Dilla University, Dilla, Ethiopia
Email [email protected]
Background: Intensive access to antiretroviral therapy improved the prognosis of HIV. As a result, a non-communicable disease risk marker known as metabolic syndrome (MS) has emerged. It is a public health issue in sub-Saharan Africa including Ethiopia. However, there is little literature on predictors of MS among people living with HIV (PLHIVs) in the study area context.
Purpose: To identify predictors of metabolic syndrome among PLHIVs, Gedeo Zone, Southern-Ethiopia.
Methods: Health institutions-based unmatched case–control study was conducted. All HIV-infected adult persons who are receiving routine care in the randomly selected two hospitals and two health centers of the Gedeo zone, southern Ethiopia were involved in the study, conducted from December 29th, 2017, to January 22nd, 2019. PLHIVs diagnosed with MS using ATP III criteria were considered as a case, and subjects free of MS in the survey were enrolled as controls. Binary logistic regression was employed to identify predictors of MS.
Results: A total of 633 (139 cases and 494 controls) PLHIVs were included in the study. The multivariable analysis result found that age (AOR=1.09, 95% CI (1.05– 1.12)); educational status being completed secondary school (AOR=0.22, 95% CI (0.02– 0.42)); occupational status being of students (AOR=0.11, 95% CI (0.24– 0.51)); wealth index being in the middle quintile (AOR=0.22, 95% CI (0.06– 0.79)); ART status exposed to ART (AOR=3.07, 95% CI (1.37– 6.89)); total physical activity state being physically active (AOR=0.36, 95% CI (0.16– 0.79)), and engaged in low levels physical activity (AOR=3.83, 95% CI (1.46– 10.05)) were the factors significantly associated with MS.
Conclusion: While education, occupation, wealth index, antiretroviral therapy status, total physical activity, and lower physical activity levels were concluded by the study as modifiable predictors of metabolic syndrome, age was found as a non-modifiable independent risk of metabolic syndrome. There is a need for an ongoing effort to realize an integrated care plan that addresses both the routine care and regular screening programs to reduce the risks associated with MS and its traits in these subjects.
Keywords: predictors, risk factors, metabolic syndrome, Gedeo zone, southern-Ethiopia
Introduction
Globally, there were 38.0 million [36.2 million adults] people living with HIV (PLHIVs), and 690 000 died from AIDS-related illnesses at the end of 2019.1 East and Southern Africa is the region hardest hit by HIV, in which there were nearly 20.6 million people infected, and around 310,000 people died of AIDS-related illnesses in 2018.2 Sub-Saharan Africa carries the highest-burden with an estimated 71% of the global total. In Ethiopia, an estimated 715 404 people were living with HIV in 2015 and this increased to 722 248 in 2017.3
Despite the continuing severity of the epidemic, as of the end of 2019, 25.4 million people were accessing antiretroviral therapy (ART) in the globe.1 In East and Southern Africa, 85% of 79% of PLHIVs were on treatment in 2018.2 In Ethiopia, 71% of PLHIVs are current on ART in 2016.4 With such expanding global access to the treatment, the prognosis of HIV/AIDS is improved.5–8
As a result, the worldwide morbidity and mortality from infectious diseases have occupied a backseat;9 instead, non-HIV-related chronic conditions, non-communicable diseases (NCDs) are increasingly emerging.9–11 The PLHIVs have high rates of NCDs5,12 due to the acquisition of non-AIDS-related comorbidities, like metabolic syndrome (MS).5
Metabolic syndrome (MS) is commonly defined as a constellation of interconnected complex diseases, like abdominal fat, high blood pressure, dyslipidemia, elevated blood sugar.7,13,14 There have been diverse standardized definitions used to measure overall MS,14 each with criteria that influence its diagnosis and complexity.9 Of which, the revised National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) was the most widely employed criteria in Africa,11,15,16 which is also used to ascertain the outcome in the current study.15,17
MS has a higher attendant economic and health system burden, resulted from the cost for identification and monitoring of it among PLHIVs, together with the routine care.13,18 It has a substantial undesirable impact on the treatment and the long-term prognosis of HIV patients due to NCDs.5
The significance of the MS is that it is a dominant predictor of future NCDs, primarily cardiovascular disease (CVDs) and type two diabetes mellitus (T2DM).14 NCDs are a major public health and medical challenges of the globe. It is rising disproportionately across countries. In 2016, over 3/4th of NCD deaths, 31.3 million deaths happenings in low- and middle-income countries.19 In Ethiopia, 52% of the total mortality in 2016 was reported to be an attribute of NCDs. The disease has a dramatic impact on out-of-pocket expenditures (OOP), accounts for 23% of total OOP expenditures.20
For this reason, there is a growing concern about what predicts MS among HIV-infected populations, yet little is known about this context. A recent crossectional study in Ethiopia Ataro and Ashenafiin, 2020 reported Age, education, and type of ART as predictors of MS.4 Another narrative review in people living with HIV in Africa Husain et al notified the following factors: female sex, age, time post-infection, exposure to ART, type of ART regimens, CD4 count, smoking, family history of diabetes, overweight, lipodystrophy, and total cholesterol as predictors of MS.13 This implies that the PLHIVs in Africa, including in the SSA share many common risk factors of NCDs, which are likely to be very large,12 encompass a combination of modifiable and non-modifiable individual-based factors.5,12
Additionally, a meta-analysis of 65 crossectional studies across five continents, including Africa documented that nevertheless, lifestyle or behavioral risks factors contribute to these conditions, additional influences unique to HIV-infected populations further rise their vulnerability to MS. Notably, disease severity indicator like the level of CD4+counts, the duration of diagnosed HIV infection, and exposure to ART was found as risk factors of MS. Furthermore, the study described that the use of ART is related to body fat redistribution and metabolic traits such as dyslipidemia, hypertension, insulin resistance, and dysglycaemia. The HIV infection itself through chronic inflammation and immune dysfunction condition is presumed to be essential predictors of dyslipidemia, atherosclerosis and type two diabetes mellitus.14
Evidence related to MS among HIV-infected individuals are compulsory to notify several HIV/AIDS programs and indicate the need for preventive and management strategies, and facilitates healthcare services design. However, there found few primary data on the predictors of MS in PLHIV in resource-limited settings.4
Unambiguously, in Ethiopia, even if there were few primary studies conducted crossectional on the study theme; there is no sufficient evidence on the predictors of MS among HIV-infected patients, using such a relatively stronger study approach. The study is perhaps vital to recognize and differentiate those, which are modifiable factors certain to reduce MS and its long-term complications (NCDs) in these subjects.
Therefore, this study sought to identify predictors of MS among PLHIVs, in the Gedeo Zone, Southern Ethiopia, and plug the gap in the existing literature.
Methods and Materials
Study Site
This study was conducted in the Gedeo zone, which is located in the Southern Nations, Nationalities, and Peoples (SNNP) region; 360km to the south of Addis Ababa, Ethiopia. It was made in the randomly chosen two hospitals (H) and two health centers (HC), namely: Dilla University referral and teaching hospital (DURH), Yirga-Cheffe primary hospital, Wonago HC, and Dilla town HC. During the study period, as the Gedeo zone ART case team Health Management Information system report reveals, there were 3597 adult PLHIVs (629 ART-naive (370 female) and 2968 current on ART (1813 female)). Of whom, while (n=135 ART naïve and n=537 ART exposed) were enrolled in the ART clinics of all public health centers, 412 and 2395 corresponding groups took services under the ART clinics of the public hospitals.
Study Design and Period
The unmatched case-control study design was conducted in between December 29th −2017 and January 22nd −2019.
Source and Study Population
Confirmed HIV-positive individuals; either previously enrolled in the randomly selected chronic HIV care clinics of the public health institutions found in the Gedeo zone, who revisited the clinics currently to take their routine care or enrolled in those clinics for the first time and participated in the prior survey arranged for one year were considered as a source population. The cases and the controls with or without the outcome of interest determined as per the criteria stipulated below were used as study populations.
Outcome Ascertainment
Metabolic Syndrome (MS)
Defined using the revised National Cholesterol Education Program (NCEP)-Adult Treatment Panel three (ATP III) criteria. Based on this criteria, the presence of three of the following criteria was employed to diagnose cases with the outcome of interest (MS): elevated waist circumferences (WC ≥ 102 cm or 40 inches (men), ≥88 cm or 35 inches (women)) or raised Body Mass Index (BMI ≥30 kg/m2); raised triglycerides: (triglyceride ≥1.7 (≥150 mg/dl) or treated; high-density lipoprotein cholesterol (HDL ≤ 40 mg/dL (Male), ≤50 mg/dL (Female)) or Treated; elevated blood pressure (systolic/diastolic BP ≥ 130/85 mmHg or treated with antihypertensive medication, and raised fasting plasma glucose (FpGl ≥110 mg/dl) or previously diagnosed with type 2 diabetes mellitus or anti-diabetic treatment).11
Case Finding
Cases were identified when they came into those randomly chosen public health institutions’ chronic HIV care clinics for their routine care and participated and eventually enumerated as a case (ie, with the outcome of interest) in the prior survey. The detail is described elsewhere.21
Eligibility Criteria for the Cases
Confirmed HIV-positive adults with or without antiretroviral therapy, who enrolled in the chronic HIV-care clinics of the public health institutions for the unrestricted period or for the first time and was recorded a case ascertained as per the revised ATP III criterion, whose age was ≥ to 18 years and below 70 years old were included in the study. The underline reason to take that age limit is due to the associate of age range more than or equal to 70 years old with metabolic dysfunction in most adults, after controlling for various factors, as suggested by various studies.4,13
Individual PLHIVs with the following conditions such as mental disorders, communication barriers, pregnant women, severely ill subjects, and have restrictions impede their participation were excluded from the study.
The control selection was accomplished using a cumulative case-control design. Accordingly, the control groups were sampled from among those who did not become cases (free of MS) or with lower than two numbers of ATP III criteria established to ascertain the outcome of interest in the prior survey.
Sample Size and Sampling Procedure
As this study is unmatched case-control, different sample sizes were calculated using Open Epi Version 3.03 with the assumption of power (% chance of detecting) of 80%, two-sided confidence level (1-alpha) of 95%, the case-control ratio of 1:3 hypothetical proportion of controls to be 19.1% and hypothetical proportion of cases with exposure to be 32.0%; estimated from a systematic review and meta-analysis study done in the globe by taking duration with HIV infection as one of the main exposure variables for MS that provide the maximum sample size.14 Accordingly, that yields a minimum sample size of 126 cases and 376 controls. Adding a 10% non-response rate, the final sample size required for the study were cases 139 and controls 414. To increase the power of the study, all cases observed during a year along with the corresponding three controls were included in the study. Concerning the sampling procedures, the detail is described elsewhere.21
Data Collection Methods and Materials
An interviewer assisted data collection method was used to accomplish the data collection procedure. Four teams were formed in each of the selected health institutions’; each with six people (a supervisor and five data collectors, ie, 3 ART nurses and two laboratory technologists). On the day of data collection, the ART nurses delivered information verbally on the survey and assess their eligibility. This was followed by securing written consent for each volunteer. After securing consent, the data collection process starting to be accomplished using a checklist and the WHO/NCD STEPS instrument version 3.2.22 The checklist was developed following reviewing the different medical records and employed to collect data from Pre ART/ART logbooks. It consists of questions regarding HIV/AIDS-related factors. The STEPS instrument was supplemented with the WHO step tool recommended show cards. In the first step: a questionnaire tool was used to gather data on respondent’s demographic and socioeconomic status; tobacco use; alcohol consumption; diet, including fruit and vegetable consumption, etc. in step 2: a physical data tool was used to build on the core data in step 1 and to determine the proportion of the study subjects with elevated blood pressure, and obesity. In step 3: a biochemical t tool was used to build on the core data in step 1 and step 2 and measure the proportion of subjects with diabetes, raised blood glucose, and abnormal lipid level. The Laboratory tests were performed with 8–12 hour overnight fast by drawing of 3–5 mL Venus blood. The detail is also presented in another place.21
Data Processing
The completed tools entered into a template formed using Epidata version 3.1 software and then transformed into Statistical Package for Social Sciences (SPSS) Version 22 for further analysis.
Data Analysis
The proportion, mean, and standard deviation measures were statistical techniques used to estimate the physical and biochemical associated risk factors. The dependent outcome variable was dichotomized as having metabolic syndrome (MS), and not having MS. Bivariable logistic regression was performed to see the crude association between the exposure and the outcome variable. All variables significant at the p<0.1 level in the bivariable analysis were moved to multivariable analysis to control the effects of confounding and to identify predictors of metabolic syndrome. All socio-economic and demographic, HIV/AIDS, and lifestyle actors with p < 0.1 in the bivariable analysis were entered once in the multivariable analysis, using an enter method, and those significant variables at (p < 0.05) were determined as predictors. For each predictor, the adjusted odds ratio (AOR) with 95% CI was estimated to assess the presence and strength of associations. All statistical tests were two-sided and considered statistically significant at a P-value of <0.05. Moreover, the goodness of fit of the model was checked using the Omnibus test of significance, model summary, Hosmer-Lemeshow goodness of fit test, and the classification table, before interpretation of the finding. Besides, multicollinearity between all the significant variables was checked using variance inflation factors (VIF), and no potential multicollinearity between independent variables was seen (VIF <10). The quantitative exposures variables were grouped into several ordered categories in the analysis. We defined some of the essential explanatory variables.
Variables and Criteria Used to Operationalize
Socio-Economic and Demographic Characteristics
Age in year categorized in to (≤34, 35–44, and ≥45); educational status grouped in to (no formal schooling, less than primary school, the primary school completed, the secondary school completed, the high school completed), and occupation considered in to (government employed, NGO-employed, self-employed, student, homemaker). Wealth index was estimated by principal component analysis based on eleven household variables measured household assets which are divided into quintiles to represent overall levels of household wealth: as quintiles, 1-lowest, quintiles 2-second, quintiles 3-middle, quintiles 4-fourth, and quintiles 5-highest, constructed as per the EDHS report (25).
HIV/AIDS-Related Factors
Antiretroviral therapy state categorized in to (ART exposed and ART naïve), and the WHO staging categorized in to (< stage III and ≥III stage).
Lifestyle/Behavioral Risk Factors
The total physical activity per day was recorded and analyzed using a continuous indicator (ie, time spent in participating in work-related, transport-related, and recreation-related activities), and then lastly grouped as (active=1, inactive=0). The percentage of respondents classified into three categories of total physical activity according to former recommendations as high, moderate, and low physical activity (PA). High physical activity was defined whenever a person reaching any of the following criteria: classified in the category of vigorous-intensity activity on at least 3 days achieving a minimum of at least 1500 MET-minutes/week or-7 or more days of any combination of walking, moderate- or vigorous-intensity activities achieving a minimum of at least 3000 MET-minutes per week. Moderate activity was defined when a person not meeting the criteria for the “high” category, but meeting any of the following criteria are classified in this category 3 or more days of vigorous-intensity activity of at least 20 minutes per day or- 5 or more days of moderate-intensity activity or walking of at least 30 minutes per day or- 5 or more days of any combination of walking, moderate- or vigorous-intensity activities achieving a minimum of at least 600 MET-minutes per week. Low activity was defined while a person not meeting any of the above-mentioned criteria falls in this category (133). Overall, an expectation and prior knowledge analysis procedures were used to handle missing interval/ratio and categorical variables, respectively, by taking the missing at random (MAR) assumptions into account. However, there were no methods used to examine subgroup analysis.
Data Quality Control
Typical data quality control procedures were implemented in each critical stage of the study. It started at the very beginning of the design stage of the study. The data collectors and supervisors were given two days of onsite training to standardize methods and ensure consistency of data collection. The tools were pre-tested in the non-considered health institutions to verify its appropriateness. The proper functioning of instruments, laboratory reagents, and technical performance was checked by using quality control samples. Standard operating procedures (SOPs) were followed starting from sample collection up to result reporting. All laboratory procedures were handled by laboratory technologists. Intensive monitoring and follow-up during each phase of data collection were undertaken by immediate supervisors. The principal investigator carried out monitoring in the study sites. A detailed explanation was found somewhere else.21
Research Ethics and Consent
All the ethical guidelines and principles placed in the Declaration of Helsinki and others, necessary to address the ethical aspects of the research initiated in humans were taken into account. Based on that, the proposal submitted to Addis Ababa University (AAU) College of Health Sciences School of public health (SPH) Research and Ethics Committee (REC) and then to the College of health science Institutional Review Board (IRB) (Meeting No.001/2017 and protocol No.0069/16/SPH) to obtain ethical clearance. Subsequently, the official letter granted from the SPH by citing the above ethical approval reference number was distributed to the respective Southern Nations Nationalities Regional health bureaus, Gedeo zone, and Woreda health bureaus, including to all of the institutions selected to conduct the study. Data were collected unlinked anonymously, without any personal identifiers. Each individual was enrolled entirely voluntarily after written consent was obtained. Any information obtained during the study was retained with the greatest confidentiality. Physical measurement was done by performing measurements at an ART clinic room that has been screened off to maintain the individual’s privacy. All biochemical analysis was performed free of charge, and results were provided to the clinicians for further investigation and possible management.
Results
Descriptive and Bivariable Analysis Results
Socio-Economic and Demographic Characteristics
A total of 633 people living with HIV (PLHIVs) (139 cases and 494 controls) were included in the analysis. Among the cases, more than half, 62.2% (87) were women and 67.6% (94) were from urban area residents. The result from the binary logistic regression analysis identified age, marital status, ethnicity, education, and occupation as significant factors associated with MS (Table 1).
 |
Table 1 Bivariable Analysis, Socio-Economic and Demographic Factors and MS, Cases and Controls, PLHIVs, Gedeo-Zone, Southern Ethiopia, 2019 |
HIV/AIDS-Related Factors
Sixty-eight point three percent (95) of the cases and 66.2% (327) of the controls were antiretroviral therapy (ART) exposed; of which higher proportion (63.3% (88) of the cases and 65.6% (324) of the control) was on the 1st line regimen. Nearly, one-third of the participants (71.2% (99) cases and 78.9% (390) controls) had been found less than three WHO staging levels. The mean viral RNA levels were 3501.7 copies per mL (+2379.3) for the cases and 1408.25 copies per mL (+1249.4) for the control. Around, 6% (9) of the cases and 2.8% (14) of the controls had more than 1500 copies per mL. A Bivariable analysis has shown that ART status, duration since diagnosed with HIV, opportunistic infections other than tuberculosis, type of ART regimen, use of 1st line and 2nd line ART were significantly associated with MS occurrence (Table 2).
 |
Table 2 Bivariable Analysis, in the HIV/AIDS-Associated Factors and MS, Cases, and Controls PLHIVs, Gedeo-Zone, Southern Ethiopia, 2019 |
Lifestyle/Behavioral Risk Factors
The mean age of smoking started for the cases and controls were (27.4 years (±7.5) vs 24.2 years (+5.0)), and the duration was (5.5 years (+2.1) vs 4.4 years (+1.8)), respectively. Nearly, 13% (19) of the case 9.7% (48) of the controls were current Khat chewer; 24.5% (34) and 14.2% (70) of the respected groups were used Khat daily. The average number of days per week on which fruit and vegetables consumed was 2.7 (±1.3) for the cases and 2.8 (±1.2) for the control group. More than half (71.2% (99)) of the cases and nearly 67% (331) of the control were consumed less than five servings of fruit and/or vegetables on average per day (Table 3). Further, around 28% (40) of the cases and 30.4% (150) of the controls did not meet the WHO recommendations on physical activity for health. Of which, nearly 80.6% (112) of the cases and 83.3% (413) of the controls were physically active; with the greatest contribution (73.4% (102) of the cases and 76.1% (376) of the control) were found from transport-related activity. The level of physical activity per day for the cases and the controls were found to be (55.8 (±72.3) and 61.1 (±70.1)) minutes per day, respectively. The mean time spent for sedentary life for the cases was 189.8minutes (+144.7) and the controls were 153.6 minutes (+90.0)(Table 3). Moreover, the result from binary logistic regression analysis revealed that smoking state, alcohol consumption status, all alcoholic consumer, the average frequency of serving fruit/vegetables/day, type of oil, total physical activity state, physical activity levels, running sedentary life, and daily chew Khat status as a factor associated with MS (Table 3).
 |
Table 3 Bivariable Analysis, in the Behavioral Risk Factors and MS, Cases and Controls, PLHIVs, in the Gedeo-Zone, Southern Ethiopia, 2019 |
Predictors of MS
The overall model fitness check statistics for the final model were as follows: (X2(df=30) = 84.83, p < 0.001, −2 Log likelihood = 314.151, Nagalkerke R2 =0.303 and Overall Percentage predicted 81.6%, and the Hosmer and Lemeshow Test of significant P= 0.905). The multi variable analysis result revealed that age (AOR=1.09, 95% CI (1.05–1.12)); educational status being completed secondary school (AOR=0.22, CI (0.02–0.42)); occupational status being of students (AOR=0.11, 95% CI (0.24–0.51)); wealth index being in the middle quintile (AOR=0.22, P=0.02, 95% CI (0.06–0.79)); ART status exposed to ART (AOR=3.07, 95% CI (1.37–6.89)); total physical activity state being physically active (AOR=0.36 95% CI (0.16–0.79)), and engaged in low levels physical activity (AOR=3.83,95% CI (1.46–10.05)) were significant predictors of MS development (Table 4).
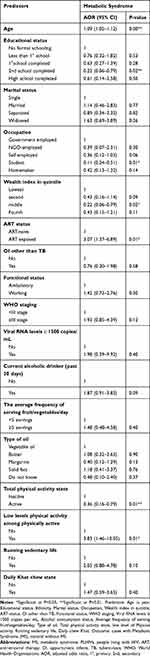 |
Table 4 Multivariable Analysis, in Between All Predictor and MS, Cases and Controls, PLHIVs, Gedeo-Zone, Southern-Ethiopia, 2019 |
Discussion
In areas where the routine screening of PLHIVs for metabolic syndrome in the chronic HIV care clinics is not regularly done, resources and evidence on HIV/AIDS comorbidities are constrained, the report from this study has public health and clinical implications that supplement the body of knowledge towards HIV-positive individuals. While education, occupation, wealth index, antiretroviral therapy status, total physical activity, and lower physical activity levels were concluded by the study as modifiable predictors of metabolic syndrome, age was found as a non-modifiable independent risk of metabolic syndrome (MS).
The finding implicated that the odds of increasing MS is 1.09 times higher with a unit increase in age in a year. Similarly, a marked disturbance in the MS with aging has also been reported by several epidemiological studies done worldwide.14,15,23–29 The observed comparability among the studies might be due to, regardless of the difference in the target population, age is also a common non-modifiable risk factor that equally likely predisposes the subjects to such health mater. In the reverse, Boshoet al 2018,30 Lívia D. Akl et al2017,31 Tesfaye et al 2014,15 and Kaduk et al 201232 were reported that age is not an independent predictor of MS. The differences across studies might be due to the variation in the studies approaches used; the differences in the standard criteria used and the variation in the characteristics of the target population, along with the time differences. Overall the finding might be highlighted, succeeding with the advance of ART, the PLHIVs have prolonged lifetimes and increasing age that eventually leads the subjects to be a victim to a chronic health problem like MS, by inflicting equivalent effect on PLHIVs, as they do in the general population. This signifies the risk of MS increases with the advance of age due to the regression of intrinsic metabolic actions.4
Inline, the study implicated that the odds of MS developing among PLHIVs completed secondary schooling was 0.22 times lower than individuals with no formal schooling. This was quite similar to a crossectional study from residents of Mizan-Aman town, South West Ethiopia, in which individuals with the educational status of below degree are up to 89% protective to develop MS than those with the educational status of degree and above.33 This finding correspondingly align with the studies from Kenya (Kiama, 2018 and Kaduk, 2012).32,34 This confirms that education is a key to open any locked secrets of life in the world that enable everybody to have a standardized and healthy lifestyle by getting a behavioral change. Unlike with our findings, few studies from Ethiopia (Ataro and Ashenafi, 2020,4 and Bosho, et al, 2018)30 were informed not enrolled in formal education and lack of formal education as a protective factor associated with metabolic syndrome. Likewise, Kagaruki, 2015,35 study from Tanzania shared, metabolic syndrome was significantly greater with a higher level of education. This might be an attribute of the differences in the measurement schemes implemented by the studies. Generally, the finding indicates the significance of the equitable distribution of education to the community to be accessed by all, regardless of their health condition, as one prevention measure.
The relation between occupation and the risk of MS is worth further discussion. Our result has shown that the odds of experiencing MS among student PLHIVs were 0.11 lower than individuals working as a government-employed. This corresponds with Mashinyaet al 201526 reports. In our view, the possible reason for the analogs report may not be out of the domains of variations on the income generated schemes in between the government employed, and not yet employed but worked as a student. This is because, since students always earn no income that they are run their life not by themselves rather by letting them dependent on either their families or any relevant others that eventually forced them to have assumed lower standards of lifestyle; which might contribute to have less risk and save from high standards of lifestyle-related health chronic diseases, including metabolic syndrome. Nonetheless, the planning of awareness creation action to all, irrespective of their occupational status may be played an essential role in the prevention of MS risks.
Our finding likewise asserted that the odds of experiencing MS were 0.22 times lower in the PLHIVs whose wealth index grouped under the middle quintile than those categorized in the lowest quintile. It was contradicted with a crossectional study done in St. Paul’s Hospital, in Ethiopia.36 This could be due to the differences in the type of study subjects involved in the study, the variation in the study approaches, and the method used to measure income by studies. This suggests, since we did not find validated evidence in the subject area, a future confirmatory study is needed to examine how wealth in quintiles influences the MS occurrences.
Notably, the odds of acquiring MS among ART exposed PLHIVs were 3.07 times higher than ART naïve individuals. Correlating with our finding, quite a lot of studies worldwide were revealed that exposure to ART as a predictor of MS, regardless of the effect of the other factors.14,15,37–41 In this regard, for example, a meta-analysis study across five continents showed that the MS prevalence in the ART exposed was significantly higher than in the ART-naïve.14 Correspondingly, a crossectional study from the southern region of Ethiopia notified that HIV patients on ART have higher risks to acquire MS than ART naïve subjects.15 Further studies from Italy,37 Spain,38 India,40 Thailand,25 Ghana,39 Burkina Faso,42 and Nigeria,41 also suggested similar reports. This could be due to the direct impacts of the mitochondrial dysfunction, oxidative stress, altered abiogenesis, and differentiations that are mostly associated with the development of every cluster of MS after exposure to a combination of treatment.27,43,44 The situations may accentuate the significances of awareness creation towards lifestyle-related risk factors such as physical activity, diet, and other harmful substance use habits reduction. However, our finding worth a confirmatory study with a strong design to be initiated to differentiate the specific effects of each drug regimen with MS incidence in these PLHIVs.
Finally, our study found that the odds of increasing MS among physically active PLHIVs were 0.36 times lower than physically inactive individuals, and 3.83 times higher in PLHIVs engaged in low levels of physical activity than individuals’ not engaged in such activity. This corresponds with different epidemiological studies.26,45,46 On the other hand, against our report, several works of literature were stated the absence association among the two variables.15,30,47 This might be partly explained due to the lower number of individuals grouped under each category that might affect the analysis. Another possible justification may be due to the nature of the data relies on self-reporting data, which is liable to response bias.
The study is one of the few primary studies conducted in the sub-Saharan Africa region, using a case-control study design among a relatively larger number of PLHIVs. To improve the power of the study, all cases seen during the one-year survey (gathered to respond for different objectives) together with the three corresponding controls were involved in the study. To decrease bias associated with measurements, the revised standardized ATP III criteria were used to identify the metabolic syndrome. Given the participants were found whenever they visited the clinics for the routine care, the inclusion probability of subjects in the study was not associated with their exposure status that there was an equivalent degree of recall among cases and controls. Besides, intensive training was given to data collectors, and there was firm supervision.
Despite all the efforts made, the reports in this study are subject to some limitations. As the study was restricted only in public hospitals, the finding may not typically be applied to patients found at the other health institutions of the study zone. Correspondingly, given the recruitment of subjects was undergone through the use of a consecutive sampling technique; this might cause the study misses its randomness that might lead one to conclude that the participants fail to represent cases of metabolic syndrome occurred in the Gedeo zone. Using secondary data for most HIV/AIDS exposure factors that could be the cause for missing of important confounding variables. But, we believe that the populations are homogenous and the selection bias is minimal. Moreover, the consideration of preexisting chronic disease state, family history, lipid-lowering drug in intake history and other clinical data related factors as part of outcome ascertainment criterion might confound the result. Hence, it is advisable to interpret the current study result carefully.
Conclusion
While education, occupation, wealth index, antiretroviral therapy status, total physical activity, and lower physical activity levels were concluded by the study as modifiable predictors of metabolic syndrome, age was found as a non-modifiable independent risk of metabolic syndrome (MS). Revising of the existing guideline for chronic HIV care and management strategy to address the routine screening of PLHIVs for MS, especially for those individuals exposed to ART with the advance of age is mandatory. Realizing an integrated care plan that addresses both the routine care given to PLHIVs and regular MS screening and management program in primary health care units is essential to reduce the potential risk related to long-term complications. In the meantime, strengthening the primary health care system towards that program will be a vital action to reduce future epidemic costs. Actions aimed at improving better access to formal education for PLHIVs at least to the level of secondary schooling are mandatory. Besides, there is a need for the provision of consistent health education to all about lifestyle modification particularly on performing low-level physical activity. Further, wide-ranging scale studies using a multi-center representative sample of HIV-infected subjects are essential to assess predictors of MS occurrence. Also, research is needed to confirm the relationship between occupational statuses, wealth indexes in quintiles with MS incidences among PLHIVs.
Note: This manuscript was prepared fully as per the guideline of the Reporting of Observational Studies in Epidemiology (STROBE).48
Data Sharing Statement
All data generated or analyzed during this study are included in this manuscript and uploaded tables.
Research Ethics and Consent
All the ethical guidelines and principles placed in the Declaration of Helsinki and others, necessary to address the ethical aspects of the research initiated in humans were taken into account. Based on that, the proposal submitted to Addis Ababa University (AAU) College of Health Sciences School of public health (SPH) Research and Ethics Committee (REC) and then to the College of health science Institutional Review Board (IRB) (Meeting No.001/2017 and protocol No.0069/16/SPH) to obtain ethical clearance. Subsequently, the official letter granted from the SPH by citing the above ethical approval reference number was distributed to the respective Southern Nations Nationalities Regional health bureaus, Gedeo zone, and Woreda health bureaus, including to all of the institutions selected to conduct the study. Data were collected unlinked anonymously, without any personal identifiers. Each individual was enrolled entirely voluntarily after written consent was obtained. Any information obtained during the study was retained with the greatest confidentiality. Physical measurement was done by performing measurements at an ART clinic room that has been screened off to maintain the individual’s privacy. All biochemical analysis was performed free of charge, and results were provided to the clinicians for further investigation and possible management.
Acknowledgments
My appreciation goes to Addis Ababa University and Dilla University for their financial support. I also would like to thank Dilla University College of medicine and health science, Yirga-Cheffe town administration health office, Wonago district health office, Dilla Town administration health office, Dilla University Referral Hospital, Yirga-Cheffe hospital, Wonago and Dilla town health center staffs, for their unreserved facilitation on the data collection. On top of that my deepest gratitude also sends to the whole study participants for their cooperation to take part in this study.
Author Contributions
All authors made a substantial contribution to conceptions and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
The study was initially funded by the Addis Ababa University College of Medicine School of public health. Additional funding was received from Dilla University. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest for this work.
References
1. UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet; 2020. Available from:https://www.unaids.org/en/resources/fact-sheet.
2. HIV/AIDS Giaeo. HIV and AIDS in East and Southern Africa; 2020. Available from: https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa.
3. Kibret GD, Ferede A, Leshargie CT, et al. Trends and spatial distributions of HIV prevalence in Ethiopia. Infect Dis Poverty. 2019;8(1):90. doi:10.1186/s40249-019-0594-9
4. Zerihun Ataro WA. Metabolic syndrome and associated factors among adult HIV positive people on antiretroviral therapy in Jugal Hospital, Harar, Eastern Ethiopia. East Afr J Health Biomed Sci. 2020;4(1):13–24.
5. Ataro Z, Ashenafi W, Fayera J, Abdosh T. Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV-positive individuals receiving highly active antiretroviral therapy at Jugal Hospital, Harar, Ethiopia. HIV/AIDS. 2018;10:181–192.
6. Han WM, Jiamsakul A, Kiertiburanakul S, et al. Diabetes mellitus burden among people living with HIV from the Asia-Pacific region. J Int AIDS Soc. 2019;22(1):e25236. doi:10.1002/jia2.25236
7. Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa—a systematic review and meta-analysis. Syst Rev. 2019;8(1):4. doi:10.1186/s13643-018-0927-y
8. David Napier A, Beverley Butler CA, Calabrese J, et al. Culture and health. Lancet. 2014;384(9954):1607–1639. doi:10.1016/S0140-6736(14)61603-2
9. Swami A. Metabolic syndrome and HIV infection. J HIV Retro Virus. 2016;2(1):9. doi:10.21767/2471-9676.100014
10. Willig AL, Overton ET. Metabolic complications and glucose metabolism in HIV infection: a review of the evidence. Curr HIV/AIDS Rep. 2016;13(5):289–296. doi:10.1007/s11904-016-0330-z
11. Paula AA, Falcao MC, Pacheco AG. Metabolic syndrome in HIV-infected individuals: underlying mechanisms and epidemiological aspects. AIDS Res Ther. 2013;10(1):32. doi:10.1186/1742-6405-10-32
12. Zenebework Getahun MA, Abuhay T, Abebe F. Comorbidity of HIV, hypertension, and diabetes and associated factors among people receiving antiretroviral therapy in Bahir Dar city, Ethiopia. J Comorbidity. 2020;10:1–12.
13. Husain NE, Noor SK, Elmadhoun WM, et al. Diabetes, metabolic syndrome and dyslipidemia in people living with HIV in Africa: re-emerging challenges not to be forgotten. HIV/AIDS. 2017;9:193–202.
14. Nguyen KA, Peer N, Mills EJ, Kengne AP. A Meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One. 2016;11(3):e0150970. doi:10.1371/journal.pone.0150970
15. Tesfaye DY, Kinde S, Medhin G, et al. Burden of metabolic syndrome among HIV-infected patients in Southern Ethiopia. Diabetes Metab Syndr. 2014;8(2):102–107. doi:10.1016/j.dsx.2014.04.008
16. Osoti A, Temu TM, Kirui N, et al. Metabolic syndrome among antiretroviral therapy-naive versus experienced HIV-infected patients without preexisting cardiometabolic disorders in western Kenya. AIDS Patient Care STDS. 2018;32(6):215–222. doi:10.1089/apc.2018.0052
17. Alencastro PR, Wolff FH, Oliveira RR, et al. Metabolic syndrome and population attributable risk among HIV/AIDS patients: comparison between NCEP-ATPIII, IDF and AHA/NHLBI definitions. AIDS Res Ther. 2012;9(1):29. doi:10.1186/1742-6405-9-29
18. Letebo M, Shiferaw F. Adapting HIV patient and program monitoring tools for chronic non-communicable diseases in Ethiopia. Global Health. 2016;12(1):26. doi:10.1186/s12992-016-0163-y
19. WHO. Global Health Observatory (GHO) data; 2020. Available from https://www.who.int/gho/hiv/en/.
20. Summary T. Addressing the impact of noncommunicable diseases and injuries in Ethiopia. Available for download at: http://www.ncdipoverty.org/ethiopia-report/. 2018.
21. Bune GT et al. The global magnitude of metabolic syndrome among antiretroviral therapy (ART) exposed and ART-naïve adult HIV-infected patients in gedio-zone, southern Ethiopia: comparative cross-sectional study, using the Adult Treatment Panel III criteria. Diabetes Metab Syndrome:Clinical Research & Reviews. 2019;13(2019):2833–2841.
22. WHO. The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS); 2005. Available from: https://www.who.int/ncds/surveillance/steps/instrument/en/. 20 Avenue Appia, 1211 Geneva 27, Switzerland. 2005.
23. Hirigo AT, Tesfaye DY. Influences of gender in metabolic syndrome and its components among people living with HIV virus using antiretroviral treatment in Hawassa, southern Ethiopia. BMC Res Notes. 2016;9(1):145. doi:10.1186/s13104-016-1953-2
24. Bekolo CE, Nguena MB, Ewane L, Bekoule PS, Kollo B. The lipid profile of HIV-infected patients receiving antiretroviral therapy in a rural Cameroonian population. BMC Public Health. 2014;14(1):236. doi:10.1186/1471-2458-14-236
25. Jantarapakde J, Phanuphak N, Chaturawit C, et al. Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDS. 2014;28(7):331–340. doi:10.1089/apc.2013.0294
26. Mashinya F, Alberts M, Van Geertruyden JP, Colebunders R. Assessment of cardiovascular risk factors in people with HIV infection treated with ART in rural South Africa: a cross sectional study. AIDS Res Ther. 2015;12(1):42. doi:10.1186/s12981-015-0083-6
27. Denue BA, Alkali MB, Abjah AU, Kida IM, Ajayi B, Fate BZ. Changes in lipid profiles and other biochemical parameters in HIV-1 infected patients newly commenced on HAART regimen. Infect Dis. 2013;6:7–14.
28. Wu PY, Hung CC, Liu WC, et al. Metabolic syndrome among HIV-infected Taiwanese patients in the era of highly active antiretroviral therapy: prevalence and associated factors. J Antimicrob Chemother. 2012;67(4):1001–1009. doi:10.1093/jac/dkr558
29. Pefura Yone EW, Betyoumin AF, Kengne AP, Kaze Folefack FJ, Ngogang J. First-line antiretroviral therapy and dyslipidemia in people living with HIV-1 in Cameroon: a cross-sectional study. AIDS Res Ther. 2011;8(1):33. doi:10.1186/1742-6405-8-33
30. Bosho DD, Dube L, Mega TA, Adare DA, Tesfaye MG, Eshetie TC. Prevalence and predictors of metabolic syndrome among people living with human immunodeficiency virus (PLWHIV). Diabetol Metab Syndr. 2018;10(1):10. doi:10.1186/s13098-018-0312-y
31. Haralambos MaRMH. Sociology: Themes and Perspectives. Oxford: Oxford University Press; 2002.
32. Kaduka LU, Kombe Y, Kenya E, et al. Prevalence of metabolic syndrome among an urban population in Kenya. Diabetes Care. 2012;35(4):887–893. doi:10.2337/dc11-0537
33. Kerie S, Menberu M, Geneto M, Oyeyemi AL. Metabolic syndrome among residents of Mizan-Aman town, South West Ethiopia, 2017: a cross sectional study. PLoS One. 2019;14(1):e0210969. doi:10.1371/journal.pone.0210969
34. Kiama CN, Wamicwe JN, Oyugi EO, et al. Prevalence and factors associated with metabolic syndrome in an urban population of adults living with HIV in Nairobi, Kenya. Pan Afr Med J. 2018;29:90. doi:10.11604/pamj.2018.29.90.13328
35. Kagaruki GB, Mayige MT, Ngadaya ES, et al. Knowledge and perception on type2 diabetes and hypertension among HIV clients utilizing care and treatment services: a cross sectional study from Mbeya and Dar es Salaam regions in Tanzania. BMC Public Health. 2018;18(1):928. doi:10.1186/s12889-018-5639-7
36. Mulugeta S, Mulugeta W. Disease burden and associated risk factors for metabolic syndrome among adults in Ethiopia. BMC Cardiovasc Disord. 2019;19(1):236. doi:10.1186/s12872-019-1201-5
37. Maloberti A, Giannattasio C, Dozio D, et al. Metabolic syndrome in human immunodeficiency virus-positive subjects: prevalence, phenotype, and related alterations in arterial structure and function. Metab Syndr Relat Disord. 2013;11(6):403–411. doi:10.1089/met.2013.0008
38. Estrada V, Martinez-Larrad MT, Gonzalez-Sanchez JL, et al. Lipodystrophy and metabolic syndrome in HIV-infected patients treated with antiretroviral therapy. Metabolism. 2006;55(7):940–945. doi:10.1016/j.metabol.2006.02.024
39. Obirikorang C, Quaye L, Osei-Yeboah J, Odame EA, Asare I. Prevalence of metabolic syndrome among HIV-infected patients in Ghana: a cross-sectional study. Nigerian Med j. 2016;57(2):86–90. doi:10.4103/0300-1652.182082
40. Kolgiri V, Nagar V, Patil V. Association of metabolic syndrome and oxidative DNA damage in HIV/AIDS patients. Indian j Clin Biochem. 2018;33(3):273–281. doi:10.1007/s12291-017-0670-5
41. Muhammad S, Sani MU, Okeahialam BN. Cardiovascular disease risk factors among HIV-infected Nigerians receiving highly active antiretroviral therapy. Nigerian Med j. 2013;54(3):185–190. doi:10.4103/0300-1652.114591
42. Guira O, Tieno H, Diendere AE, et al. Features of metabolic syndrome and its associated factors during highly active antiretroviral therapy in Ouagadougou (Burkina Faso). J Int Assoc Provid AIDS Care. 2016;15(2):159–163. doi:10.1177/2325957415601503
43. Srinivasa S, Grinspoon SK. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur j Endocrinol. 2014;170(5):R185–R202. doi:10.1530/EJE-13-0967
44. Palios J, Kadoglou NP, Lampropoulos S. The pathophysiology of HIV-/HAART-related metabolic syndrome leading to cardiovascular disorders: the emerging role of adipokines. Exp Diabetes Res. 2012;2012:103063.
45. Alvarez C, Salazar R, Galindez J, et al. Metabolic syndrome in HIV-infected patients receiving antiretroviral therapy in Latin America. Br j Infect Dis. 2010;14(3):256–263. doi:10.1016/S1413-8670(10)70053-2
46. Tiozzo E, Konefal J, Adwan S, et al. A cross-sectional assessment of metabolic syndrome in HIV-infected people of low socio-economic status receiving antiretroviral therapy. Diabetol Metab Syndr. 2015;7(1):15. doi:10.1186/s13098-015-0008-5
47. Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30(1):113–119.
48. von Elmg E, Altmanb DG, Eggera M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Vandenbrouckef, for the STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi:10.1016/j.ijsu.2014.07.013
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
