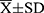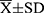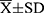Back to Journals » Clinical Interventions in Aging » Volume 17
Predictors of Adverse Outcomes in Healthy Aging Adults: Coronary Artery Disease, Lower Educational Status and Higher P-Selectin Levels
Authors Batko-Szwaczka A , Francuz T, Kosowska A, Cogiel A, Dudzińska-Griszek J, Wilczyński K , Hornik B , Janusz-Jenczeń M , Włodarczyk I, Wnuk B , Szołtysek J, Durmała J , Dulawa J , Szewieczek J
Received 24 February 2022
Accepted for publication 2 July 2022
Published 5 August 2022 Volume 2022:17 Pages 1173—1185
DOI https://doi.org/10.2147/CIA.S363881
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Agnieszka Batko-Szwaczka,1 Tomasz Francuz,2 Agnieszka Kosowska,2 Anna Cogiel,1 Joanna Dudzińska-Griszek,1 Krzysztof Wilczyński,1 Beata Hornik,3 Magdalena Janusz-Jenczeń,3 Iwona Włodarczyk,3 Bartosz Wnuk,4 Joanna Szołtysek,4 Jacek Durmała,4 Jan Dulawa,5 Jan Szewieczek1
1Department of Geriatrics, Faculty of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 2Department of Biochemistry, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 3Department of Internal Nursing, Faculty of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 4Department of Rehabilitation, Faculty of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 5Department of Internal Medicine and Metabolic Diseases, Faculty of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Correspondence: Agnieszka Batko-Szwaczka, Department of Geriatrics, Faculty of Health Sciences in Katowice, Medical University of Silesia, Poniatowskiego 15, Katowice, 40-055, Poland, Tel +48323598239, Fax +48322059483, Email [email protected]
Background: Societal aging – as a global demographic phenomenon – shows no indication of abating. As a result, the problem of age-associated disability and related long-term care is emerging as a major public health challenge. It is important that methods for identifying older adults at risk of adverse outcomes are implemented early.
Methods: The study group consisted of 145 individuals, 44.1% women, who were randomized from community-dwelling 60– 74-year-old adults. A comprehensive geriatric assessment was supplemented with Fried frailty phenotype evaluation and blood tests (including adhesion molecules, matrix metalloproteinases and neurotrophic factors). A follow-up by phone call was made for at least 3 years after the initial examination. Composite endpoint (CE) included falls, hospitalization, institutionalization and death.
Results: Mean study group age was 66.5 ± 4.1 years () and mean number of diseases was 3.7 ± 2.2. Functional status of the subjects was good, as indicated by high Barthel Index scores of 99.1 ± 2.4, MMSE scores of 29.0 ± 1.5 and no frailty case. During a three-year follow-up, 71 participants (49.0%) experienced any CE-events. The Wilcoxon-Gehan test indicates that a higher probability of three-year CE completion was associated with an age > 65 years (P = 0.006), coronary artery disease (CAD) (P = 0.008), 6-Minute Walk Test < 432 m (P = 0.034), serum glucose > 120 mg/dL (P = 0.047), serum cortisol > 10 μg/dL (P = 0.011), leptin ≥ 15 ng/mL (P = 0.018), P-selectin ≥ 23 ng/mL (P = 0.006) and GDNF ≥ 20 pg/mL (P = 0.004). CAD (OR = 3.64, 95% CI = 1.53− 8.69, P = 0.004), educational status (OR = 0.87, 95% CI = 0.77− 0.98, P = 0.022) and P-selectin levels (OR = 1.07, 95% CI = 1.02− 1.13, P = 0.013) were independent measures predicting three-year CE occurrence in multivariate logistic regression analysis adjusted for clinical and functional measures, and blood tests.
Conclusion: Coronary artery disease, poorer lower educational status and higher P-selectin levels were predictive of adverse outcomes in the community-dwelling healthy-aging early-old adults during three-year follow-up.
Keywords: comprehensive geriatric assessment, frailty phenotype, community-dwelling older adults, healthy aging, adverse events, coronary artery disease, education, P-selectin
Introduction
Elderly populations have been disproportionately affected by the COVID-19 pandemic.1 However, societal aging – a global demographic phenomenon – shows no indication of abating. As a result, age-associated disability and long-term care are emerging as major public health challenges facing society.2 Therefore, there is an urgent need to develop prevention programs that reduce the scale of age-related disability and dependence on care. For these programs to be effective, practically useful methods are needed to identify persons who are particularly at risk of adverse health events and who can significantly benefit from participating in these programs.3,4 Age-dependent adverse events are associated with biological rather than chronological age.5 Significant discrepancies between these two measures may exist in each individual older person.6,7 Unfortunately, no widely accepted measure of biological age and associated risk of adverse outcomes is agreed upon.5,6 Multiple studies in this area have been performed with regard to frailty,8–13 while less is known on the subject with regard to healthy aging.14 The most serious adverse health outcomes of age-associated frailty include falls, hospitalization, admission to long-term care and mortality.11 These complications of frailty seem to be a reasonable clinical measure of biological aging. We applied these four events as a combined end-point to assess the usefulness of different clinical and functional measures in predicting the three-year risk of significant adverse outcomes in a population of healthy aging early-old adults. It might seem inappropriate to combine such different events, like falls and mortality at a single composite end-point. However, falls among the elderly, even if non-injurious, should be considered potentially serious outcomes because they are common manifestations of underlying significant health disorders, and they are associated with a high risk of injuries, psychological complications (the post-fall syndrome, depression) social consequences (social isolation), and ultimately premature death.15,16 Among the wide spectrum of clinical and functional measures that were analyzed as potential candidates for prediction of adverse outcomes, we also considered a range of adhesion molecules, matrix metalloproteinases and neurotrophic factors. There is increasing evidence of their contribution to the changes related to aging and age-related morbidity. This paper is a continuation of our previous studies17,18 directed towards the identification of markers useful for the prediction of age-associated adverse health outcomes in healthy aging early-old adults, in the context of the development of efficient preventive programs.
Participants and Methods
Detailed subject enrolment, study methodology, measurements, statistical analysis and ethics statement were described in our previous papers.17,18
Participants
This analysis includes 145 participants (44.1% women) randomized out of 843,278 community-dwelling 60–74 years old inhabitants of the Silesian Voivodeship, Poland, who presented good functional status (Barthel Index score ≥85, Mini-Mental State Examination score ≥24, frailty excluded according to the Fried et al criteria19) and had performed extended laboratory tests at the initial examination.
Measurements
A comprehensive geriatric assessment that included a structured interview with analysis of past medical history, Charlson Comorbidity Index20 calculation, physical examination, functional assessment, blood sampling and Fried et al frailty phenotype assessment19 were recorded as described in our previous papers.17,18 Barthel Index of Activities of Daily Living (Barthel Index)21 and Instrumental Activities of Daily Living Scale (IADL)22 were used to assess functional status. Mini-Mental State Examination (MMSE)23 was used to assess cognitive function. Geriatric Depression Scale – Short Form (GDS-SF) was used to screen for depression.24 Norton Scale for pressure sore risk assessment was also used. The scale is scored from 20 points (minimum risk) to 5 points (maximum risk).25 To assess the overall physical capacity, the 6-Minute Walk Test (6MWT), measuring the maximum distance that a subject can walk in 6 minutes, was used.26,27 Tinetti Performance-Oriented Mobility Assessment (Performance-Oriented Mobility Assessment, POMA)28 and Timed Up and Go (TUG) test were used to assess both functional capacity and the risk of falls.29 Blood tests are listed in Table 1. Serum samples for leptin, adiponectin, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), P-selectin, E-selectin, matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 2 (MMP-2), matrix metalloproteinase 9 (MMP-9), brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF) assessment were stored at −80°C in polypropylene tubes and assayed in panels, using magnetic Luminex assay according to manufacturer’s instruction. Human serum was diluted according to manufacturer’s recommendations and assayed immediately. R&D Systems human magnetic assays were used. The results were read using BioRad Bio-Plex 200 System and Bio-Plex Manager 6.2 for data processing. C-reactive protein (CRP) was quantified by latex-enhanced immunoturbidimetric high-sensitivity CRP assay in serum samples. Patient examination was performed at the Department of Geriatrics of the Leszek Giec Upper-Silesian Medical Centre of the Silesian Medical University in Katowice either at the hospital outpatient clinic. A phone call follow-up was made at least for 3 years after the initial examination. Composite endpoint (CE) included falls, hospitalization, institutionalization and death.
Statistical Analysis
Data were analyzed using Statistica version 13 (StatSoft Polska). The nonparametric Mann–Whitney U-test for quantitative variables, and chi-square test, V-square test or Fisher’s exact test for categorical variables were used. The Kaplan–Meier method was used to estimate three-year CE-free probability for subjects classified according to selected clinical and functional variables, while differences between these subgroups were assessed with the Wilcoxon-Gehan statistic. Spearman’s rank correlation coefficient was used to assess associations between selected variables. Multivariate logistic regression was used to assess measures predictive of CE occurrence. Analysis with backward elimination included variables with P values of 0.1 or lower in the initial univariate analysis that included age, sex, educational level and clinical measures listed in Table 2, functional measures listed in Table 3, and blood tests listed in Table 1. The total number of drugs taken orally was included in the analysis, but not the individual drugs used by the participants. Collinearity and redundancy of variables were eliminated before odds ratios (OR) calculation. P values <0.05 were considered to be statistically significant.
Ethics
The study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Bioethical Committee of the Medical University of Silesia in Katowice, Poland (KNW/0022/KB1/1/14). All participants provided informed consent.
Results
Mean study group age was 66.5 ± 4.1 years (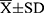 ). The mean number of diseases or comorbidities (with predominant osteoarthritis and hypertension) was 3.7 ± 2.2, and the mean number of oral medications used by the participants was 3.8 ± 3.2 (Table 2). Functional status of the cohort was good, as indicated by high MMSE scores of 29.0 ± 1.5, Barthel Index scores of 99.1 ± 2.4 and no frailty case (Table 3).
). The mean number of diseases or comorbidities (with predominant osteoarthritis and hypertension) was 3.7 ± 2.2, and the mean number of oral medications used by the participants was 3.8 ± 3.2 (Table 2). Functional status of the cohort was good, as indicated by high MMSE scores of 29.0 ± 1.5, Barthel Index scores of 99.1 ± 2.4 and no frailty case (Table 3).
During a three-year follow-up, 71 participants (49.0%; 95% Confidence Interval (CI) = 40.8−57.1%) experienced any of CE-events; 54 participants (37.2%; 95% CI = 29.4−45.1) were hospitalized, 33 participants (22.8%; 95% CI = 15.9−29.6) experienced falls, 1 participant died (0.7%; 95% CI = 0.0−2.0). No institutionalization was reported. Patients who met CE were characterized by higher age, higher coronary artery disease (CAD) morbidity (Table 2), shorter distance in the 6MWT (Table 3) and higher serum levels of glucose, cortisol, leptin, P-selectin, MMP-1 and GDNF (Table 1).
The Wilcoxon-Gehan test indicates that a higher probability of three-year CE completion was associated with an age >65 years (P = 0.006), CAD (P = 0.008), 6MTW <432 m (P = 0.034), serum glucose >120 mg/dL (P = 0.047) (Figure 1), serum cortisol >10 μg/dL (P = 0.011), leptin ≥15 ng/mL (P = 0.018), P-selectin ≥23 ng/mL (P = 0.006) and GDNF ≥20 pg/mL (P = 0.004) (Figure 2).
6MTW correlated with TUG (Spearman R=−0.468; P < 0.001) and total number of diseases (R=−0.357; P < 0.001). Leptin correlated with body mass index (R = 0.353; P < 0.001), total body fat percentage (R = 0.456; P < 0.001), total fat mass (R = 0.382; P < 0.001). P-selectin correlated with MMP-1 (R = 0.593; P < 0.001), GDNF (R = 0.580; P < 0.001), E-selectin (R = 0.415; P < 0.001), VCAM-1 (R = 0.273; P < 0.001), BDNF (R = 0.269; P = 0.001), MMP-2 (R=−0.252; P = 0.002), ICAM-1 (R = 0.250; P = 0.002). GDNF, beyond P-selectin, correlated with MMP-1 (R = 0.847; P < 0.001), E-selectin (R = 0.676; P < 0.001), MMP-2 (R=−0.628; P < 0.001), adiponectin (R=−0.313; P < 0.001) and ICAM-1 (R = 0.289; P < 0.001).
After eliminating collinearity and redundancy, the following variables were included in the multivariate logistic regression: number of completed school classes, coronary artery disease, total number of oral medications, fat-free mass, Tinetti POMA, Norton Scale, Fried et al frailty criterion for slowness, and P-selectin level. In this analysis, CAD (OR = 3.64, 95% CI = 1.53−8.69, P = 0.004), educational status (OR = 0.87, 95% CI = 0.77−0.98, P = 0.022) and P-selectin levels (OR = 1.07, 95% CI = 1.02−1.13, P = 0.013) appeared independent measures predicting three-year CE occurrence.
Subjects with CAD accounted for 26.2% of all participants. CAD was associated with an increased risk of hospitalization: 63.2% hospitalizations occurred in subjects with CAD (P < 0.001). 13.2% of subjects with CAD experienced subsequently falls (P = 0.156). CAD was associated with age (68.6 ± 4.1 years in subjects with CAD vs 65.8 ± 3.8 in those without CAD; P < 0.001), and hypertension that coexisted in 84.2% of subjects with CAD (P < 0.001). The following diseases were significantly associated with CAD: 73.7% cases of chronic heart failure (P < 0.001), 66.7% cases of cerebral vascular disease (P = 0.014), 66.7% cases of peripheral artery disease (P < 0.001) and 53.3% cases of cancer (P = 0.012).
Less than 15 years of education was associated with an increased risk of falls (P = 0.020): 78.8% of falls occurred in subjects with lower level of education, while this group accounted for 61.4% of all subjects. Education <≤10 years was associated with an increased risk of hospitalizations (P = 0.026): 20.4% of hospitalizations occurred in subjects with lower level of education, while this group accounted for 12.4% of all subjects. Education level correlated with raw MMSE score (R = 0.357, P < 0.001) and Tinetti POMA score (R = 0.173, P = 0.037).
P-selectin ≥19 ng/mL was associated with an increased risk of falls (P = 0.039): 87.9% of falls occurred in subjects with higher levels of this molecule, while this group accounted for 72.4% of all subjects. P-selectin ≥30 ng/mL was associated with an increased risk of hospitalization (P = 0.026): 20.4% of hospitalizations occurred in subjects with higher levels of the molecule, while this group accounted for 12.4% of all subjects. P-selectin level was higher in subjects who subsequently experienced falls (25.5 ± 7.4 vs 22.2 ± 6.8 ng/mL; P = 0.032) and non-significantly higher in subjects who subsequently were hospitalized (24.4 ± 8.2 vs 22.1 ± 6.2 ng/mL; P = 0.136). P-selectin concentrations correlated with levels of the ICAM-1 (R = 0.250, P = 0.002), VCAM-1 (R = 0.273, P = 0.001), E-selectin (R = 0.415, P < 0.001), MMP-1 (R = 0.593, P < 0.001), MMP-2 (R=−0.251, P = 0.002), BDNF (R = 0.269, P = 0.001) and GDNF (R = 0.580, P < 0.001).
Discussion
Despite multi-morbidity and polypharmacy, the studied cohort represented a healthy ageing population, as indicated by good functional status. According to the WHO, healthy ageing is defined as “the process of developing and maintaining the functional ability that enables wellbeing in older age”.30 Being disease-free is not a requirement for the WHO definition of healthy ageing provided these health conditions are managed appropriately and functional capacity allows elder persons “to be and do what they have reason to value” (such as meeting basic needs, learning, growing and making decisions, being mobile, building and maintaining relationships, and contributing to society).30
Identification of persons who are most likely to benefit from early preventive interventions is important from a public health perspective.4,9,14 In our cohort of healthy aging individuals, measures of frailty phenotype were not predictive of three-year risk for the predefined composite endpoint (CE) of adverse outcomes. Other functional measures, beyond 6-Minute Walk Test, also appeared incompetent for the screening of potentially vulnerable persons. In particular, we found no direct relationship between physical activity (measured with the Minnesota Leisure Time Activity Questionnaire which is a component of the Fried et al frailty phenotype assessment19) and the endpoint occurrence. This is worth noting because physical activity is an important component of the prevention of age-related cardiovascular disease,5 a factor determining fall risk8,15 and healthy aging.30 Fried et al frailty phenotype criteria are considered one of two main frailty assessment instruments.11 However, the measure of physical activity being a component of these criteria appear not sensitive enough to detect significant differences in the risk of endpoint occurrence in the studied cohort characterized by good functional status. Other possible reasons for the lack of this association might be too short of follow-up time. Instead, we found an association between the endpoint occurrence and 6MWT which is commonly used as a measure of functional exercise capacity for the management of patients with moderate-to-severe pulmonary disease, capturing also manifestations of extra-pulmonary disorders, including cardiovascular disease, frailty, sarcopenia, cancer and others.31,32 Although the 6MWT distance was consistently associated with symptoms and health-related quality of life across different disease groups, the relationships of this test with patient-reported outcomes were of weak to moderate strength.33 Most of the studies in patients with chronic pulmonary diseases demonstrated that lower 6MWT distance is consistently associated with increased mortality, and – to a lesser extent – with hospitalization.33 An advantage of the test is its simplicity, safety, and that it does not require any complex equipment nor technical expertise.31 In our analysis 6MWT negatively correlated with TUG and total number of diseases reflecting a general health status. Therefore, as our study indicates, 6MWT may be useful for the selection of relevant candidates for prevention of adverse outcomes in the healthy aging early-old cohort, although it does not appear to be an independent predictive measure in this analysis. Serum glucose, cortisol, leptin, MMP-1 and GDNF levels were other measures that distinguished the group of subjects who developed CE from those who CE did not meet, although these factors did not prove to be independent predictors of adverse outcomes in logistic regression analysis. These results are generally consistent with other studies. Fasting glucose levels of 115 mg/dL or more were associated with an increased cardiovascular risk in community-dwelling 65-year and older adults.34 Increased blood glucose is associated with increased mortality in elderly populations.35 Elevated levels of cortisol are associated with aging, frailty and age-related diseases.36 Our results concerning higher levels of leptin in subjects who subsequently experienced CE may seem controversial. In the Framingham Heart Study (mean age 62 years) higher leptin levels were associated with lower risk of incident heart failure.37 However, obesity and the aging process may lead to leptin resistance followed by increased leptin concentration, associated with pro-inflammatory cytokine production and insulin resistance that may impair muscle function.38 In the Seniors-ENRICA study (mean age 67.7 years), higher leptin concentration was associated with increased risk of impaired physical function.38 In our analysis, leptin correlated positively with body mass index, total body fat percentage and total fat mass, which supports the conclusion of the seniors-ENRICA study. A proposed interpretation of higher levels of MMP-1 and GDNF in the group of subjects who experienced CE is presented below, discussing the positive correlations between MMP-1 and P-selectin, as well as GDNF and P-selectin.
Coronary artery disease (CAD), lower educational level and increased serum P-selectin concentration appear to be major predictors of CE. CAD diagnosis nearly quadrupled the risk of the CE, primarily the risk of hospitalization. CAD diagnosis did not significantly increase the risk of falls. An association between CAD and other cardiovascular diseases, as well as between CAD and cancer, was observed in our group. Aging is an important risk factor for CAD39,40 and CAD is one of the leading causes of morbidity and the single most common cause of death.40–43 CAD morbidity is higher in persons with cancer than in the general population.44 CAD and cancer share numerous risk factors and cancer patients carry a higher prevalence of cardiovascular risk factors than cancer-free controls.44 The predictive value of CAD for morbidity and mortality is well documented; however, it was not previously considered in the context of elderly patient CE. Promotion of healthy aging should be primarily based on long-life prevention of cardiovascular risk factors. This issue has a wide social context.45 Promotion of healthy lifestyle in the population from the earliest age should be considered the most important task of the national health policy, as it is of fundamental importance for reducing the burden on society with the enormous effects of morbidity and disability of the elderly.
Educational level emerged as another factor with social dimension, less significant but also independent, as a prognostic marker for the trajectory of aging. In our study, poorer education was associated both with increased risk of falls and hospitalization. As might be expected, educational level correlated with raw MMSE scores and also with fall risk as assessed by Tinetti POMA. The most likely explanation for these relationships seems to be the impact of education on health-promoting lifestyle and access to health care, although our analysis failed to demonstrate this because the study was not designed to analyze such relationships. Associations between educational level and risk of fall have been documented in several studies.46–48 Higher levels of education were associated with better cardiovascular health knowledge, risk perception, intention to physical activity and to healthy diet in vulnerable communities of high-income countries.49 Low educational status is one socioeconomic determinant of worsening cardiovascular health in midlife.50 Lower educational level was associated with an increased risk of coronary artery disease and cardiovascular disease in general in more than 130 thousand participants of the National Health Interview Surveys (Italy), aged 30–74 years, during 10-year follow-up.51 A higher level of education may lead to better perception of changes associated with aging (physical, professional), although it can be associated with a higher distress level.52 Higher levels of education significantly increased the probability of reversion of mild cognitive impairment to normal cognition versus progression to dementia.53 This issue points to the importance of universal access to the highest possible level of education. It is important that the level of general education achieved at a young age should be effectively complemented with health education provided for adults and the elderly. Multiple studies indicate the effectiveness of health education in the prevention of frailty,54 falls and other age-related disorders.
P-selectin was predictive of CE in our analysis. This protein belongs to the family of adhesion molecules and is expressed on platelets and endothelial cells in response to various inflammatory mediators. P-selectin and P-selectin glycoprotein ligand-1 interaction initiate the recruitment of leukocytes, attachment, rolling, and extravasation at inflammation foci.55 There is increasing knowledge about the pivotal role that P-selectin plays in the pathogenesis of atherosclerosis and cardiovascular disease,55 cancer,56 and infections, including Covid-19.57 P-selectin levels in community-dwellers aged 70 years or more with physical frailty and sarcopenia were higher as compared to the non-sarcopenic, non-frail controls.58 Higher expression of P-selectin in the platelet membrane was revealed in frail older adults, indicating that up-regulation of platelet activity may lead to increased thrombosis risk and aspirin resistance in these populations.59 Our cohort did not include participants with frailty and yet P-selectin appeared to be an independent predictor of increased risk of CE-events. In our study, P-selectin correlated positively with E-selectin, matrix metalloproteinase MMP-1, adhesion molecules VCAM-1 and ICAM-1, and neurotrophic factors GDNF and BDNF, and negatively – with matrix metalloproteinase MMP-2. These associations may have different meanings. Some of these factors may be involved in platelet activation, endothelial inflammation and related pathology; others may only be markers of these pathophysiological processes. Soluble forms of both P-selectin and E-selectin in blood characterize the state of endothelial and platelet activation in atherosclerosis.60 Matrix metalloproteinases are inflammatory mediators that degrade various components of extracellular matrix (ECM) and non-ECM molecules, thus promoting recruitment of stem/progenitor cells and facilitating migration and invasion of endothelial cells and vascular smooth muscle cells. They may also participate in regulation of vascular cell proliferation and apoptosis.61 It is hypothesized, that lower MMP-2 activity may be associated with increased bioavailability of proinflammatory cytokines normally inactivated by MMP-2, leading to the stimulation of systemic inflammation.62 Intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) promote leukocyte adhesion and migration to the activated endothelium, mediating endothelial inflammation.63 BDNF plays an important role in the control of neuronal and glial development, neuroprotection, and modulation of synaptic interactions in central and peripheral nervous systems,64 but the role of peripheral BDNF is less known.65 This neurotrophin is abundant in platelets,65 and its serum concentration correlates positively with soluble P-selectin that may be considered a marker of platelet activation.66 The role of GDNF in the growth and maintenance of the central and peripheral nervous systems is also well described.67 However, it is also expressed in peripheral tissues, including digestive, respiratory, hematopoietic and urogenital systems, heart, blood, muscles and skin.68 Different types of immune and epithelial cells have the capacity to release GDNF and express their receptors, leading to the participation in the repair of epithelial barrier damage after inflammation.68 Serum GDNF levels correlate with serum BDNF levels.69 Since serum BDNF derives mainly from platelets65 and serum GDNF may originate from peripheral tissues,68 their plasma concentrations do not necessarily correlate with their activity in the central nervous system.
To our knowledge, this is the first study showing the significance of P-selectin for prediction of adverse outcomes in healthy aging early-old adults.
The main limitation of this study is the small sample size. This was caused by unforeseen difficulties in recruiting volunteers for the study, despite the fact that we offered the opportunity to perform the examination both at our medical center and at the examinee’s place of residence. This issue was discussed in our previous publication,17 where we also pointed out that the sample is not representative of the general older adult population, but rather of a cohort of healthy ageing early-old adults. Nevertheless, we believe that our results may be useful in developing effective preventive programs for adverse outcomes in healthy ageing early-old adults. Our observations indicate that coronary artery disease is a major predictor of risk in this cohort, and the level of education is another important factor. In addition, we showed that P-selectin is also a promising predictive measure for adverse outcomes. Although the determination of biochemical indices, such as adhesion molecules, due to low availability and high cost currently remains limited to scientific research, we believe that these tests may be introduced to clinical practice in the near future. In the face of rapidly aging populations, promotion of healthy aging and development of effective programs to prevent adverse outcomes of aging are necessary to meet the challenges facing medical and social care systems. Further research is needed to define measures most useful for the identification of people who will achieve the greatest benefit from early preventive interventions.
Conclusion
Coronary artery disease, lower educational status and higher P-selectin levels were predictive of adverse outcomes in community-dwelling, healthy-aging early-old adults during a three-year follow-up.
Funding
This project was funded by Medical University of Silesia grants to statutory work (contracts KNW-1-014/K/6/Z, KNW-1-055/K/7/Z and KNW-1-026/K/8/Z and PCN-1-030/K/0/2).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Madrazo Cabo JM, Monter Valera NA, Hernández Sánchez EJ, Ruiz Sánchez M, Sánchez Machorro G, Kurezyn Díaz C. Demographic variables associated with Covid-19 mortality. J Public Health Res. 2020;9(4):1827. doi:10.4081/jphr.2020.1827
2. Ogura S, Jakovljevic MM. Editorial: global population aging - health care, social and economic consequences. Front Public Health. 2018;6:335. doi:10.3389/fpubh.2018.00335
3. Puig J, Biarnes C, Pedraza S, et al. The Aging Imageomics Study: rationale, design and baseline characteristics of the study population. Mech Ageing Dev. 2020;189:111257. doi:10.1016/j.mad.2020.111257
4. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229–234. doi:10.1111/jgs.15216
5. Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):919–930. doi:10.1016/j.jacc.2019.11.062
6. Colloca G, Di Capua B, Bellieni A, et al. Biological and functional biomarkers of aging: definition, characteristics, and how they can impact everyday cancer treatment. Curr Oncol Rep. 2020;22(11):115. doi:10.1007/s11912-020-00977-w
7. Brown PJ, Wall MM, Chen C, Levine ME, Yaffe K, Roose SP, Rutherford BR. Biological age, not chronological age, is associated with late-life depression. J Gerontol a Biol Sci Med Sci. 2018;73(10):1370–1376. doi:10.1093/gerona/glx162
8. Lusardi MM, Fritz S, Middleton A, et al. Determining risk of falls in community dwelling older adults: a systematic review and meta-analysis using posttest probability. J Geriatr Phys Ther. 2017;40:1–36. doi:10.1519/JPT.0000000000000099
9. Liotta G, Ussai S, Illario M, et al. Frailty as the future core business of public health: report of the activities of the A3 action group of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA). Int J Environ Res Public Health. 2018;15(12):2843. doi:10.3390/ijerph15122843
10. Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Predicting risk and outcomes for frail older adults: an umbrella review of frailty screening tools. JBI Database System Rev Implement Rep. 2017;15:1154–1208. doi:10.11124/JBISRIR-2016-003018
11. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi:10.1016/S0140-6736(19)31786-6
12. Ekram ARM, Woods RL, Britt C, Espinoza S, Ernst ME, Ryan J. The association between frailty and all-cause mortality in community-dwelling older individuals: an Umbrella review. J Frailty Aging. 2021;10(4):320–326. doi:10.14283/jfa.2021.20
13. Chu W, Chang SF, Ho HY. Adverse health effects of frailty: systematic review and meta-analysis of middle-aged and older adults with implications for evidence-based practice. Worldviews Evid Based Nurs. 2021;18(4):282–289. doi:10.1111/wvn.12508
14. Chen LK. Challenges of promoting healthy aging and healthy longevity in the communities. Arch Gerontol Geriatr. 2022;99:104624. doi:10.1016/j.archger.2022.104624
15. Khow KSF, Visvanathan R. Falls in the Aging Population. Clin Geriatr Med. 2017;33(3):357–368. doi:10.1016/j.cger.2017.03.002
16. Lee J, Negm A, Peters R, Wong EKC, Holbrook A. Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: a systematic review and meta-analysis. BMJ Open. 2021;11(2):e035978. doi:10.1136/bmjopen-2019-035978
17. Batko-Szwaczka A, Dudzińska-Griszek J, Hornik B, et al. Frailty phenotype: evidence of both physical and mental health components in community-dwelling early-old adults. Clin Interv Aging. 2020;15:141–150. doi:10.2147/CIA.S238521
18. Batko-Szwaczka A, Wilczyński K, Hornik B, et al. Predicting adverse outcomes in healthy aging community-dwelling early-old adults with the timed up and go test. Clin Interv Aging. 2020;15:1263–1270. doi:10.2147/CIA.S256312
19. Fried LP, Tangen CM, Walston J, et al. Cardiovascular health study collaborative research group. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi:10.1093/gerona/56.3.M146
20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8
21. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:56–61.
22. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi:10.1093/geront/9.3_Part_1.179
23. Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6
24. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. doi:10.1300/J018v05n01_09
25. Norton D. Calculating the risk: reflections on the Norton Scale. Decubitus. 1989;2(3):24–31. PMID: 2775471.
26. Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837–841. doi:10.1016/S0003-9993(99)90236-8
27. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Phys Ther. 2002;82:128–137. doi:10.1093/ptj/82.2.128
28. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–126. doi:10.1111/jgs.1986.34.issue-2
29. Podsiadlo D, Richardson S. The timed ”Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi:10.1111/j.1532-5415.1991.tb01616.x
30. World Health Organization. Ageing: healthy ageing and functional ability. Available from: https://www.who.int/news-room/q-a-detail/ageing-healthy-ageing-and-functional-ability.
31. Agarwala P, Salzman SH. Six-minute walk test: clinical role, technique, coding, and reimbursement. Chest. 2020;157(3):603–611. doi:10.1016/j.chest.2019.10.014
32. Zhang H, Hu D, Xu Y, Wu L, Lou L. Effect of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2022;54(1):262–273. doi:10.1080/07853890.2021.1999494
33. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European respiratory society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. doi:10.1183/09031936.00150414
34. Smith NL, Barzilay JI, Shaffer D, et al. Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the cardiovascular health study. Arch Intern Med. 2002;162(2):209–216. PMID: 11802755. doi:10.1001/archinte.162.2.209
35. Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: a meta-analysis of prospective cohort studies. Medicine. 2017;96(45):e8491. PMID: 29137039; PMCID: PMC5690732. doi:10.1097/MD.0000000000008491
36. Marcos-Pérez D, Sánchez-Flores M, Maseda A, et al. Serum cortisol but not oxidative stress biomarkers are related to frailty: results of a cross-sectional study in Spanish older adults. J Toxicol Environ Health A. 2019;82(14):815–825. PMID: 31405343. doi:10.1080/15287394.2019.1654639
37. Ho JE, Lyass A, Courchesne P, et al. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc. 2018;7(14):e008108. PMID: 30006491; PMCID: PMC6064847. doi:10.1161/JAHA.117.008108
38. Lana A, Struijk E, Guallar-Castillón P, Martín-Moreno JM, Rodríguez Artalejo F, Lopez-Garcia E. Leptin concentration and risk of impaired physical function in older adults: the Seniors-ENRICA cohort. Age Ageing. 2016;45(6):819–826. PMID: 27515676. doi:10.1093/ageing/afw142
39. Nakou ES, Parthenakis FI, Kallergis EM, Marketou ME, Nakos KS, Vardas PE. Healthy aging and myocardium: a complicated process with various effects in cardiac structure and physiology. Int J Cardiol. 2016;209:167–175. doi:10.1016/j.ijcard.2016.02.039
40. Triposkiadis F, Xanthopoulos A, Parissis J, Butler J, Farmakis D. Pathogenesis of chronic heart failure: cardiovascular aging, risk factors, comorbidities, and disease modifiers. Heart Fail Rev. 2022;27(1):337–344. doi:10.1007/s10741-020-09987-z
41. Cassar A, Holmes DR Jr, Rihal CS, Gersh BJ. Chronic coronary artery disease: diagnosis and management. Mayo Clin Proc. 2009;84(12):1130–1146. doi:10.4065/mcp.2009.0391
42. García-Blas S, Cordero A, Diez-Villanueva P, et al. Acute Coronary Syndrome in the Older Patient. J Clin Med. 2021;10(18):4132. PMID: 34575243. doi:10.3390/jcm10184132
43. World Health Organization. The top 10 causes of death. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
44. Lange SA, Reinecke H. Coronary artery disease and cancer: treatment and prognosis regarding gender differences. Cancers. 2022;14(2):434. doi:10.3390/cancers14020434
45. Vellakkal S, Khan Z, Alavani H, Fledderjohann J, Stuckler D. Effects of public policies in the prevention of cardiovascular diseases: a systematic review of global literature. Public Health. 2022;207(73–81):73–81. PMID: 35567826. doi:10.1016/j.puhe.2022.03.021
46. Li YH, Song GX, Yu Y, Zhou de D, Zhang HW. Study on age and education level and their relationship with fall-related injuries in Shanghai, China. Biomed Environ Sci. 2013;26(2):79–86. doi:10.3967/0895-3988.2013.02.001
47. Yoo JS, Kim CG, Yim J, Jeon MY. Factors influencing falls in the frail elderly individuals in urban and rural areas. Aging Clin Exp Res. 2016;28(4):687–697. doi:10.1007/s40520-015-0469-2
48. Petersen N, König HH, Hajek A. The onset of falls and its effects on perceived social exclusion and loneliness. Evidence from a nationally representative longitudinal study. Arch Gerontol Geriatr. 2022;100:104622. doi:10.1016/j.archger.2022.104622
49. Hassen HY, Bowyer M, Gibson L, Abrams S, Bastiaens H. Level of cardiovascular disease knowledge, risk perception and intention towards healthy lifestyle and socioeconomic disparities among adults in vulnerable communities of Belgium and England. BMC Public Health. 2022;22(1):197. doi:10.1186/s12889-022-12608-z
50. Lassale C, Cené CW, Asselin A, Sims M, Jouven X, Gaye B. Sociodemographic determinants of change in cardiovascular health in middle adulthood in a bi-racial cohort. Atherosclerosis. 2022;346:98–108. doi:10.1016/j.atherosclerosis.2022.01.006
51. Petrelli A, Sebastiani G, Di Napoli A, et al. Education inequalities in cardiovascular and coronary heart disease in Italy and the role of behavioral and biological risk factors. Nutr Metab Cardiovasc Dis. 2021;32:918–928. doi:10.1016/j.numecd.2021.10.022
52. Belo P, Navarro-Pardo E, Pocinho R, Carrana P, Margarido C. Relationship between mental health and the education level in elderly people: mediation of leisure attitude. Front Psychol. 2020;11:573. doi:10.3389/fpsyg.2020.00573
53. Iraniparast M, Shi Y, Wu Y, et al. Cognitive reserve and mild cognitive impairment: predictors and rates of reversion to intact cognition vs progression to dementia. Neurology. 2022;98:e1114–e1123. doi:10.1212/WNL.0000000000200051
54. Billot M, Calvani R, Urtamo A, et al. Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging. 2020;15:1675–1690. PMID: 32982201; PMCID: PMC7508031. doi:10.2147/CIA.S253535
55. Lyngbakken MN, Myhre PL, Røsjø H, Omland T. Novel biomarkers of cardiovascular disease: applications in clinical practice. Crit Rev Clin Lab Sci. 2019;56(1):33–60. doi:10.1080/10408363.2018.1525335
56. Borsig L. Selectins in cancer immunity. Glycobiology. 2018;28(9):648–655. doi:10.1093/glycob/cwx105
57. Campo G, Contoli M, Fogagnolo A, et al. Over time relationship between platelet reactivity, myocardial injury and mortality in patients with SARS-CoV-2-associated respiratory failure. Platelets. 2020;32:1–8.
58. Marzetti E, Picca A, Marini F, et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: the frailty ”cytokinome” at its core. Exp Gerontol. 2019;122:129–138. doi:10.1016/j.exger.2019.04.019
59. Arauna D, García F, Rodríguez-Mañas L, et al. Older adults with frailty syndrome present an altered platelet function and an increased level of circulating oxidative stress and mitochondrial dysfunction biomarker GDF-15. Free Radic Biol Med. 2020;149:64–71. doi:10.1016/j.freeradbiomed.2020.01.007
60. Zinellu A, Mangoni AA. Systematic review and meta-analysis of the effect of statins on circulating E-Selectin, L-Selectin, and P-Selectin. Biomedicines. 2021;9(11):1707. doi:10.3390/biomedicines9111707
61. Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. PMID: 23840100. doi:10.1155/2013/928315
62. Hardy E, Hardy-Sosa A, Fernandez-Patron C. Fernandez-Patron C. MMP-2: is too low as bad as too high in the cardiovascular system? Am J Physiol Heart Circ Physiol. 2018;315(5):H1332–H1340. PMID: 30118342. doi:10.1152/ajpheart.00198.2018
63. Oates J, Russell DL, Van Beusecum JP. Endothelial cells: potential novel regulators of renal inflammation. Am J Physiol Renal Physiol. 2022;322:F309–F321. PMID: 35129369. doi:10.1152/ajprenal.00371.2021
64. Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38(3):579–593. PMID: 28623429; PMCID: PMC5835061. doi:10.1007/s10571-017-0510-4
65. Chacón-Fernández P, Säuberli K, Colzani M, Moreau T, Ghevaert C, Barde YA. Brain-derived neurotrophic factor in megakaryocytes. J Biol Chem. 2016;291(19):9872–9881. PMID: 27006395; PMCID: PMC4858990. doi:10.1074/jbc.M116.720029
66. Bélanger JC, Bouchard V, Le Blanc J, et al. Brain-derived neurotrophic factor mitigates the association between platelet dysfunction and cognitive impairment. Front Cardiovasc Med. 2021;8:739045. PMID: 34557534; PMCID: PMC8452906. doi:10.3389/fcvm.2021.739045
67. Saarma M, Sariola H. Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF). Microsc Res Tech. 1999;45(4–5):292–302. PMID: 10383122. doi:10.1002/(SICI)1097-0029(19990515/01)45:4/5<292::AID-JEMT13>3.0.CO;2-8
68. Morel L, Domingues O, Zimmer J, Michel T. Revisiting the role of neurotrophic factors in inflammation. Cells. 2020;9(4):865. PMID: 32252363; PMCID: PMC7226825. doi:10.3390/cells9040865
69. Shen Z, Zhu J, Yuan Y, et al. The roles of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) in predicting treatment remission in a Chinese Han population with generalized anxiety disorder. Psychiatry Res. 2019;271:319–324. PMID: 30529313. doi:10.1016/j.psychres.2018.08.111
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.