Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 9
Predictors of hypertension in an urban HIV-infected population at the University of Calabar Teaching Hospital, Calabar, Nigeria
Authors Okpa HO, Bisong EM, Enang OE, Monjok E , Essien EJ
Received 2 November 2016
Accepted for publication 11 January 2017
Published 14 February 2017 Volume 2017:9 Pages 19—24
DOI https://doi.org/10.2147/HIV.S126374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Henry Ohem Okpa,1 Elvis Mbu Bisong,2 Ofem Egbe Enang,1 Emmanuel Monjok,2,3 Ekere James Essien3
1Department of Internal Medicine, 2Department of Family Medicine, University of Calabar and University of Calabar Teaching Hospital, Calabar, Nigeria; 3Institute of Community Health, University of Houston, Texas Medical Center, Houston, TX, USA
Background: The introduction of highly active antiretroviral therapy (HAART) has remarkably improved the prognosis of human immunodeficiency virus (HIV)-infected patients, at the expense of the development of long-term complications such as cardiovascular and renal diseases. Hypertension (HTN) is a major risk factor for cardiovascular diseases and its associated mortality. In this study, we aimed to determine the prevalence of HTN and to identify possible predictors among HIV-infected patients attending the HIV Special Treatment Clinic at the University of Calabar Teaching Hospital, Calabar.
Materials and methods: A cross-sectional study was carried out over a 5-month period from February to July 2016. A total of 112 HIV-infected persons were consecutively recruited and their blood pressures were measured in two consecutive clinic visits. They were compared with the HIV-negative control group (n=309). Data collected were analyzed with SPSS 18, and statistical significance was set at P<0.05.
Results: There was a female preponderance in both the HIV-infected individuals and HIV-negative control group (57.5% vs. 57.4%). The mean ages were 39.3 and 33.9 years in HIV-infected and HIV-negative subjects, respectively. The risk factors that were associated with HTN in both groups were older age (>40 years), increased weight and body mass index (BMI), and presence of obesity. Male sex and duration of exposure to HAART and CD4 count levels >200 cells/mm3 were associated with HTN in HIV-infected patients, whereas the absence of family history of HTN was significantly associated with HTN in both groups. However, in a multivariate logistic regression, the predictors of HTN in both groups are absence of family history of HTN and older age in HIV-infected patients and HIV-negative subjects, respectively.
Conclusion: Traditional risk factors such as older age, increased BMI, and obesity were linked to HTN in both HIV-infected and HIV-negative subjects, but higher CD4 count level and cumulative HAART exposure were associated with HTN in HIV-positive individuals. In a multivariate logistic regression, the predictors of HTN in both groups are absence of family history of HTN and older age in HIV-infected patients and HIV-negative subjects, respectively.
Keywords: hypertension, urban, HIV, population, Calabar, Nigeria
Introduction
The survival of human immunodeficiency virus (HIV)-infected patients has increased significantly since the introduction of combination antiretroviral therapy (ART), leading to the development of important long-term complications including cardiovascular disease (CVD) and renal diseases.1 Hypertension (HTN) is the leading risk factor for cardiovascular and cerebrovascular mortality worldwide2 and is thought to be responsible for 45% of deaths due to heart disease and 51% of deaths due to stroke in 2013.3,4 HIV is more prevalent in sub-Saharan Africa than anywhere else in the world,5 but with improved survival of these patients with the use of highly active antiretroviral therapy (HAART), morbidity and mortality are associated with non-communicable diseases such as dyslipidemia, insulin resistance, and HTN.6,7
The mechanisms of HTN in HIV patients prior to the introduction HAART were often associated with complications related to HIV such as renal failure and vasculopathy.8 Also, the use of carotid intima/media thickness, an early marker of atherosclerotic disease, seems to progress more rapidly in HIV-infected patients than in the general population.9 The cause of such progressive vascular damage in HIV-infected patients is not clear. However, in the post-HAART period, some studies have suggested the possibility that HAART may induce HTN.10–12
There are various conflicting reports from the developed countries and sub-Saharan Africa on the prevalence of HTN in adults HIV-infected patients as compared to HIV-negative (HIV-N) individuals in the general population. Some studies in the developed countries reported higher HTN prevalence in HIV-positive (HIV-P) compared with HIV-N adults;13–15 others, also in developed countries, reported no significant association between HIV status and HTN.12,16,17 On the other hand, in sub-Saharan Africa, some studies reported higher prevalence of HTN in HIV-P patients as compared to HIV-N adults,18 some others found HIV infection to be associated with lower prevalence of HTN,19,20 whereas others reported HIV infection to be positively associated with CVD as a whole.21,22 Studies from Ethiopia and Uganda among the adult general population reported older age, male sex, family history, physical inactivity, salt-rich diet, and obesity as risk factors for HTN.18,23 Similar results were recently reported in a study in Uganda among HIV-infected patients.18
In view of the decline in HIV-related morbidity and mortality due to the availability of HAART, early detection of HTN might identify HIV-infected patients at high risk for developing CVD or kidney disease. To our knowledge, data on the HIV–HTN relationship and comparison with the general population in our environment are scarce.
This study was conducted to determine the blood pressure (BP) and prevalence of HTN in HIV-P persons attending the HIV special clinic at the University of Calabar Teaching Hospital (UCTH), Calabar, Nigeria, and comparing them with matched HIV-N controls to identify the possible predictors of HTN in both populations.
Materials and methods
Study settings, design, and population
This was a cross-sectional study carried out in the adult HIV clinic of the UCTH, Calabar. The UCTH is one of the major HIV treatment centers in Nigeria that is largely supported by family health international and receives referrals mainly from states in South–East and South–South geopolitical zones of Nigeria.
The study was conducted over a 5-month period from February to July 2016. Consecutive HIV patients attending clinic were recruited after meeting the study criteria, and informed written consent was obtained. Also, corresponding consecutive HIV-N (HIVN) controls were recruited in the study. The controls recruited were non-hypertensive health workers in the hospital such as doctors, nurses, pharmacists, laboratory scientists, and administrative staff.
Data on HIV infection, CD4 count, and ART were obtained from medical records. Duration since HIV testing was calculated from the first positive HIV test. Duration of ART was calculated from the ART starting date to the date of the first clinical visit, and patients were grouped according to the CD4 count levels. All participants were aged 18 years and above. Pregnant women and patients with signs of active AIDS mainly affecting the central nervous system where the patients present with alteration in the level of consciousness (either confused, restless, or comatose) or hospitalization in the previous 2 months were excluded.
Demographic parameters were obtained from the patients’ records. Height and weight were measured at the first clinical visit for estimation of the body mass index (BMI) in kg/m2. We used BMI to classify participants as underweight or normal weight (<25 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30 kg/m2).
The BP was measured with an Accoson sphygmomanometer and a standard sized cuff using the usual methods with the patient sitting quietly. The mean of two readings taken at least 2 min apart after the patient had rested in a relaxed sitting position for 5 min in a quiet room was recorded as the BP.
Diagnosis of HTN was confirmed on a second visit, not >2 months later, when two BP measurements were taken in an upper limb, and mean systolic and diastolic pressures were calculated. HTN was defined as systolic blood pressure (SBP) 140 mmHg at least and/or diastolic blood pressure (DBP) 90 mmHg at least, or history of HTN with the use of antihypertensive medications. Patients were further classified according to the criteria of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),24 which considers the following classifications of BP: normal (SBP <120 mmHg and DBP <80 mmHg), pre- HTN (SBP between 120 and 139 mmHg or DBP between 80 and 89 mmHg), and HTN (SBP ≥140 mmHg and DBP ≥90 mmHg).
Data analysis
The data obtained were analyzed using Statistical Package for Social Sciences (SPSS) software (version 18.0; SPSS, Inc., Chicago, IL, USA). Quantitative data are presented as mean±SD and categorical variables as percentages. Statistical comparisons were done using independent sample t-test, Pearson’s chi-square test, or Fisher’s exact test as appropriate. For comparisons between multiple groups, one-way analysis of variance (ANOVA) was used to test continuous variables. Significant levels were set at p<0.05.
Ethical consideration
Ethical approval was obtained from the Health Research Ethical Committee (HREC) of UCTH, Calabar, Nigeria. Verbal informed consent was obtained from all the participants in the study, and participants were informed that they had the right to discontinue or refuse to participate in the study. All data from the study were handled confidentially.
Results
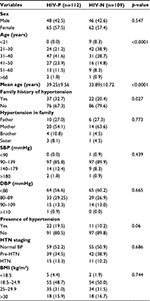
Table 1 shows that majority of the participants were females in both HIV-P and HIV-N group with 57.5% and 57.4%, respectively. Also, majority of the participants in both groups were in the 21–40 age category as compared to the 41–60 age category, p<0.05, while the mean age was significantly higher in HIV-P subjects. There was no family history of HTN in the majority of participants in both groups, and this was statistically significant (p<0.05).
In Table 2, all the parameters were higher in HIV patients with HTN (≥140/90 mmHg) as compared to those with normal BP and pre-HTN, except for the waist–hip ratio and CD4 count levels, which were higher in pre-HTN patients as compared to those with normal BP and HTN. All these were not statistically significant (p>0.05).
In Table 3, the mean age, weight, and BMI were significantly higher in HIV patients with HTN as compared to those without HTN, p<0.05. A proportion of HIV patients with a family history of HTN and obesity had HTN, and these were statistically significant (p<0.05). Also, the mean waist–hip ratio, duration of illness, exposure to ART, and CD4 count levels were higher in HIV patients with HTN as compared to those without HTN, but these were not statistically significant.
Table 4 shows the logistic regression model in HIV-infected patients, with HTN as the dependent variable, patients with the absence of family history of HTN were more likely to develop HTN than those with the presence of family history of HTN (p<0.05). The other variables in the model were not statistically significant.
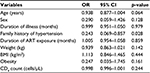  | Table 4 Logistic regression analysis for the predictors of hypertension in HIV patients Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; OR, odds ratio. |
Table 5 shows the regression model among the HIV-N subjects, and older age was significantly associated with the development of HTN (p<0.05). The other parameters were not significantly associated with the development of HTN.
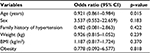  | Table 5 Logistic regression analysis for the predictors of hypertension in HIV-N subjects Abbreviations: BMI, body mass index; CI, confidence interval; HIV-N, HIV-negative; |
Discussion
Although there are various studies that have looked at cardiovascular risks among HIV-infected patients,25,26 but to the best of our knowledge, the study of the prevalence of HTN and its predictors in HIV patients as compared to the HIV-N subjects in our environment has not been investigated.
There was a female preponderance in both HIV-P and HIV-N patients with 57.5% and 57.4%, respectively. Also, HIV infection was commoner in the younger age group of 21–40 years constituting a little >60% of the HIV population, and this age group has been shown to be the sexually active component of the population. These findings are consistent with other studies in Africa18 and outside Africa.13,15
Using the criteria of the JNC 7,24 the prevalence of HTN was 13.3% in HIV-infected patients, similar and higher proportions have been observed in several studies.13,15,18
However, with the World Health Organization (WHO) criteria, the prevalence of HTN is 19.5% among HIV-infected persons. On the other hand, the prevalence of pre-HTN in HIV-P and HIV-N participants is 34.5% and 38.9%, respectively. Arruda et al observed similar proportion of pre-HTN among HIV-infected patients.15 These figures are clear indication that a lot more attention should be given to these group in terms of follow-up and regular check of BP. In the HIV-N control group, the prevalence of HTN was 10.2%, which is just a little lower than in the HIV population, and the finding is in in-line with the reports from other studies.14,27 The higher prevalence of HTN in HIV patients has been shown to be associated with the use of ART;14,28 however, there are contrasting evidence that the use of ART appears to lower the prevalence of HTN.13
Family history of HTN, which is one of the known traditional risk factors for HTN, was not associated with the presence of HTN in our study. It is possible that most of the participants were not conversant with the medical history of their family members, so most participants simply deny the presence of HTN in their family even when they were not sure. For the participants with the family history of HTN, most were in their mothers both in HIV-P and HIV-N patients, which accounted for 54.1% and 63.6%, respectively.
In our study, the risk factors that were associated with HTN in HIV-infected patients and the HIV-N controls were older age, increased weight and BMI, and presence of obesity. Male sex was associated with HTN in the HIV-infected population, but this was, however, not so in the general population. Our findings are consistent with reports from other studies.15,18 However, in a multivariate logistic regression analysis done in our study, the absence of family history of HTN was a predictor of HTN in HIV-infected patients, while older age was a predictor of HTN in HIV-N subjects.
In this study, obesity was a risk factor strongly associated with HTN, as in other studies that deal with HTN in a similar population.15,18 Obesity in individuals with HIV/AIDS may be associated with the weight gain that follows the use of HAART over a reasonable period of time.
Studies have shown that in the pre-HAART era, elevated BP was not common, and when present, it was frequently associated with HIV-related complications such as renal failure.8 We observed that the duration of HAART exposure was longer in HIV-P patients with HTN; although there are similar observations,13,15 there were also contrasting views in which the prevalence of HTN was low in patients treated with HAART.13,28 Some believe that the elevated BP observed may be due mainly to the metabolic side effects of ART, others have linked lipodystrophy to elevated BP, which was not looked at in this study.
The levels of CD4 lymphocytes <200 cells/mm3 are less associated with HTN in the HIV-infected patients, whereas the higher levels of CD4 >200 cells/mm3 are more likely to be linked to the presence of HTN. The reason for this may not be farfetched, as the use of HAART increases the levels of CD4 count with concurrent association with HTN due to this exposure to ART, as mentioned earlier. Arruda et al noted a similar observation in terms of the CD4 count category.15 Also, the mean values of CD4 count were lower in HIV-infected patients with HTN in our study. In view of these findings, it is therefore possible to deduce that lower CD4 count levels may be associated with some level of protection against the development of HTN.
Limitations
This study is not without limitations, as we mainly looked at the association of clinical parameters with HTN, while biochemical parameters such as various abnormalities in lipid profile and microalbuminuria have been observed to be strongly associated with HTN in HIV-infected patients.13,15 Also, another limitation of the study is that the diet and sodium intake of the participants and renal function of the participants which have been shown in several studies to have effect on BP were not looked at in our study.8,18,23 Moreover, this study was a cross-sectional single-center study and would have been better if the patients were followed up after the commencement of HAART especially in large multicenter studies. Lack of properly selected aged and sex-matched HIV-N control subjects may have affected some of the conclusions drawn from this study.
Conclusion
In conclusion therefore, our study indicates that HTN in HIV patients and HIV-N controls is associated with traditional risk factors such as older age, increased BMI, and obesity. However, male sex and duration of exposure to HAART and higher CD4 levels are associated with HTN in HIV-infected patients. In a multivariate logistic regression, the predictors of HTN in both groups are absence of family history of HTN and older age in HIV-infected patients and HIV-N subjects, respectively.
Acknowledgments
The authors acknowledge the University of Calabar Teaching Hospital HIV special clinic staff and the medical record staff for their unreserved support during the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Baekken M, Os I, Sandvik L, Oektedalen O. Microalbuminuria associated with indicators of inflammatory activity in an HIV-positive population. Nephrol Dial Transplant. 2008;23(10):3130–3137. | ||
Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. The World Health Organization; 2009. Available from: http://www.who.int/healthinfo/global_burden_disease/Global HealthRisks_report. Accessed October 20, 2016. | ||
Lloyd-Sherlock P, Ebrahim S, Grosskurth H. Is hypertension the new HIV epidemic? Int J Epidemiol. 2014;43(1):8–10. | ||
A Global Brief on Hypertension. The World Health Organization; 2013. Available from: http://ish-world.com/downloads/pdf/global_brief_hypertension. Accessed October 20, 2016. | ||
UNAIDS Report on the Global AIDS Epidemic 2013. UNAIDS; 2013. Available from: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013. Accessed October 20, 2016. | ||
Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32(1):130–139. | ||
Petersen M, Yiannoutsos CT, Justice A, Egger M. Observational research on NCDs in HIV-positive populations: conceptual and methodological considerations. J Acquir Immune Defic Syndr. 2014;67(Suppl 1):S8–S16. | ||
Winston J, Klotman PE. HIV-associated nephropathy. Mt Sinai J Med. 1998;65(1):27–32. | ||
Hsue PY, Lo JC, Franklin AR, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–1608. | ||
Aoun S, Ramos E. Hypertension in the HIV-infected patient. Curr Hypertens Rep. 2000;2(5):478–481. | ||
Chow DC, Souza SA, Chen R, Richmond-Crum SM, Grandinetti A, Shikuma C. Elevated blood pressure in HIV-infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials. 2003;4(6):411–416. | ||
Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naive and HIV negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22(12):731–736. | ||
Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV-positive population compared with the general population: influence of combination antiretroviral therapy. J Hypertens. 2008;26(11):2126–2133. | ||
Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21(7):1377–1382. | ||
Arruda Junior ER, Lacerda HR, Moura LC, et al. Risk factors related to hypertension among patients in a cohort living with HIV/AIDS. Braz J Infect Dis. 2010;14(3):281–287. | ||
Mattana J, Siegal FP, Sankaran RT, Singhal PC. Absence of age-related increase in systolic blood pressure in ambulatory patients with HIV infection. Am J Med Sci. 1999;317(4):232–237. | ||
Jerico C, Knobel H, Montero M, et al. Hypertension in HIV-infected patients: prevalence and related factors. Am J Hypertens. 2005;18(11):1396–1401. | ||
Dalsone K, Laura B, David H, et al. Population-based assessment of hypertension epidemiology and risk factors among HIV-positive and general populations in rural Uganda. PLoS One. 2016;11(5):e0156309. | ||
Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol. 2012;42(6):1754–1771. | ||
Schutte AE, Schutte R, Huisman HW, et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5-year prospective study. Int J Epidemiol. 2012;41(4):1114–1123. | ||
Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Afr. 2012;23(2):90–97. | ||
Schwartz T, Magdi G, Steen TW, Sjaastad I. HIV as a risk factor for cardiac disease in Botswana: a cross-sectional study. Int Health. 2012;4(1):30–37. | ||
Helelo TP, Gelaw YA, Adane AA. Prevalence and associated factors of hypertension among adults in Durame Town, Southern Ethiopia. PLoS One. 2014;9(11):e112790. | ||
Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. | ||
Friis-Moller N, Weber R, Reiss P, et al; DAD study group. Cardiovascular disease risk factors in HIV patients – association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–1193. | ||
Glass TR, Ungsedhapand C, Wolbers M, et al; Swiss HIV Cohort Study. Prevalence of risk factors for cardiovascular disease in HIV-infected patients over time: the Swiss HIV Cohort Study. HIV Med. 2006;7(6):404–410. | ||
Seaberg EC, Munoz A, Lu M, et al; Multicenter AIDS Cohort Study. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19(9):953–960. | ||
Palacios R, Santos J, Garcia A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7(1):10–15. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.