Back to Journals » Patient Preference and Adherence » Volume 15
Predictors of Health-Related Quality of Life in Patients with Ankylosing Spondylitis in Southwest China
Received 11 June 2021
Accepted for publication 22 August 2021
Published 28 August 2021 Volume 2021:15 Pages 1887—1894
DOI https://doi.org/10.2147/PPA.S324097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Naifeng Liu
Yuqing Song, Hong Chen
West China School of Nursing/West China Hospital, Sichuan University, Chengdu, Sichuan, 610041, People’s Republic of China
Correspondence: Hong Chen
West China School of Nursing/West China Hospital Sichuan University, No. 37, Guoxuexiang, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China
Tel +86 28 8542 2684
Email [email protected]
Purpose: The purpose of this study was to evaluate health-related quality of life (QoL) and explore its predictors in patients with ankylosing spondylitis (AS) in Southwest China.
Patients and Methods: We recruited AS patients from a tertiary hospital in Chengdu, China. Data were collected by self-reported questionnaires, including sociodemographic and disease-related variables, the Medical Outcomes Study 36-Item Short Form (SF-36), Beck Depression Inventory-Second Edition (BDI-II), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), and Bath Ankylosing Spondylitis Global score (BAS-G). Stepwise multiple linear regression analysis was used to determine the factors affecting physical component summary (PCS) and mental component summary (MCS) of SF-36.
Results: A total of 125 patients with AS were included in the current study. The PCS, MCS scores of SF-36 were 41.06± 9.12, 47.82± 9.84, respectively. Stepwise multiple linear regression analysis showed that higher educational level (β=0.237, P< 0.001) and income (β=0.141, P=0.017), lower BASDAI (β=− 0.195, P=0.006), BASFI (β=− 0.317, P< 0.001) and BAS-G (β=− 0.288, P< 0.001) scores were associated with higher PCS scores of SF-36. Higher BDI-II (β=− 0.444, P< 0.001) and fatigue (β=− 0.293, P< 0.001) scores were associated with worse MCS scores of SF-36.
Conclusion: AS patients in Southwest China had impaired health-related QoL. Healthcare providers should take effective strategies to modify the factors affecting health-related QoL, which may prompt disease management and increase QoL.
Keywords: ankylosing spondylitis, health-related quality of life, physical function, disease activity, depression, fatigue
Introduction
Ankylosing spondylitis (AS), as a subtype of spondyloarthritis (SpA), is a common inflammatory rheumatic disease with an unknown etiology.1–3 The main symptoms of AS patients are inflammatory back pain, morning stiffness, fatigue, enthesitis, extra-arthritis manifestations.2,4 During the disease process, AS may cause structural and functional limitation, psychological disorders (eg, depression, anxiety), reduced work productivity, and lead to impaired health-related quality of life (QoL) and substantial disease burden.5–8 The goal of AS management is to maximize long-term health-related QoL through controlling inflammation and improving function.1,2 Thus, health-related QoL of AS patients should be well studied before health professionals develop target interventions for improving health-related QoL.
Health-related QoL is a multi-domain concept that reflects the impact of illness and treatment on a person’s perception of his/her daily life, including physical and mental well-being.8–10 Existing evidence suggested that AS patients have poorer health-related QoL than the general population.7,8 Health-related QoL of AS patients was mainly assessed by the generic instrument- the Medical Outcomes Study 36-Item Short Form (SF-36) and the disease-specific instrument- the Ankylosing Spondylitis Quality of Life (ASQoL).7,8 Although ASQoL was developed for AS patients use,11 it may not reflect the multi-domain concept of Health-related QoL well. SF-36 contains 8 domains that can reflect the physical and mental health among different people.12,13 Thus, SF-36 can be used to assess the level and different aspects of health-related QoL among AS patients.
Although many previous studies have explored health-related QoL in AS patients,7,8,14–17 health-related QoL among AS patients in Southwest China is not well studied. QoL is a broad concept that can be affected by individual’s physical health, psychological state, social relationship, and the relationships with the environment.8,10 Previous studies indicated that disease activity and functional ability are the main factors affecting health-related QoL.7,8,18 However, the impacts of psychological disorders (eg, depression), sociodemographic variables (eg, age, educational level, marital status), health behaviors (eg, exercise, smoking status) on health-related QoL have not been well explored.
The aims of this analysis were to (1) assess health-related QoL of AS patients; (2) explore the associations between health-related QoL and sociodemographic variables, health behaviors, depression, and disease-specific variables among patients with AS in Southwest China.
Materials and Methods
Study Design and Participants
The data of this study were derived from baseline data of a randomized controlled trail (ChiCTR-IPR-16009293) in Chengdu, China.19 The patients meeting the following criteria were included in the current study: (1) patients were diagnosed as AS according to the modified New York criteria for the diagnosis of AS; (2) patients were ≥14 years old; (3) patients can communicate or read in Chinese language; (4) patients were willing to attend this study. Exclusion criteria were: having severe cognitive impairment and other rheumatic diseases.
Ethical Consideration
This study was conducted in accordance with the Declaration of Helsinki and approved by West China Hospital Medical Ethics Committee in China (ID 20160364). All participants were informed of the content and procedure of this study, and completed written informed consents.
Measures and Data Collection
All participants independently completed the questionnaires. Research assistants would help participants if they had difficulty in completing the questionnaires. The participants reported their information regarding sociodemographic characteristics (ie age, gender, educational level, marital status, monthly per capita income), disease-related information (peripheral involvement, extra-arthritis manifestation, disease activity, physical function, global well-being), behaviors (ie exercise, smoking status), and health-related QoL.
Evaluation of Disease Activity
We evaluated disease activity using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).20 It is a 6-item scale to measure fatigue, spinal and peripheral joint pain, localized tenderness, the severity and duration of morning stiffness.20 This scale reported good test-retest reliability.20 The BASDAI is scored in a range from 0 to 10. Lower scores represents less disease activity.
Evaluation of Physical Function
AS patients’ physical function was evaluated using the Bath Ankylosing Spondylitis Functional Index (BASFI). It is a 10-item scale to measure function in AS and reflect the patient’s ability to cope with everyday life.21 The total score of BASFI is the mean of 10 items with higher scores indicating worse physical function.21
Evaluation of Global Well-Being
We used the Bath Ankylosing Spondylitis Patient Global Score (BAS-G) to reflect the effects of the disease on patients’ well-being over the past week/6 month.22 The final BAS-G is scored from 0 (none) to 10 (very severe). Evidence suggested that this scale had good test–retest reliability, construct validity and predictive validity.22
Evaluation of Health-Related QoL
Health-related QoL was evaluated using the Chinese version of the Medical Outcomes Study Short Form 36-item Health Survey (SF-36).23,24 This 36-item instrument contains 8 domains: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH).24 The first 4 domains can be summarized into the physical component summary (PCS) and the last 4 domains can be summarized into the mental component summary (MCS).13 The 8 domains and 2 components are scored from 0 to 100 after recoding, and higher scores indicate better health-related QoL.12,13
Evaluation of Depression
Patients’ depressive symptoms were evaluated using the Chinese version of Beck Depression Inventory-Second Edition (BDI-II).25,26 This instrument includes 21 items assessing the severity of depressive symptoms over the past 2 weeks.27 Total scores of BDI-II range from 0 to 63, with higher scores reflecting more severe depressive symptoms.27
Statistical Analysis
All statistical analyses were conducted using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). We used descriptive statistics (eg, means, standard deviations, frequencies, and percentages) to describe the participants’ characteristics. The Spearman rank correlation, independent sample t-test and analysis of variance (ANOVA) test were used to explore factors associated with PCS and MCS of SF-36. Finally, stepwise multiple linear regression analyses were performed to explore the predictors of PCS and MCS scores. A P value less than 0.05 was considered to be statistically significant.
Results
We invited 140 patients with AS to participate in this study, and 132 patients were willing to participate in our study. Seven patients who did not meet the inclusion and exclusion criteria were excluded. Finally, 125 patients were included and completed the questionnaire.
Patients Characteristics
A total of 125 AS patients with a mean age of 29.8 years were enrolled in the current study. The majority of the participants had no extra-arthritis manifestation (103, 82.4%), did not smoke (85, 68.0%), and had no exercise habit (70, 56.0%). Around half of the patients had a high educational level (64, 51.2%) and peripheral involvement (53, 42.4%), and were married (60, 48.0%). Characteristics of the participants are shown in Table 1.
 |
Table 1 Characteristics of Participants and Relationship Between Sociodemographic, Disease-Related Variables and Health-Related QoL (N=125) |
Clinical Variables and Health-Related QoL
The scores of SF-36 and other clinical variables are shown in Table 2. For the 8 domains of SF-36, the most affected domains were role physical (45.40±43.00), general health (48.94±21.09). The mean (standard deviations, SD) PCS and MCS scores of SF-36 were 41.06±9.12, 47.82±9.84, respectively.
 |
Table 2 Clinical Variables and Health-Related QoL of AS Patients (N=125) |
Factors Associated with Health-Related Quality of Life
Patients with peripheral involvement and exercise habits had higher PCS scores than the others (P=0.001, 0.011, Table 1). The PCS scores varied by educational level, monthly per capita income (P<0.05, Table 1). Spearman’s test showed that BDI-II, BASFI, BASDAI, BAS-G and fatigue scores were negatively associated with PCS and MCS scores (all P<0.05, Table 3). However, age was only associated with PCS scores (P=0.46, Table 3).
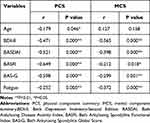 |
Table 3 Correlations Between Variables and Health-Related QoL (N=125) |
The results of stepwise multiple linear regression analyses using PCS and MCS scores as dependent variables are shown in Tables 4 and 5. The results showed that higher educational level (β=0.237, P<0.001) and income (β=0.141, P=0.017), lower BASDAI (β=−0.195, P=0.006), BASFI (β=−0.317, P<0.001) and BAS-G (β=−0.288, P<0.001) predicted higher PCS scores. The whole model could explain 62.9% of the total variance in PCS. For MCS as the dependent variable, BDI-II (β=−0.444, P<0.001) and fatigue (β=−0.293, P<0.001) scores negatively predicted the MCS scores. This model could explain 35.6% of the total variance in MCS.
 |
Table 4 Multiple Linear Regression Analyses with the PCS Score of SF-36 as Dependent Variable (N=125) |
 |
Table 5 Multiple Linear Regression Analyses with the MCS Score of SF-36 as Dependent Variable (N=125) |
Discussion
This study evaluated health-related QoL using the generic instrument SF-36 and explored their predictors among AS patients in Southwest China. We found that the most affected domains of SF-36 were role physical, general health, and bodily pain. Our results were similar to previous studies on AS patients, revealing that role physical and general health were the most affected domain of SF-36.28,29 Meanwhile, we found that the PCS scores were lower than the MCS scores. Previous studies revealed that physical component of health-related QoL is worse compared to mental component.9,28–30 AS patients often suffer from pain, morning stiffness, functional limitation, which may negatively affect physical component of health-related QoL.7 Thus, our findings emphasize that specific and effective intervention should be developed to improve health-related QoL of AS patients, particularly physical component.
AS patients with higher educational level reported higher PCS scores of SF-36. Mielck et al’s study among people with chronic disease found that people with low educational level reported more problems compared with the high education group.31 Lu’s cross-sectional study for AS patients revealed that patients with a lower educational level had a poor physical function QoL.14 Bodur et al32 also revealed that advanced educational attainment positively affects QoL. More/better education is associated with better income, occupational choice and disease management, leading to improved health-related QoL.14,33 Meanwhile, we found that patients with higher income had better PCS scores of health-related QoL, similar to previous study.31 AS causes a great economic burden for patients, such as medical expense.6,34 Economic status is an important factor of medication adherence and persistence.35 AS patients with good economic status may adhere to long-term treatment and management, which may lead to better physical health QoL. Thus, health professionals should pay more attention to patients with low educational level and household income.
We found that higher disease activity, worse physical function and global well-being predicted lower PCS scores of health-related QoL. Bodur et al32 conducted a cross-sectional study enrolling 962 AS patients and found that health-related QoL measured by SF-36 were strongly correlated with BASDAI and BASFI scores. Previous studies and reviews revealed that high disease activity, functional limitation were the most important determinants of a worse PCS score in AS patients.7,9,36 Additionally, some previous studies also reported that patients with worse overall wellbeing were correlated with worse PCS scores.8,18,36 Clinical variables, such as disease activity and symptom severity, influence the progress of the disease, which in turn affects physical component of health-related QoL.8 Pharmacological treatment, exercise, and self-management interventions are effective to increase AS patients’ health outcomes and QoL.3,37,38 Thus, the combined management is required to modify clinical variables and prompt health-related QoL of AS patients.
In the current study, depression and fatigue were negatively associated with the MCS scores of health-related QoL. Previous studies found that depressive symptoms were associated with impaired QoL.8,39 Depression leads to enhanced symptom burden, medication non-adherence, disability and negative clinical outcomes, which adversely affects mental health of QoL.8 Alkan et al40 found that fatigue was significantly associated with all SF-36 domains. Law et al’s study revealed that high level of fatigue was a factor associated with worse MCS scores.9 Zhou et al41 found that fatigue significantly reduced physical and mental aspects of QoL in AS patients. Fatigue, as a common complaint among AS patients, influences patients’ social life, relationships and work,42 which may have a negative impact on the mental component of health-related QoL. We suggest that the management of depression and fatigue may contribute to better mental component of QoL in AS patients.
Limitations
Several limitations in our study should be taken into account in future studies. The main limitation is that this analysis did not have a control population, such health population, patients with other rheumatic diseases. Secondly, the current study was also limited by a cross-sectional study design and a small sample size. Thirdly, health-related QoL may be different between the newly diagnosed patients and patients in the follow-up period. However, we included both of the patients and no recorded information can be used to compare health-related QoL of the newly diagnosed patients and patients in the follow-up period. This may limit the finding of this study. Finally, we did not have adequate financial support, so we only collected self-reported outcomes.
Conclusion
This study demonstrated that AS patients in Southwest China had an impaired health-related QoL. The most affected domains of SF-36 were role physical, general health, and the PCS scores were worse than MCS scores. Educational level, household income, disease activity, functional status, and global well-being were predictors of PCS of health-related QoL. Severe depressive symptom and fatigue predicted lower MCS score. Healthcare providers should take effective strategies to modify the factors affecting health-related QoL, which may prompt disease management and increase QoL.
Data Sharing Statement
The data used in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors appreciate all researchers who contribute to this research work and thank all the patients who participated in this study. A part of this study has been published in Arthritis Research & Therapy, 2021, 23(1):72. https://doi.org/10.1186/s13075-021-02453-7.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896–904. doi:10.1136/ard.2011.151027
2. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. doi:10.1136/annrheumdis-2016-210770
3. Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic Axial spondyloarthritis. Arthritis Rheumatol. 2019;71:1599–1613. doi:10.1002/art.41042
4. Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. doi:10.1016/S0140-6736(07)60635-7
5. Martindale J, Shukla R, Goodacre J. The impact of ankylosing spondylitis/axial spondyloarthritis on work productivity. Best Pract Res Clin Rheumatol. 2015;29:512–523. doi:10.1016/j.berh.2015.04.002
6. Malinowski KP, Kawalec P. The indirect costs of ankylosing spondylitis: a systematic review and meta-analysis. Expert Rev Pharmacoecon Outcomes Res. 2015;15:285–300. doi:10.1586/14737167.2015.1001370
7. Yang X, Fan D, Xia Q, et al. The health-related quality of life of ankylosing spondylitis patients assessed by SF-36: a systematic review and meta-analysis. Qual Life Res. 2016;25:2711–2723. doi:10.1007/s11136-016-1345-z
8. Kotsis K, Voulgari PV, Drosos AA, et al. Health-related quality of life in patients with ankylosing spondylitis: a comprehensive review. Expert Rev Pharmacoecon Outcomes Res. 2014;14:857–872. doi:10.1586/14737167.2014.957679
9. Law L, Rehnman JB, Deminger A, et al. Factors related to health-related quality of life in ankylosing spondylitis, overall and stratified by sex. Arthritis Res Ther. 2018;20:284. doi:10.1186/s13075-018-1784-8
10. Skevington SM, Lotfy M, O’Connell KA; WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310. doi:10.1023/B:QURE.0000018486.91360.00
11. Doward LC, Spoorenberg A, Cook SA, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis. 2003;62:20–26. doi:10.1136/ard.62.1.20
12. Ware JE
13. Ware J, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: a User’s Manual. Boston, MA: Health Assessment Lab, 1994.
14. Lu MC, Huang KY, Tung CH, et al. Factors associated with disease-specific quality of life in Taiwanese patients with ankylosing spondylitis: a cross-sectional study. BMJ Open. 2019;9:e028966. doi:10.1136/bmjopen-2019-028966
15. Sato T, Yonezawa I, Inoue H, et al. Relationship between characteristics of spinopelvic alignment and quality of life in Japanese patients with ankylosing spondylitis: a cross-sectional study. BMC Musculoskelet Disord. 2020;21:41. doi:10.1186/s12891-020-3040-z
16. Sang Y, Dong C, Fu T, et al. Associated factors with adherence to standard exercise therapy and health-related quality of life in Chinese patients with ankylosing spondylitis. Mod Rheumatol. 2020;30:149–154. doi:10.1080/14397595.2018.1559966
17. Chiowchanwisawakit P, Thaweeratthakul P, Wattanamongkolsil L, et al. Relationship between health-related quality of life and patient acceptable symptom state with disease activity and functional status in patients with ankylosing spondylitis in Thailand. J Clin Rheumatol. 2019;25:16–23. doi:10.1097/RHU.0000000000000750
18. Dean LE, Macfarlane GJ, Jones GT. Five potentially modifiable factors predict poor quality of life in ankylosing spondylitis: results from the Scotland Registry for ankylosing spondylitis. J Rheumatol. 2018;45:62–69. doi:10.3899/jrheum.160411
19. Song Y, Xie X, Chen Y, et al. The effects of WeChat-based educational intervention in patients with ankylosing spondylitis: a randomized controlled trial. Arthritis Res Ther. 2021;23:72. doi:10.1186/s13075-021-02453-7
20. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–2291.
21. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol. 1994;21:2281–2285.
22. Jones SD, Steiner A, Garrett SL, et al. The Bath Ankylosing Spondylitis Patient Global Score (BAS-G). Br J Rheumatol. 1996;35:66–71. doi:10.1093/rheumatology/35.1.66
23. Li L, Wang H, Shen Y. Development and psychometric tests of a Chinese version of the SF-36 Health Survey Scales. Chin J Prev Med. 2002;36:109–113.
24. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi:10.1136/bmj.305.6846.160
25. Wang Z, Yuan CM, Huang J, et al. Reliability and validity of the Chinese version of Beck Depression Inventory-II among depression patients. Chin Mental Health J. 2011;25:476–480.
26. Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Braz J Psychiatry. 2013;35:416–431. doi:10.1590/1516-4446-2012-1048
27. Erford BT, Johnson E, Bardoshi G. Meta-analysis of the English version of the Beck Depression Inventory–Second Edition. Meas Eval Couns Dev. 2017;49:3–33. doi:10.1177/0748175615596783
28. Huang JC, Qian BP, Qiu Y, et al. Quality of life and correlation with clinical and radiographic variables in patients with ankylosing spondylitis: a retrospective case series study. BMC Musculoskelet Disord. 2017;18:352. doi:10.1186/s12891-017-1711-1
29. Chen HH, Chen DY, Chen YM, et al. Health-related quality of life and utility: comparison of ankylosing spondylitis, rheumatoid arthritis, and systemic lupus erythematosus patients in Taiwan. Clin Rheumatol. 2017;36:133–142. doi:10.1007/s10067-016-3471-y
30. Chen MH, Lee MH, Liao HT, et al. Health-related quality of life outcomes in patients with rheumatoid arthritis and ankylosing spondylitis after tapering biologic treatment. Clin Rheumatol. 2018;37:429–438. doi:10.1007/s10067-017-3965-2
31. Mielck A, Vogelmann M, Leidl R. Health-related quality of life and socioeconomic status: inequalities among adults with a chronic disease. Health Qual Life Outcomes. 2014;12:58. doi:10.1186/1477-7525-12-58
32. Bodur H, Ataman S, Rezvani A, et al. Quality of life and related variables in patients with ankylosing spondylitis. Qual Life Res. 2011;20:543–549. doi:10.1007/s11136-010-9771-9
33. Cutler D, Lleras-Muney A. Education and health: evaluating theories and evidence. National Bureau of Economic Research (NBER) Working Paper No. 12352.Cambridge, 2006.
34. Tu L, Rai JC, Cao S, et al. Costs and work limitation of patients with ankylosing spondylitis in China. Clin Exp Rheumatol. 2014;32:661–666.
35. Anghel LA, Farcaş AM, Oprean RN. Medication adherence and persistence in patients with autoimmune rheumatic diseases: a narrative review. Patient Prefer Adherence. 2018;12:1151–1166. doi:10.2147/PPA.S165101
36. Ibn Yacoub Y, Amine B, Laatiris A, et al. Health-related quality of life in Moroccan patients with ankylosing spondylitis. Clin Rheumatol. 2011;30:673–677. doi:10.1007/s10067-010-1613-1
37. Marques A, Santos E, Nikiphorou E, et al. Effectiveness of self-management interventions in inflammatory arthritis: a systematic review informing the 2021 EULAR recommendations for the implementation of self-management strategies in patients with inflammatory arthritis. RMD Open. 2021;7:e001647. doi:10.1136/rmdopen-2021-001647
38. Regnaux JP, Davergne T, Palazzo C, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019;10:CD011321.
39. Hyphantis T, Kotsis K, Tsifetaki N, et al. The relationship between depressive symptoms, illness perceptions and quality of life in ankylosing spondylitis in comparison to rheumatoid arthritis. Clin Rheumatol. 2013;32:635–644. doi:10.1007/s10067-012-2162-6
40. Alkan BM, Fidan F, Erten S, et al. Fatigue and correlation with disease-specific variables, spinal mobility measures, and health-related quality of life in ankylosing spondylitis. Mod Rheumatol. 2013;23:1101–1107. doi:10.3109/s10165-012-0800-0
41. Zhou W, Guo J, He M, et al. Fatigue and contributing factors in Chinese patients with ankylosing spondylitis. Clin Rheumatol. 2020;39:2337–2344. doi:10.1007/s10067-020-04976-x
42. Farren W, Goodacre L, Stigant M. Fatigue in ankylosing spondylitis: causes, consequences and self-management. Musculoskeletal Care. 2013;11:39–50. doi:10.1002/msc.1029
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
