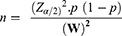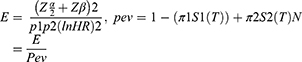Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Predictors of Anemia Among Adult HIV Positive Patients on First-Line Antiretroviral Therapy in Northwest Ethiopia: A Retrospective Follow-Up Study
Authors Agegnehu CD , Merid MW, Yenit MK
Received 26 November 2020
Accepted for publication 13 April 2021
Published 29 April 2021 Volume 2021:13 Pages 455—466
DOI https://doi.org/10.2147/HIV.S280338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Chilot Desta Agegnehu,1 Mehari Woldemariam Merid,2 Melaku Kindie Yenit2
1School of Nursing, College of Medicine and Health Sciences and Comprehensive Specialized Hospital, University of Gondar, Gondar, Ethiopia; 2Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Chilot Desta Agegnehu
School of Nursing, College of Medicine and Health Sciences and Comprehensive Specialized Hospital, University of Gondar, P.O. Box: 196, Gondar, Ethiopia
Tel +251 9-18-62-74-03
Email [email protected]
Background: Globally, anemia is a common hematological disorder among HIV-infected patients. People with anemia often suffer from impaired physical functioning, psychological distress, and poor quality of life. Therefore, the aim of this study was to determine the incidence of anemia and its determinants among HIV positive individuals in northwest Ethiopia.
Methods: A total of 486 adult HIV positive patients on the first-line ART with complete information were enrolled in the adult care clinics of northwest Amhara referral hospitals from December 2015 to December 2018. EpiData version 4.2 was used for data entry and Stata version 14 for analysis. Variables having time to event nature were presented with the Kaplan–Meier function. The Cox regression model was used to identify predictors of anemia. Variables with P-values less than 0.2 in the bivariable analysis were considered in the multivariable regression. Adjusted hazard ratio with 95% CI was computed, and variables with less than 0.05 P-values in the multivariable Cox regression were taken as significant predictors of anemia.
Results: This study noted an overall 26.4 per 100 person-year observations (95% CI: 23.46, 30.74) incidence rate of anemia. According to the multivariable Cox regression, TB co-infection (AHR =1.99, 95% CI: 1.45, 2.74), zidovudine-based regimen (AHR=1.39, 95CI: 1.1, 1.85), CD4 level (AHR= 1.7, 95% CI: 1.23, 2.35), advanced WHO stage (AHR=1.32, 95% CI: 1.01, 1.74), and being underweight (AHR= 1.53, 95% CI: 1.14, 2.07) were predictors of anemia.
Conclusion: Anemia is a burden among HIV patients in the study setting. Baseline clinical variables, TB co-infection, and zidovudine-based were predictors of anemia. Therefore, early identification of anemia and addressing significant predictors are highly suggested to the study setting.
Keywords: anemia incidence, HIV, first-line HAART, Ethiopia
Background
Anemia is the frequent hematological disorder among people living with HIV.1–3 It is a condition occurred when the number of red blood cells (RBCs) or the amount of hemoglobin decreases in the blood. Red blood cells and hemoglobin are responsible for carrying oxygen to vital organs throughout the body.4 Patients with anemia experience easy fatigability, increase heartbeat, shortness of breath, and dizziness.5 These symptoms occurred when the body attempts to deliver poorly oxygenated blood to all of the vital organs. The cause of anemia in people with HIV is multifactorial and results in poor treatment outcomes.6 The virus itself can infect and induce the myelosuppressive effect on the bone marrow, resulting in decreased RBC.7 Several pathogenetic mechanisms have been implicated for the myelosuppressive effect on the hematopoietic system occurring during the course of HIV infection. Some of which includes altered cytokine production by infected CD4+marrow cells, production of inhibitory factors by infected marrow stromal cells, antibody-mediated suppression of hematopoietic progenitors, direct infection of hematopoetic progenitors and/or precursors, and HIV-1 mediated suppression of CD34+ cell growth in the absence of a complete viral replication cycle.
Besides, opportunistic infections, nutritional deficiencies, bleeding, and multiple drugs of antiretroviral therapy results in the development of anemia.8
The use of highly active antiretroviral therapy (HAART) has the capacity of reducing the incidence of severe anemia by suppressing viral replication and increase CD4 cell count. In spite of this, mild-to-moderate forms of anemia continue to be common problem. Although Antiretroviral Therapy (ART) increases hemoglobin concentrations and are effective in viral load reduction and restoration of the immune system, people with anemia often suffer from impaired physical functioning, psychological distress and poor quality of life.9 Anemia also facilitates the progression of HIV to acquired immune deficiency syndrome (AIDS) despite the initiation of HAART. This would happen because of anemic patients could face failure to make neutralising antibodies, develop persistent viremia, and chronic red cell aplasia all of which lead to immune-compromised state and thereby increased risk of progression of the disease.8,10 Besides, the adverse drug reaction, malabsorption, and nutritional abnormalities associated with anemia subsequently result in the occurrence of opportunistic infections, neoplasm, and death. It is suggested that if anemia is not treated, it will accelerate disease progression and decrease the chance of survival.11
More than two billion people are estimated to have anemia worldwide where young children bear the world’s highest prevalence rate of anemia.12 The problem is more devastating among people living with HIV. About 30% of patients with an asymptomatic infection and as many as 75–80% of those with AIDS have anemia.13 Crudely, the magnitude of anemia among HIV patients varies significantly, ranging from 1.3% to 95%.8,14 In Africa, where HIV/AIDS is a high burden, severe anemia accounted for 3% to 8%.15 Cognizant of this, anemia is a public health problem that affects significantly resource limited settings including Ethiopia. Consequently, it causes significant adverse health consequences and detrimental effects on countries’ social and economic development.
Despite the consensus on anemia as the global public health problem, its incidence has been diverse and affected by several predictors.6 A number of scholars indicated that the occurrence of anemia is mainly related to an advanced stage of HIV, lower CD4 level, co-infection,16 duration of infection,17 age, and sex.8 For instance, studies from Ethiopia at Menelik II18 and Zewditu Memorial II,8 hospitals indicated that initiation of HAART decreases the incidence of anemia. On the contrary, there is evidence that showed multiple drugs used for the treatment of HIV/AIDS results in side-effects including anemia. For instance, drugs such as AZT, cotrimoxazole, amphotericin, interferon, dapsone, and hydroxyurea-based drugs can cause anemia.19
Our country, Ethiopia is one of the Sub-Saharan Africa countries that are severely affected by HIV/AIDS. It is estimated that more than 610,335 people were living with HIV in 2018.20 The prevalence of anemia among people living with HIV/AIDS in Ethiopia ranged from 21% to 70%. A study at Tikur Anbessa Specialized Hospital, Ethiopia indicated that more than one-third (34.6%) of people living with HIV developed anemia. A similar study at the University of Gondar, Ethiopia indicated that the prevalence of anemia was 1.7% among children who had ART follow-up.21
Hence, anemia becomes a critical challenge in patients with HIV/AIDS. However, there is a dearth of literature on the incidence of anemia and its predictors in Ethiopia. Hence, this study aimed to determine the incidence of anemia and its predictors among HIV-infected individuals at referral hospitals in the Amhara region, Ethiopia. Understanding the predictors of anemia in HIV will help practitioners and policymakers to develop cost-effective interventions that would improve survival and reduce morbidity and mortality due to anemia among HIV patients.
Methods
Study Design and Setting
An institutional-based retrospective follow-up study was conducted from September 2015 to December 2018 at three referral hospitals in Amhara regional state including; the University of Gondar Comprehensive Specialized Hospital, Bahir Dar Felege Hiwot Referral Hospital, and Debre Markos Referral Hospital. As part of the national AIDS control Program, in Amhara regional referral hospitals have been providing free ART services from 2005 to date. The hospitals provide clinical care, including laboratory and pharmacy services.
Population, Sample Size Determination, and Sampling Procedures
The source population includes all patients ≥15 years of age enrolled in ART clinic for first-line ART treatment in Amhara regional state referral hospitals. Whereas the patients enrolled in the selected referral hospitals of ART clinic from September 2015 to December 2018 were the study population.
For the first objective, the sample size was determined by using single population proportion formula with considerations of the following statistical assumption; confidence level 95%, incidence rate of anemia =35% (on a study conducted at a tertiary hospital in Addis Ababa among adult HIV positive patients on first-line ART) and 5% margin of error (W).
Where;
n= sample size
Z=1.96 the corresponding Z-score for the 95% CI
W= error of margion
α=confidence level (95%)
p = proportions of virological failure on ART
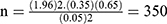 by considering the incomplete patient record, 10%35 was added from the initial sample size and the final sample was 385.
by considering the incomplete patient record, 10%35 was added from the initial sample size and the final sample was 385.
To check the adequacy of the sample for the second objective: we used a survival sample size calculation power approach using Stata 14.1 software with Cox proportional hazard assumptions. The sample size was calculated for the three predictor variables including CD4 count (HR= 5.9), baseline ART (HR= 1.52), and chronic diarrhea (HR= 1.85) from retrospective follow up study done in territory hospital Addis Ababa22 (Table 1) and by using Schoenfeld23 formula.
 |
Table 1 Sample Size Determination of Adult HIV/AIDS Patients on First-Line ART in Northwest Amhara Referral Hospitals, Ethiopia from December 2015 to December 2018 |
where E= number of event
N= number of sample size
P1 = proportion of event among exposed. Prev. = probability of an event.
P2 = proportion of event among non-exposed.
By considering 10% incomplete data The minimum sample size was 498. A simple random sampling technique was used in each hospital based on their allocation.
Variables of the Study
The outcome variable, anemia was defined as a hemoglobin (Hgb) level of less than 12 g/dl in women and less than 13 g/dl in men.24 It was collected by reviewing the patient card and medical registration in the ART care units. The independent variables include sociodemographic characteristics (age, sex, residency, marital status, belonging to support group and HIV disclosure status), antiretroviral medication-related and Clinical ART-related characteristics such as adherence, type of ART regimen, baseline BMI, baseline WHO clinical stage, baseline CD4 count, TB/HIV co-infection status, past opportunistic infection and baseline functional status. Survival time was defined as the time in year from the start of first-line ART treatment to the development of anemia. The event was defined as patients who developed anemia during the follow-up time.
Data Collection Procedures and Quality Control
Data were extracted by using the appropriate data extraction tool adapted from the national HIV intake and follow-up care form. Data were collected by six nurses working in the ART clinics and training was given about the objective of the study. Structured data extraction tools were used to extract data from patient’s medical records, pre ART intake, follow up, and laboratory request forms. Medical charts of patients and the ART unit registration book were the sources of data extraction. To ensure the quality of data, a one-day training was given to six data collectors and three supervisors on the significance, variables of the research, and how to review the documents by using the extraction tool. Furthermore, after collecting the data from the registration book its consistency was re-checked from the patients’ chart by clinicians working in the ART treatment centres.
Data Analysis
Data was entered into EpiData version 4.2.0.0 and exported to Stata 14 software for analysis. Descriptive statistics were carried out and incidence rate (IR) was calculated for the events of anemia. The Kaplan–Meier (KM) failure curve and Log rank test were used to describe the survival experiences among groups of key categorical variables. The proportional hazard assumption was checked both graphically and using a Schoenfeld residual test. The goodness of model fitness was also checked using the Cox-Snell residual plot. Variables having P-values ≤0. 2 in the bivariable analysis were entered into the multivariable analysis and variables with P-value ≤0. 05 and an adjusted hazard ratio (AHR) with a 95% confidence interval (CI) were considered as statistically significant predictors of anemia.
Result
Description of Study Participants
A total of 2230 adult HIV/AIDS patients on the first-line ART were enrolled between December 2015 and December 2018 in northwest Amhara referral hospitals. Based on our sample size determination, 498 HIV/AIDS patients on the first-line ART would have been included for this study. But, we were unable to access medical follow-up charts of 5 patients. Another 7 charts were also excluded due to the development of the event (anemia) at baseline. Finally, 486 patients were included in the analysis.
Socio-Demographic Characteristics of the Patients
The median age of the patients was 32 with IQR26,40 years. Of all the patients, 437 (89.9%) were orthodox Christian. Regarding sex, 207 (42.6%) were male. More than half (55.5%) of the patients were self-employed and 348 (71.6%) were urban dwellers at the moment. Nearly half (48.8%) of the patients were secondary and above by educational status. Among the total, 126 (40.1%) of the patients were married. More than half (60.5%) of patients disclosed their status with 80% of the patients had also support group/caregiver (Table 2).
 |
Table 2 Socio-Demographic Characteristics of Adult HIV Patients on First-Line ART in Amhara Regional Referral Hospitals, Ethiopia from December 2015 to December 2018 (N=486) |
Baseline Clinical and Antiretroviral Medication-Related Characteristics of Patients
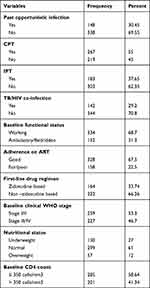
Of the total 486 patients, 183 (37.5%) had taken Isoniazid Preventive Therapy (IPT). Nearly one-third 142 (29.2%) of the patients had TB/HIV co-infection. About 30.45% of the patients had a past opportunistic infection and more than 55.5% of the patients had taken Cotrimoxazole Preventive Therapy (CPT). Among the total, 164 (37.4%) of the patients were treated by a zidovudine-based ART regimen. More than two-thirds of 328 (67.5%) of the patients had good treatment adherence. More than half (53.3%) and (58.64%) of the patients were on stage I/II and had CD4 count less than 350 cells/mm3, respectively. Only 130 (27%) of their nutritional status were categorized as underweight (Table 3).
The Incidence Rate of Anemia
Four hundred eighty-six (486) adult HIV patients on first-line ART were followed for a total of 841.31 Person-Year (PY) of observations.
A total of 225 (46.29% (95% CI, 41. 88, 50.76)) patients developed anemia during the follow-up period. The overall incidence rate of anemia among people living with HIV in the follow-up period was 26.4 (95% CI: 23.46, 30.74) per 100 PY of observations. The median time to develop anemia was 30.63 months (95% CI: 28.33, 32.53).
A graph of the Kaplan–Meier (KM) failure function was used to describe the cumulative probability of the occurrence of anemia over the follow-up period. The cumulative hazard of anemia at 10, 20, 30, and greater than 30 months after starting ART was 1.1, 2.3, 3.3, and 11.2 per 100 person-month observation, respectively (Figure 1).
Predictors of Time to Anemia
The Log rank test and Kaplan–Meier failure function were used to show differences in survival experiences among different groups of categorical variables at baseline.
In case of survival experience without adjusting other covariate, patients who had CD4 count less than 350 cells/mm3 had a higher rate of anemia as compared to CD4 count greater than 350 cells/mm3 (P-value <0.001). Those patients who were treated in Non-zidovudine-based regimen had a lower rate of anemia than the zidovudine-based regimen (p-value <0.001) (Figure 2).
The survival curve plotted below indicated that the estimated hazard curves of the hospital and the Log rank test used for checking the differences in hazard curves displayed. There was no overall difference among the hazard curves of the hospitals and supported by the Log rank test (Log-rank Chi-square2 = 0.86, p= 0.16) (Figure 3).
The Proportional Hazard Assumption
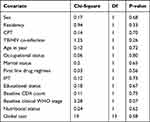
To fit the appropriate model, we have to describe the nature of the data. In this setting, the main aim of testing proportional hazard assumption and measuring the overall goodness of the fit model. The global test of proportional hazards assumption based on the Schoenfeld residuals was done and it was found that all of the covariates and full model satisfy the proportional hazard assumption (Chi-square=19, p-value=0.58) (Table 4).
Cox Regression Analysis
The bivariable cox-regression analysis revealed that age, sex, educational status, occupation, residency, marital status, TB/HIV co-infection, IPT prophylaxis, first-line drug regimen, baseline CD4 count, baseline WHO stage, CPT, and nutritional status were significantly associated with anemia (p-value <0.2).
However, TB/HIV co-infection, zidovudine-based first-line drug regimen, baseline CD4 count, WHO stage, and nutritional status were significantly associated with anemia after controlling for the confounders.
After adjusting all other variables constant, the hazards of developing anemia among TB/HIV co-infection patients were 1.99 times (AHR =1.99, 95% CI: 1.45, 2.74) more likely than their counterparts.
Keeping other variables constant, patients who were treated with a zidovudine-based first-line drug regimen had 1.39 times (AHR=1.39, 95CI: 1.1, 1.85) the hazard of developing anemia compared to patients who were treated in a non-zidovudine regimen.
The hazard of developing anemia among patients who had CD4 count ≤350 cells/mm3 was 1.7 times (AHR= 1.7, 95% CI: 1.23, 2.35) higher than patients having CD4 count >350 cells/mm3, with adjusting all other variables constant.
Holding other variables constant, patients with WHO stage III/IV had 1.32 times (AHR=1.32, 95% CI: 1.01, 1.74) higher hazard of developing anemia compared to WHO stage I/II.
The hazard of developing anemia among patients with underweight nutritional status was 1.53 times (AHR= 1.53, 95% CI: 1.14, 2.07) more likely than patients with normal nutritional status (Table 5).
Discussion
This study investigated the incidence and predictors of anemia among adult HIV/AIDS patients on the first-line ART in northwest Amhara referral hospitals.
Accordingly, the overall incidence rate of anemia among HIV patients in this study was 26.4 per 100 PY observations (95% CI: 23.46, 30.74). This finding was comparable with the figure generated from similar studies done in South India,25 Uganda,26 and at Bahir Dar, Ethiopia.27 The agreement of our study finding with others such as the study in Bahir Dar might have been due to the similarity of population characteristics as the two studies were conducted in the same region, similar study setting where both were conducted in tertiary hospitals, and relatively similar duration of the studies, and similar definition for anemia used in both studies.
However, the incidence of anemia in our study was lower compared to studies conducted in Nigeria28 and Addis Ababa.22 The cut-off value used for the definition of anemia was one of the possible explanations for the observed difference in the incidence rate of anemia between our findings and the other studies. For instance, the higher incidence rate of anemia in Nigerian study can be explained in that a study in Nigeria defined anemia as patients having hemoglobin level of less than 10.5gm/dl irrespective of gender whereas a cut-off value less than 12 gm/dl hemoglobin level for women and less than 13 gm/dl for men was used to define anemia in our study. The type of ART regimen patients were taking could also be the other justification for the variations in the incidence rate of anemia across studies. For example, the proportion of patients on the Zidovudine (AZT)-based regimen in our study was 33.74% but 50% in the study from Addis Ababa. Hence, the known myelosuppressive effect of AZT-based regimen might have been responsible for the higher incidence rate of anemia in the study from Addis Ababa compared to our finding.19
Different factors are found to affect the occurrence of anemia among people living with HIV. Accordingly, TB/HIV co-infection, zidovudine-based regimen, baseline CD4 count (≤350 cells/mm3, WHO stage III/IV, and underweight nutritional status were significantly associated with the occurrence of anemia.
This study presented that the hazards of developing anemia among patients with TB/HIV co-infection were nearly two times higher than that of their counterparts. This finding was supported by studies done in Addis Ababa Zewditu Memorial Hospital,8 and North Wollo, Ethiopia.29 Increased risk of anemia had been observed in patients with TB/HIV co-infection. These could be different explanations for the observed association of HIV/TB co-infection and anemia. For instance, tuberculosis by itself is a chronic infection which causes a moderate degree of anemia.30 It is the fact that TB/HIV co-infection predispose patients for further infection by suppressing the immune system, and also have a direct impact on decreasing and hemoglobin level.31,32 Zidovudine-based regimen was found to be the other important predictor of anemia. The risk of developing anemia among patients who were treated with zidovudine based first-line drug regimen was nearly one and a half times higher than that of patients who were treated with non-zidovudine-based regimen. This finding was in agreement with studies done in South Africa,33 Addis Ababa,22 Nigeria,28 and Debre Tabor, Ethiopia.34,35 Zidovudine containing regimen is associated with suppression of bone marrow and will be risk factor for developing anemia. It causes low production of the red blood cells and platelets in the bone marrow.15,36
The hazard of developing anemia among patients who had CD4 count ≤350 cells/mm3 was 1.7 times higher than patients having CD4 count >350 cells/mm3. This result was in agreement with studies done in South Africa,33 Addis Ababa,22 Wolaita Sodo, Ethiopia,37 Debre Tabor, Ethiopia,34,35 and Gondar.38 There might be several explanations for this relationship. For instance, it is well known that HIV patients with low CD4 count are known to be at risk of various opportunistic infections which are known to be the cause of anemia. Besides, immune-compromised patients are also likely to have a high viral load which could lead to viral infiltration of the bone marrow and causes anemia.
This result presented that patients with WHO stage III/IV were at 1.32 times higher hazard of developing anemia compared to WHO stage I/II. This result is supported by studies done in South Africa,33 Ethiopia,39 Debre Birhan, Ethiopia.40 The possible explanation could be that advanced clinical stage is characterized by marked weight loss, consistent diarrhea, systemic bacterial infection, oral candidiasis, cytomegalovirus, and other complications.41 Therefore, all these complications in stage III/IV of HIV patients might lead to anemia because of excessive fluid loss due to continued diarrhea as a result of different opportunistic infections of bacterial and viral origins that cause immune suppression.
The hazard of developing anemia among underweight nutritional status patients was nearly one and a half times more likely than patients with normal nutritional status. This result is supported by studies done in Rwanda,42 and Debre Birhan.40 This can be justified in that HIV/AIDS causes malnutrition in many individuals due to loss of appetite, opportunistic infection, and treatment side effects. This, in turn, might lead to diarrhea, which might limit the uptake of nutrients including iron malabsorption that results in the development of anemia.
The strength of this study is its multicenter nature which gives replicable results. However, our study was not without its limits. As the study was based on secondary data, some follow-up data such as CD4 count, T-staging, and viral load does not test as predictors. Besides, it was not possible to address the behavioral characteristics of the patients like smoking, and alcohol.
Conclusion
The incidence rate of anemia was lower among HIV patients on the first line ART at the Northwest Amhara regional referral hospitals, Northwest, Ethiopia. TB/HIV co-infection, first-line drug regimen, baseline CD4 count, WHO stage, and nutritional status were significantly associated with anemia. The problem of anemia remains a public health challenge in the study setting, with serious consequences for the most vulnerable populations such as HIV patients. Therefore, to reduce the burden of anemia among ART patients, policies, strategies, and programs should consider the identified predictors of anemia and emphasize after two years of treatment to reduce the complication of HIV patients.
Abbreviations
AHR, adjusted hazard ratio; AIDS, acquired immune deficiency syndrome; AZT, azidothymidine; CHR, crude hazard ratio; HARRT, highly active antiretroviral therapy; HIV, human immune virus; TB/HIV, tuberculosis/human immune virus; WHO, World Health Organization; IPT, isoniazid preventive therapy; CPT, cotrimoxazole preventive therapy.
Data Sharing Statement
All relevant data supporting the current study are available upon reasonable request from the lead author.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the Ethical Review Committee of the Institute of Public Health, University of Gondar. A permission letter was obtained from the University of Gondar Comprehensive Specialized Hospital, Debre Markos Referral Hospital, and Bahir Dar Felege Hiwot Referral Hospital management and the HIV care clinic’s focal person. To ensure the confidentiality, personal identifiers were not included in the data. Our study was based on a retrospective review of secondary data from medical records of patients. Hence, the Ethical Review Committee of the University of Gondar waived the requirement for consent. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
We would like to thank the University of Gondar Comprehensive Specialized Hospital, Bahir Dar Referral Hospital, Debre Markos Referral Hospital for their cooperation, and permitting the data access and we would like to give great appreciation for data collectors for their great collaboration and tolerance for this research work.
Author Contributions
All the listed authors made a substantial contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, studying or critically revising the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the and any significant changes introduced at the proofing stage, agree to take responsibility and be accountable for the contents of the article. Finally approved the content of the manuscript.
Funding
The study has no funding institution.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Zon LI, Arkin C, Groopman JE. Haematologic manifestations of the human immune deficiency virus (HIV). Br J Haematol. 1987;66(2):251–256. doi:10.1111/j.1365-2141.1987.tb01307.x
2. Erhabor O, Babatunde S, Uko K. Some haematological parameters in plasmodial parasitized HIV-infected Nigerians. Niger J Med. 2006;15(1):52–55. doi:10.4314/njm.v15i1.37116
3. De Santis GC, Brunetta DM, Vilar FC, et al. Hematological abnormalities in HIV-infected patients. Int J Infect Dis. 2011;15(12):e808–e11. doi:10.1016/j.ijid.2011.08.001
4. WHO/CDC. Worldwide Prevalence of Anemia 1993–2005: WHO Global Database on Anemia. Switzerland: WHO Press; 2008.
5. Anemia: causes, symptoms, diagnosis, treatments; 2020. Available from: https://www.webmd.com/a-to-z-guides/understanding-anemia-basics#1.
6. Ndlovu Z, Chirwa T, Takuva S. Incidence and predictors of recovery from anaemia within an HIV-infected South African Cohort, 2004–2010. Pan Afr Med J. 2014;19:19. doi:10.11604/pamj.2014.19.114.3600
7. Re M, Zauli G, Furlini G, Giovannini M, La MP. HIV-1 infection and hematologic picture. Microbiologica. 1991;14(2):165–176.
8. Assefa M, Abegaz WE, Shewamare A, Medhin G, Belay M. Prevalence and correlates of anemia among HIV infected patients on highly active anti-retroviral therapy at Zewditu Memorial Hospital, Ethiopia. BMC Hematol. 2015;15(1):6. doi:10.1186/s12878-015-0024-6
9. Ferede G, Wondimeneh Y. Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Blood Disord. 2013;13(1):8. doi:10.1186/2052-1839-13-8
10. Gedefaw L, Yemane T, Sahlemariam Z, Yilma D. Anemia and risk factors in HAART naive and HAART experienced HIV positive persons in south west Ethiopia: a comparative study. PLoS One. 2013;8(8):e72202. doi:10.1371/journal.pone.0072202
11. Zunke P, Ghat V, Waran M, Tyagi A. A study of prevalence of anemia among HIV patients and its correlation with clinical stage of aids, Cd4 count and antiretroviral therapy. Int J Med Sci Clin Invent. 2017;4(2):2698–2701.
12. World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia; 2005. Available from: https://apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf;jsessionid=817CE198213C8B5704631DAEF4AA48B7?sequence=1.
13. Levine AM, Berhane K, Masri-Lavine L, et al. Prevalence and correlates of anemia in a large cohort of HIV-infected women: women’s interagency HIV Study. J Acquir Immune Defic Syndr. 2001;26(1):28–35. doi:10.1097/00042560-200101010-00004
14. Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(7):27–43. doi:10.1016/j.amjmed.2003.12.010
15. Zhou J, Jaquet A, Bissagnene E, et al. Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific, and central and South America. J Int AIDS Soc. 2012;15(1):5. doi:10.1186/1758-2652-15-5
16. Kerkhoff AD, Wood R, Vogt M, Lawn SD. Predictive value of anaemia for tuberculosis in HIV-infected patients in sub-Saharan Africa: an indication for routine microbiological investigation using new rapid assays. J Acquir Immune Defic Syndr. 2014;66(1):33. doi:10.1097/QAI.0000000000000091
17. Weldegebreal F, Mitiku H, Teklemariam Z. Magnitude of adverse drug reaction and associated factors among HIV-infected adults on antiretroviral therapy in Hiwot Fana specialized university hospital, eastern Ethiopia. Pan Afr Med J. 2016;24(1). doi:10.11604/pamj.2016.24.255.8356
18. Adane A, Desta K, Bezabih A, Gashaye A, Kassa D. HIV-associated anaemia before and after initiation of antiretroviral therapy at Art Centre of Minilik II Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50(1):13–21.
19. Moyle G. Anaemia in persons with HIV infection: prognostic marker and contributor to morbidity. AIDS Rev. 2002;4(1):13.
20. EthiopiaCountry Operational Plan. Strategic direction summary. 2018.
21. Enawgaw B, Alem M, Addis Z, Melku M. Determination of hematological and immunological parameters among HIV positive patients taking highly active antiretroviral treatment and treatment naïve in the antiretroviral therapy clinic of Gondar University Hospital, Gondar, Northwest Ethiopia: a comparative cross-sectional study. BMC Hematol. 2014;14(1):8. doi:10.1186/2052-1839-14-8
22. Wolde HM. Incidence and risk factors of anemia among HIV/AIDS patients taking anti-retroviral therapy at tertiary hospitals in Addis Ababa, Ethiopia: a Retrospective Cohort Study. J HIV AIDS Infect Dis. 2014;2:1–06.
23. Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503. doi:10.2307/2531021
24. World Health Organization. Nutritional anaemias: tools for effective prevention and control. 2017.
25. Subbaraman R Anemia among HIV-infected individuals in South India. 2007.
26. Ramezani A, Aghakhani A, Sharif MR, Banifazl M, Eslamifar A, Velayati AA. Anemia prevalence and related factors in HIV-infected patients: a cohort study. Iran J Pathol. 2008;3(3):125–128.
27. Manaye Y, Asrat A, Mengesha EW. Time to development of anemia and predictors among HIV-infected patients initiating ART at Felege Hiwot Referral Hospital, Northwest Ethiopia: a Retrospective Follow-Up Study. Biomed Res Int. 2020;2020:1–7. doi:10.1155/2020/7901241
28. Ikunaiye N, Denue B, Aderemi-Williams R, Rawizza H. Incidence of anaemia among HIV-infected patients treated with zidovudine-containing antiretroviral therapy in northeastern Nigeria. Ann Ib Postgrad Med. 2018;16(2):115–124.
29. Beletew B, Mengesha A, Ahmed M, Fitwi A, Wudu M. Determinants of anemia among HIV-positive children on highly active antiretroviral therapy attending hospitals of North Wollo Zone, Amhara Region, Ethiopia, 2019: a Case-Control Study. Anemia. 2020;2020:2020. doi:10.1155/2020/3720572
30. Lee SW, Kang Y, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21(6):1028–1032. doi:10.3346/jkms.2006.21.6.1028
31. Schaible UE, Stefan H. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4(5):e115. doi:10.1371/journal.pmed.0040115
32. Killewo J. Poverty, TB, and HIV infection: a vicious cycle. J Health Popul Nutr. 2002;281–284.
33. Takuva S, Maskew M, Brennan AT, Sanne I, MacPhail AP, Fox MP. Anemia among HIV-infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. J Trop Med. 2013;2013:1–6. doi:10.1155/2013/162950
34. Zerihun KW, Bikis GA, Muhammad EA. Prevalence and associated factors of anemia among adult human immune deficiency virus positive patients on anti-retroviral therapy at Debre Tabor Hospital, Northwest Ethiopia. BMC Res Notes. 2019;12(1):168. doi:10.1186/s13104-019-4214-3
35. Melese H, Wassie MM, Woldie H, Tadesse A, Mesfin N. Anemia among adult HIV patients in Ethiopia: a hospital-based cross-sectional study. HIV AIDS (Auckl). 2017;9:25.
36. Mata-Marín JA, Gaytán-Martínez JE, Martínez-Martínez RE, Arroyo-Anduiza CI, Fuentes-Allen JL, Casarrubias-Ramirez M. Risk factors and correlates for anemia in HIV treatment-naïve infected patients: a cross-sectional analytical study. BMC Res Notes. 2010;3(1):1–5. doi:10.1186/1756-0500-3-230
37. Ageru TA, Koyra MM, Gidebo KD, Abiso TL. Anemia and its associated factors among adult people living with human immunodeficiency virus at Wolaita Sodo University teaching referral hospital. PLoS One. 2019;14(10):e0221853. doi:10.1371/journal.pone.0221853
38. Yesuf T, Muhie OA, Shibru H. Prevalence and predictors of anemia among adult HIV infected patients at the University of Gondar Hospital, Northwest Ethiopia. HIV AIDS (Auckl). 2019;11:211.
39. Gebremedhin KB, Haye TB. Factors associated with anemia among people living with HIV/AIDS taking ART in Ethiopia. Adv Hematol. 2019;2019:2019. doi:10.1155/2019/9614205
40. Aynalem YA, Shibabaw Shiferaw W, Woldiye Z. Prevalence of anemia and its associated factors in antiretroviral-treated HIV/AIDS-positive adults from 2013 to 2018 at Debre Berhan Referral Hospital, Ethiopia. Adv Hematol. 2020;2020:1–7. doi:10.1155/2020/2513578
41. World Health Organization. Interim WHO Clinical Staging of HVI/AIDS and HIV/AIDS Case Definitions for Surveillance: African Region. World Health Organization; 2005.
42. Masaisa F, Gahutu JB, Mukiibi J, Delanghe J, Philippé J. Anemia in human immunodeficiency virus–infected and uninfected women in Rwanda. Am J Trop Med Hyg. 2011;84(3):456–460. doi:10.4269/ajtmh.2011.10-0519
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.