Back to Journals » Journal of Inflammation Research » Volume 15
Predictive Value of the Baseline and Early Changes in Blood Eosinophils for Short-Term Mortality in Patients with Acute Respiratory Distress Syndrome
Authors Peng J, Tang R, Qi D, Yu Q, Hu H, Tang W, He J, Wang D
Received 1 December 2021
Accepted for publication 7 March 2022
Published 14 March 2022 Volume 2022:15 Pages 1845—1858
DOI https://doi.org/10.2147/JIR.S350856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Video abstract of "Baseline and early changes in blood eosinophils in ARDS" [ID 350856].
Views: 229
Junnan Peng,* Rui Tang,* Di Qi, Qian Yu, Hao Hu, Wen Tang, Jing He, Daoxin Wang
Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Daoxin Wang; Jing He, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Chongqing Medical University, No. 76 Linjiang Road, Yuzhong District, Chongqing, 400010, People’s Republic of China, Email [email protected]; [email protected]
Background: Eosinophils play an essential role in the pathogenesis of acute respiratory distress syndrome (ARDS). We aimed to assess the association between the baseline blood eosinophils, eosinophil changes during the first week in the intensive care unit (ICU) and short-term patient outcomes.
Methods: All patients meeting the Berlin definition of ARDS from the Medical Information Mart for Intensive Care IV database were retrospectively analyzed. We used logistic regression, Kaplan–Meier survival and random forest analysis to determine the association between the baseline eosinophils and short-term mortality. Then the trends in eosinophils over time were compared between the survivors and non-survivors using a generalized additive mixed model (GAMM), which is a common approach used for analysis of repeated measurement data. Area under the receiver-operating characteristic curve (AUC) was used to evaluate the predictive value.
Results: A total of 1685 patients were included, and the 30-day mortality was 25.1%. Kaplan–Meier analysis showed that patients with high baseline eosinophils (> 0.3%) had lower 30-day mortality (p < 0.001). Random forest model selected the baseline eosinophils as an important factor associated with 30-day mortality. Multivariable logistic regression analysis identified high baseline eosinophils as an independent factor for 30-day mortality (OR 0.743, 95% CI 0.568– 0.970). The GAMM result showed that the levels of eosinophils were increased in both survival and non-survival groups, and the between-group differences increased over time, with an average of 0.154 daily after adjusting for confounders. The AUC of changes in eosinophils within the first week was significantly higher than that of baseline eosinophils.
Conclusion: There is a negative association between the baseline eosinophils and short-term mortality in ARDS patients, and the differences in eosinophils increased over time between the survivors and non-survivors. Higher increase in eosinophils is associated with decreased short-term mortality, and dynamic monitoring of eosinophils could better predict the survival of ARDS patients.
Keywords: ARDS, eosinophils, 30-day mortality, generalized additive mixed model, change
Introduction
Acute respiratory distress syndrome (ARDS) is a major contributing cause of hospitalization to the intensive care unit (ICU), with a high mortality rate of 30–40%.1 It is characterized mainly by massive inflammation, increased permeability of alveolar-capillary barrier, and subsequent non-cardiogenic pulmonary edema and refractory hypoxia.2 For decades, researchers and clinicians have dedicated significant efforts to treat ARDS, but most of them have met disappointing results, largely as a consequence of the marked heterogeneity of ARDS.3 Therefore, accelerating precision medicine in ARDS has become a top priority, and biomarkers are suggested to play an essential role in this field, due to their potential implications in molecular phenotype.4
Inflammation is critically involved in the development of ARDS.5 Innate immune cells play crucial roles in the modulation of inflammatory responses.6 Neutrophils are the classical effector cells of the innate immune response during ARDS, which is associated with type-1 inflammation. In contrast, eosinophils, an effector cell of type-2 inflammation, have recently attracted much interest, as their potential diagnostic and prognostic abilities in ARDS.7–13 About 40 years ago, eosinopenia among patients with ARDS was first described and may be a predictor of impending ARDS.7–9 Subsequently, the accumulation of eosinophils in lung was found to be a favorable prognostic indicator for patients with ARDS.10 Moreover, recent studies have found that eosinophils could ameliorate neutrophilic inflammation and evert a protective effect against ARDS.11–13 It is therefore plausible to speculate that “eosinophilic” ARDS is a distinct phenotype, and may present a better clinical prognosis. Unfortunately, the collection of biopsies and bronchoalveolar lavage requires invasive and laborious process, which is often infeasible in clinics. Thus, the measurement of peripheral blood eosinophils seems to be a more simple, efficient and secure option. Actually, blood eosinophils have been proposed as a surrogate biomarker to identify the eosinophil-mediated phenotype in various respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD).14,15 In our previous study, we have demonstrated that blood eosinophils have predictive utility for clinical outcomes in patients with COPD.16
To date, the predictive value of eosinophils, especially considering longitudinal monitoring data, for patients with ARDS has not been fully elucidated. Therefore, we aimed to evaluate the association between the baseline eosinophils, early changes (the first week of ICU admission) in its level and adverse patient outcomes.
Method
Study Design
We performed this retrospective study using a sizeable US-based critical care database called the Medical Information Mart for Intensive Care (MIMIC)-IV. The MIMIC-IV database is an updated version of MIMIC-III and currently contains 76,540 patients in the ICU of the Beth Israel Deaconess Medical Center in Boston, Massachusetts between 2008 and 2019.17 These data were collected before the coronavirus disease 2019 (COVID-19) outbreak. This database was approved by the Institutional Review Boards of the Massachusetts Institute of Technology. We have completed the National Institutes of Health Web-based training course and the Protecting Human Research Participants examination (no. 9555299) to gain access to the database. The approval of our institution and informed consent were exempted given the retrospective nature of the study on an anonymized database. Our study was conducted and reported according to the Reporting of Studies Conducted using Observational Routinely Collected Health Data (RECORD) statement.18
Selection of Patients
We reviewed and retrieved the patient clinical data from the database. Patients who were≥16 years of age, stayed more than 72 hours in the ICU, and met the Berlin definition of ARDS at the time of ICU admission were considered eligible for study inclusion. We excluded the patients who lacked eosinophil values within 24 hours after ICU admission. Additionally, if a patient had multiple ICU admissions, we used the data from his first admission.
Berlin definitions are as follows:19 (1) acute onset of respiratory symptoms; (2) presence of bilateral infiltrates on chest radiograph; (3) arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) <300mmHg with a minimum requirement for positive end-expiratory pressure (PEEP) ≥5 cmH2O; (4) absence of heart failure. The severity of ARDS was classified into mild (200 mmHg < PaO2/FiO2 ratio ≤ 300 mmHg), moderate (100 mmHg < PaO2/FiO2 ratio ≤ 200 mmHg), or severe (PaO2/FiO2 ratio ≤ 100 mmHg) according to the Berlin criteria.
Data Extraction
We used the Structured Query Language with Navicat Premium (version 15) to extract the data. The following variables were extracted or calculated: baseline characteristics (age, gender, body mass index, ethnicity, smoking history, admission type), comorbidities (hypertension, atherosclerosis, diabetes, COPD, acute kidney failure, tumor), the risk factors of ARDS (pneumonia, sepsis, trauma, shock, pancreatitis and others, like transfusion, pulmonary contusion or inhalation of gastric contents), the severity of organ dysfunction (Acute Physiology Score III, APS III; Oxford Acute Severity of Illness Score, OASIS; Logistic Organ Dysfunction System, LODS), the vital signs during the first 24 hours in the ICU (heart rate, mean arterial pressure, respiratory rate, temperature, SpO2), PaO2/FiO2 at diagnosis, PEEP at diagnosis, the laboratory findings during the first 24 hours in the ICU (bicarbonate, blood urea nitrogen, creatinine, lactate, platelet, glucose, white blood cell), and treatment received (mechanical ventilation, antibiotic therapy, vasopressor therapy, renal replacement therapy, corticosteroids therapy). If a variable was recorded more than once in the first 24 hours, we used the value at the first measurement. All comorbidities were diagnosed based on ICD-9 or ICD-10 codes in the MIMIC-IV database. The organ dysfunction scores were estimated during the first 24 hours in the ICU for all patients. The baseline level of eosinophils was obtained based on the first laboratory value within 24 hours after ICU admission. The repeated measurements of eosinophils were performed within the first 7 days after ICU admission, and were irregularly spaced over time.
Missing Data
Details of missing data can be found in Supplementary Table S1. To avoid loss of statistical power, all missing data were assumed to be missing at random and imputed using a random Forest-based imputation method.20 Specifically, we used the R package MissForest, which handles missing data by iteratively using Random Forests. The superiority of the MissForest method over other methods for imputation of missing values has been reported, especially when faced with heavy missingness.21
Outcomes
The primary outcome in the present study was 30-day mortality, which was defined as the occurrence of death at 30 days. Secondary outcomes included in-hospital mortality, length of ICU and hospital stay, which were defined as the occurrence of death during the hospital, and the duration of patients’ ICU and hospital stays measured in days, respectively.
Statistical Analysis
We used R statistical software (version 3.6.1), GraphPad Prism (version 8.0, San Diego, CA) or SPSS software (version 26.0, IBM, USA) to perform statistical analysis and create pictures. We first analyzed the normality of continuous data using the Kolmogorov–Smirnov test. We described continuous variables by mean with standard deviation (SD) if they are normally distributed or median with interquartile range (IQR) if they are not, and categorical variables by count with percentage (%). We estimated the significance of variations between groups using Student’s t-test and Mann–Whitney U-test for normally and non-normally distributed continuous variables, respectively, and chi-squared test for categorical variables when appropriate.
Then, we assessed the relationship between the baseline eosinophils and adverse clinical outcomes in patients with ARDS. We arbitrarily divided the patients into two groups according to the median instead of the mean value of the baseline eosinophils, due to its skewed distribution. We used Kaplan–Meier survival analysis to compare the overall survival between the two groups. We further performed multivariable logistic regression analysis and smooth curve fitting to test the independent effects of the baseline eosinophils and 30-day and in-hospital mortality with crude and adjusted models. We purposefully selected potential confounders for this study based on their possible relationships with the outcome or a p-value less than 0.1 in the univariate analysis. Variables for inclusion were carefully chosen, given the number of events available, to ensure parsimony of the final model. As vital signs and laboratory parameters are usually included in the disease severity scores, we did not enter them into the multivariate model except for PaO2/FiO2 and PEEP. The following variables were included: age, gender, BMI, ethnicity, tobacco history, admission type, hypertension, diabetes, asthma, acute kidney failure, tumor, sepsis, trauma, shock, APS III, OASIS, LODS, PaO2/FiO2, PEEP, mechanical ventilation, antibiotics, vasopressor, CRRT, and glucocorticoid. Besides, we built a random forest Random model to determine the feature importance of baseline eosinophils. Because comorbidities and certain medications may influence the baseline value of eosinophils, we therefore conducted a subgroup analysis for hypertension, coronary atherosclerosis, diabetes, COPD, asthma, acute kidney failure, antibiotic and corticosteroids therapy on the first day of ICU admission.
Lastly, we analyzed the relationship between the early changes of eosinophils and 30-day mortality. We compared the difference of eosinophils between survivors and non-survivors during the first 7 days in ICU. Receiver operating characteristic (ROC) curves with area under the curve (AUC) values were used to evaluate the predictive value. We used a generalized additive mixed model (GAMM) to examine the temporal variation of early changes in eosinophils and mortality with crude and adjusted models. The GAMM is often applied to analyze the results of repeated measurements, especially when the interval between repeated measurements is irregular and some data is missing.22
All tests were two-tailed. A p-value <0.05 was considered significant.
Results
Basic Characteristics
After reviewing the data of 76,540 ICU patients in the MIMIC-IV database, we finally identified a total of 1685 patients in our study. Among them, 423 (25.1%) patients died within 30 days after admission to ICU. The flow chart of patient selections is presented in Figure 1.
 |
Figure 1 Flow chart of this study. Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; MIMIC, Medical Information Mart for Intensive Care. |
Baseline characteristics of 30-day survivors and non-survivors are presented in Table 1. In general, survivors were more likely to be younger, male, non-white, and had lower BMI than non-survivors. A higher proportion of survivors were diagnosed with hypertension and diabetes, while a higher proportion of non-survivors were diagnosed with acute kidney failure and tumor. Concerning risk factors of ARDS, survivors were more likely to have trauma and less likely to have sepsis and shock than non-survivors. The severity scores, included APS III, OASIS, LODS, were all lower in the group of survivors, which indicated that non-survivors were more critically ill than the survivors upon ICU admission. On day 1, the median value of eosinophils was 0.3% (IQR, 0–1.0%) in all patients, and survivors had higher value of eosinophils than non-survivors [0.4% (IQR, 0–1.1%) vs 0.1% (IQR, 0–0.6%), p<0.001]. Moreover, we found that there were significant differences in terms of vital signs (heart rate, respiratory rate, SpO2, PaO2/FiO2), ventilator and laboratory parameters (PEEP, bicarbonate, BUN, creatinine, lactate), and treatment (mechanical ventilation, antibiotics, vasopressor, CRRT, and glucocorticoid) between the two groups.
 |
Table 1 Baseline Characteristics of Survivors and Non-Survivors at Day 30 |
Association Between Baseline Eosinophils and Mortality
We divided all the patients into two groups based on the median of baseline eosinophils. As shown in Table 2, patients with high eosinophils (>0.3%) had shorter length of ICU and hospital stay, and lower 30-day and in-hospital mortality. The Kaplan–Meier curve also revealed significant differences in the 30-day and in-hospital mortality among patients stratified according to baseline eosinophils (Figure 2A and B). Besides, the random forest model selected the baseline eosinophils as the 8th important factor in predicting mortality (Figure 3). The subgroup analyses suggested that the significant inverse relationship between baseline eosinophils and the risk of mortality still existed except for patients with COPD, asthma and corticosteroids therapy on the first day of ICU admission (Supplementary Table S2).
 |
Table 2 Relationship Between Baseline Eosinophils and Clinical Outcomes |
 |
Figure 2 Survival curves for the relationship between baseline eosinophils and risk of 30-day mortality (A)/in-hospital mortality (B). |
We performed a smooth curve fitting to illustrate the association between baseline eosinophils and the risk of in-hospital and 30-day mortality. Taking eosinophils on admission as a continuous variable, we observed a significant negative correlation between eosinophils and short-term mortality in the adjusted model (Figure 4A and B).
We also conducted the multivariable logistic regression analysis to further test our findings, which was shown in Table 3. When we treated eosinophils as a categorical variable, patients with high eosinophils (>0.3%) had a significantly lower risk of 30-day mortality (OR 0.743, 95% CI 0.568–0.970, p=0.029), as well as a lower but not significant risk of in-hospital mortality (OR 0.794, 95% CI 0.607–1.040, p=0.094) than patients with low eosinophils (≤0.3%) in the full adjusted model. However, when we treated eosinophils as a continuous variable, this association was no longer present (all p>0.05).
 |
Table 3 Multivariate Logistic Regression Analysis of Baseline Eosinophils for Mortality |
Association Between Early Changes in Eosinophils and Mortality
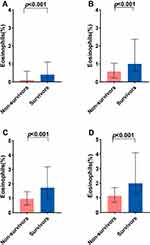
We compared the changes in eosinophils at early time points between the 30-day survivors and non-survivors. In univariate analyses, we observed significant differences in eosinophils on admission, the 2–3rd, the 4–5th, and the 6–7th day between the two groups (Figure 5A–D, Table 4). Compared to the baseline eosinophils in the analysis of ROC curves, the change in eosinophils within the first week after ICU admission had a significantly higher AUC for 30-day mortality (p < 0.001; Supplementary Figure S1).
 |
Table 4 Comparison of the Early Changes (0–7 Days) in Eosinophils Between 30-Day Survivors and Non-Survivors |
We next applied GAMM to characterize the changes in eosinophils over time (the first week of ICU admission) between 30-day survivors and non-survivors after adjustment for the listed confounders. As shown in Figure 6A and B, we found that although eosinophils increased over time in both groups, the trend in survival group was much more apparent. Moreover, the between-group difference was gradually increased with time elapse.
Furthermore, we explored the early longitudinal changes of eosinophils for prediction of 30-day mortality in patients with ARDS. As shown in Table 5, the level of eosinophils in the survival group was significantly higher than that in the non-survival group, and particularly the between-group difference presented an increasing trend during the first week of ICU admission, with an average of 0.154 per day. This association remained significant after adjusting for confounding factors (β=0.154, 95% CI 0.138–0.169, p < 0.001). The same results were observed for in-hospital mortality.
 |
Table 5 Relationship Between Early Changes (0–7 Days) in Eosinophils and Mortality in Patients with ARDS Derived from a Generalized Additive Mixed Model (GAMM) |
Discussion
The present study is the first to explore the dynamic changes in eosinophils for prediction of adverse outcomes in ICU patients with ARDS. Our results show that high eosinophils at ICU admission was associated with lower 30-day and in-hospital mortality, as well as shorter ICU and hospital stays in patients with ARDS. Moreover, survivors had a persistently high eosinophil levels from ICU admission to day 7, and the between-group difference was gradually increased over time. This association remained materially unchanged after adjusting for potential confounders. Therefore, dynamic monitoring of eosinophils could help identify patients with better survival probabilities.
ARDS is a heterogeneous syndrome with a variety of risk factors.1 Inflammation plays a pivotal role in the pathogenesis of ARDS,5 which is associated with a concerted response of the innate immune system.6 Eosinophil is an important innate immune cell. Bass et al23 have shown that eosinophils can be activated by acute inflammation contributing from the infectious or non-infectious sources, instead of a specific type of pathogen. In ARDS, the level of eosinophil cationic protein (ECP), a specific granule protein of eosinophils, was increased,7 and lavage ECP levels correlated strongly with the severity of lung damage.9 Taken together, these findings indicate a pathophysiologic role for eosinophils in ARDS.
It has been recognized that the level of eosinophil was associated with the short-term prognosis in critical illness.24,25 Abidi et al24 found that eosinophils remained significantly lower in non-survivors from ICU admission to seventh day than that in survivors, and lower eosinophil level was an independent predictor of 28-day all-cause mortality. Of note, the study population in their report was general hospitalized ICU patients and the number of included patients was relatively small (n=200), limiting the ability to extrapolate their findings to a specific population. Up to date, there are only two published studies that have described the relationship between blood eosinophils and clinical outcomes in patients with ARDS.11,13 Zhu et al11 observed that following clinical treatment, patients with increased levels of blood eosinophils at discharge are associated with increased survival. Moreover, Chen et al13 found that patients with higher baseline eosinophils had lower 28-day mortality. However, in the two studies, the blood eosinophils were only detected at a single time point, and were not evaluated for their dynamic changes. Our study further provided evidence of the relationship between early changes in eosinophils and mortality in patients with ARDS, which may better reflect the dynamic process of the disease situation.
In present study, we found that patients with high level of baseline eosinophils (>0.3%) had significantly lower 30-day mortality than non-survivors in univariate and multivariate analyses, which is similar to previous studies.13,24 The results from Kaplan–Meier curve and random forest model also supported the above findings. Of note, when we regarded blood eosinophils as a continuous variable, no significant association was found in the multivariate logistic regression analysis. The reason might be the distribution of baseline eosinophil values was skewed, with a high proportion of zero or extremely low data, and this was also evident in previous studies.11,13 The statistical power for the logistic regression would be considerably influenced in the case of highly skewed data.26 By using a categorical variable, the data processing is significantly simplified, which leads to improved robustness of the model. Moreover, in the subgroup analysis, we found that the protective effect of high baseline eosinophils on short-term mortality still existed, except for patients with COPD, asthma and corticosteroids therapy on the first day of ICU admission. However, after excluding patients with these conditions, the significant differences in baseline eosinophils between the survivors and non-survivors remained, indicating that these factors might be surrogate markers but not necessarily the main reason for the difference in baseline eosinophils between the two groups.
Subsequently, we used the GAMM to explore the temporal variation of eosinophils in ARDS patients and its relationship with short-term outcomes. Our result showed that the levels of eosinophils were increased over time in both survival and non-survival groups, and the between-group differences of eosinophils remained significant within the first week after ICU admission. Further, we found the between-group difference presented an increasing trend (an average of 0.154 per day) during the first week in ICU based on the GAMM analysis. The AUC of early changes in eosinophils was significantly higher than that of baseline eosinophils, suggesting a better predictive value for mortality. Taken together, these results indicate that dynamic monitoring of eosinophils can predict the survival probabilities of patients with ARDS. Moreover, several studies have also suggested that the longitudinal changes in eosinophils are helpful to predict prognosis in some diseases, such as COVID-19, COPD and septic shock.25,27,28
Our study suggests that eosinophils exert a possible protective role in ARDS, and some possible mechanisms might explain this protective effect. Zhu et al11 found that CD101− eosinophils, a subtype of eosinophils, could attenuate neutrophil recruitment and accelerate clean-up of neutrophil debris through the production of protectin D1 in lipopolysaccharide (LPS)-induced ARDS murine model. Besides, Krishack et al12 observed that IL-33–mediated eosinophilia protects against acute lung injury, by reducing neutrophil-associated proinflammatory cytokines and mobilizing tissue repair responses in the bacteria-induced pneumonia model. Eosinophils have been reported to participate in the process of tissue regeneration and repair by producing growth factors.29 Moreover, Willetts et al10 found that surviving patients with ARDS had higher lung eosinophil numbers and increased eosinophil degranulation than non-surviving patients. Taken together, these findings indicate that the protective effect of eosinophils is mainly achieved by alleviating neutrophil-dependent inflammation and accelerating lung repair.
There are several limitations in our present study. First, we only used the blood eosinophil concentrations for analysis. The blood eosinophil counts were not discussed, as a large proportion of them are missed in the MIMIC-IV database. The study would be more complete if we take blood eosinophil counts into account together. Second, we did not analyze the outcomes stratified by different subtypes of eosinophils (eg CD101+ and CD101−), because the blood specimens were unavailable in this study. Third, we did not have information about the treatment regimens (especially for corticosteroids) before hospitalization, which might alter the value of eosinophils. Fourth, due to the retrospective nature of the study, missing data and a possible selection bias could have affected the outcome. Our results should be considered exploratory rather than definitive. Therefore, more in-depth studies with prospective design are required to validate and further expand our findings.
Conclusions
Our present study revealed that increased baseline eosinophils were associated with better clinical outcomes, such as decreased 30-day and in-hospital mortality as well as shortened ICU and hospital length of stay, in patients with ARDS. Moreover, a significant difference in the changes of eosinophils over time were observed between survivors and non-survivors. Further, we found a relationship between a higher increased trend of eosinophils and lower short-term mortality in patients with ARDS. Taken together, our results indicate the potential benefit of monitoring early changes in eosinophils for prediction of short-term mortality in patients with ARDS. As a peripheral biomarker, blood eosinophil concentration may reflect lung pathology in this cohort of ARDS patients. Further studies may be suggested to clarify the role of eosinophils as a protective factor.
Abbreviations
APS III, Acute Physiology Score III; ARDS, acute respiratory distress syndrome; AUC, area under the curve; BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; ECP, eosinophil cationic protein; GAMM, generalized additive mixed model; ICU, intensive care unit; IQR, interquartile range; LODS, Logistic Organ Dysfunction System; LOS, length of hospital stay; LPS, lipopolysaccharide; MAP, mean arterial pressure; MIMIC, Medical Information Mart for Intensive Care; OASIS, Oxford Acute Severity of Illness Score; OR, odds ratio; PaO2/FiO2, arterial oxygen partial pressure/fraction of inspired oxygen; PEEP, positive end-expiratory pressure; ROC, receiver operating characteristic; RR, respiratory rate; SD, standard deviation; WBC, white blood cells.
Ethics Approval and Consent to Participate
The establishment of this database was approved by the Institutional Review Boards of the Massachusetts Institute of Technology, and consent was obtained for the original data collection. The study was reviewed and considered exempt by the Ethics Committees of the Second Affiliated Hospital of Chongqing Medical University (NO. 2021-113).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
References
1. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi:10.1001/jama.2016.0291
2. Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi:10.1038/s41572-019-0069-0
3. Meyer NJ, Calfee CS. Novel translational approaches to the search for precision therapies for acute respiratory distress syndrome. Lancet Respir Med. 2017;5(6):512–523. doi:10.1016/s2213-2600(17)30187-x
4. Beitler JR, Thompson BT, Baron RM, et al. Advancing precision medicine for acute respiratory distress syndrome. Lancet Respir Med. 2021. doi:10.1016/s2213-2600(21)00157-0
5. Famous KR, Delucchi K, Ware LB, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195(3):331–338. doi:10.1164/rccm.201603-0645OC
6. Xiao TS. Innate immunity and inflammation. Cell Mol Immunol. 2017;14(1):1–3. doi:10.1038/cmi.2016.45
7. Hällgren R, Borg T, Venge P, Modig J. Signs of neutrophil and eosinophil activation in adult respiratory distress syndrome. Crit Care Med. 1984;12(1):14–18. doi:10.1097/00003246-198401000-00004
8. Modig J, Samuelsson T, Hällgren R. The predictive and discriminative value of biologically active products of eosinophils, neutrophils and complement in bronchoalveolar lavage and blood in patients with adult respiratory distress syndrome. Resuscitation. 1986;14(3):121–134. doi:10.1016/0300-9572(86)90116-4
9. Hällgren R, Samuelsson T, Venge P, Modig J. Eosinophil activation in the lung is related to lung damage in adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135(3):639–642. doi:10.1164/arrd.1987.135.3.639
10. Willetts L, Parker K, Wesselius LJ, et al. Immunodetection of occult eosinophils in lung tissue biopsies may help predict survival in acute lung injury. Respir Res. 2011;12(1):116. doi:10.1186/1465-9921-12-116
11. Zhu C, Weng QY, Zhou LR, et al. Homeostatic and early-recruited CD101− eosinophils suppress endotoxin-induced acute lung injury. Eur Respir J. 2020;56(5):1902354. doi:10.1183/13993003.02354-2019
12. Krishack PA, Hollinger MK, Kuzel TG, et al. IL-33-mediated eosinophilia protects against acute lung injury. Am J Respir Cell Mol Biol. 2021;64(5):569–578. doi:10.1165/rcmb.2020-0166OC
13. Chen HT, Xu JF, Huang XX, Zhou NY, Wang YK, Mao Y. Blood eosinophils and mortality in patients with acute respiratory distress syndrome: a propensity score matching analysis. World J Emerg Med. 2021;12(2):131–136. doi:10.5847/wjem.j.1920-8642.2021.02.008
14. Nadif R, Siroux V, Oryszczyn MP, et al. Heterogeneity of asthma according to blood inflammatory patterns. Thorax. 2009;64(5):374–380. doi:10.1136/thx.2008.103069
15. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
16. Peng J, Yu Q, Fan S, et al. High blood eosinophil and YKL-40 levels, as well as low CXCL9 levels, are associated with increased readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:795–806. doi:10.2147/copd.S294968
17. Johnson A, Bulgarelli L, Pollard T, et al. “MIMIC-IV” (version 1.0). PhysioNet. 2020. doi:10.13026/s6n6-xd98
18. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi:10.1371/journal.pmed.1001885
19. Ranieri V, Rubenfeld G, Thompson B, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi:10.1001/jama.2012.5669
20. Pantanowitz A, Marwala T. Missing data imputation through the use of the random forest algorithm. In: Yu W, Sanchez EN, editors. Advances in Computational Intelligence. Advances in Intelligent and Soft Computing. Vol. 116. Berlin, Heidelberg: Springer; 2009. doi:10.1007/978-3-642-03156-4_6
21. Tang F, Ishwaran H. Random forest missing data algorithms. Stat Anal Data Min. 2017;10(6):363–377. doi:10.1002/sam.11348
22. Lin X, Zhang D. Inference in generalized additive mixed models by using smoothing splines. J R Stat Soc Ser B Stat Methodol. 1999;61:381–400. doi:10.1111/1467-9868.00183
23. Bass DA. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest. 1975;56(4):870–879. doi:10.1172/jci108166
24. Abidi K, Belayachi J, Derras Y, et al. Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med. 2011;37(7):1136–1142. doi:10.1007/s00134-011-2170-z
25. Xie G, Ding F, Han L, Yin D, Lu H, Zhang M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy. 2021;76(2):471–482. doi:10.1111/all.14465
26. Jennings DE. Outliers and residual distributions in logistic regression. J Am Stat Assoc. 1986;81(396):987–990. doi:10.1080/01621459.1986.10478362
27. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. 2017;50:4. doi:10.1183/13993003.00853-2017
28. Merino CA, Martínez FT, Cardemil F, Rodríguez JR. Absolute eosinophils count as a marker of mortality in patients with severe sepsis and septic shock in an intensive care unit. J Crit Care. 2012;27(4):394–399. doi:10.1016/j.jcrc.2011.10.010
29. Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17(12):746–760. doi:10.1038/nri.2017.95
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.