Back to Journals » International Journal of Women's Health » Volume 14
Predictive Value of Serum Cholic Acid and Lithocholic Acid for the Diagnosis in an Intrahepatic Cholestasis of Pregnancy Population with High Levels of Total Bile Acids and the Correlation with Placental Hypoxia-Inducible Factor-1α
Authors Cheng CY, Zeng GY, Wang T, Su YH, Xu FD, Luo H, Zhong HT, Chen XL
Received 21 December 2021
Accepted for publication 21 April 2022
Published 9 May 2022 Volume 2022:14 Pages 687—696
DOI https://doi.org/10.2147/IJWH.S355156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Chu-Yun Cheng,1 Guan-Yin Zeng,1 Tong Wang,1 Yan-Hua Su,1 Feng-Dan Xu,2 Hong Luo,1 Hui-Ting Zhong,1 Xiu-Lan Chen1
1Department of Obstetrics, Dongguan Eighth People’s Hospital (Dongguan Children’s Hospital), Dongguan, Guangdong, 523325, People’s Republic of China; 2Department of Neonatal Clinic, Dongguan Eighth People’s Hospital (Dongguan Children’s Hospital), Dongguan, Guangdong, 523325, People’s Republic of China
Correspondence: Chu-Yun Cheng, Department of Obstetrics, Dongguan Eighth People’s Hospital (Dongguan children’s Hospital), No. 68, West Lake 3rd Road, Shilong Town, Dongguan, Guangdong, 523325, People’s Republic of China, Tel +86 13688988412, Email [email protected]
Objective: This study aimed to investigate the ability of serum cholic acid (CA) and lithocholic acid (LCA) in the diagnosis and perinatal prognosis assessment of intrahepatic cholestasis of pregnancy (ICP), and the relationship between both indicators and hypoxia-inducible factor-1α (HIF-1α).
Methods: Between March 2020 and March 2021, pregnant women with high levels of total bile acid (TBA) in the late pregnancy with TBA ≥ 10 μmol/L and TBA < 10 μmol/L (control group) were included for the retrospective study. Those with TBA ≥ 10 μmol/L were divided into the ICP group and the asymptomatic hypercholanaemia of pregnancy (AHP) group based on ICP symptoms. The comparison of the bile acid profiles, the receiver operating characteristic (ROC) curve analysis, and Pearson correlation analysis were conducted successively.
Results: Nine types of bile acids were significantly higher in ICP and AHP than in the control group, while CA and LCA serum levels in the AHP group were significantly lower than those in the ICP group (P < 0.05). The ROC curve analysis showed that LCA, CA, and LCA+CA were all diagnostic indicators for ICP, and LCA+CA displayed the greatest diagnostic value (area under the curve (AUC), 0.923). Subgroup analysis using the LCA+CA cut-off point (3.28 μmol/L) as the subgroup indicator proved that the incidence of adverse perinatal outcomes and the placental HIF-1α positivity were significantly higher in the high LCA+CA group than in the low LCA+CA group (P < 0.05). Pearson correlation analysis revealed significant positive correlations of HIF-1α expression levels to LCA, CA and LCA+CA (r = 0.473, 0.537, 0.619, respectively. P < 0.05 in all).
Conclusion: This study confirmed that CA and LCA have a predictive diagnostic value for ICP in pregnant women, and the combined evaluation is associated with adverse perinatal outcomes, and LCA+CA positively correlates to placental HIF-1α expression levels.
Keywords: cholic acid, lithocholic acid, intrahepatic cholestasis of pregnancy, hypoxia-inducible factor-1α
Corrigendum for this paper has been published
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a common disorder in middle to late pregnancy, characterized by clinical manifestations, such as pruritus and jaundice.1,2 The incidence is correlated with population and geographic factors.3 Some studies have confirmed that ICP is correlated with genetic factors,4 and the pathogenesis is not fully understood, which may be correlated with abnormal bile acid metabolism due to elevated estrogen levels during pregnancy.5–7 Several national and international studies have demonstrated that ICP has a relatively better prognosis.8 However, with elevated bile acids (BA), ICP may result in severe adverse perinatal outcomes, such as preterm birth, fetal growth retardation, fetal distress, and even intrauterine death.9,10 In recent years, injuries directly caused by BA accumulation have become a hot topic of research in this field. For example, elevated BA levels may lead to preterm delivery by enhancing the biological effects of oxytocin11 and also result in fetal growth restriction or even intrauterine death by inducing chorionic vasoconstriction, oxidative stress, promoting apoptosis of the placenta and fetal hepatocytes, or impairing fetal cardiomyocytes function.12–14 Fetus monitoring in middle to late pregnancy to avoid adverse outcomes is the primary purpose of ICP management. Therefore, it is essential to find highly sensitive and specific monitoring indicators and clarify the pathogenesis of ICP. Studies have shown that high levels of total bile acid (TBA), an important indicator for liver function, are a risk factor for poor prognosis in perinatal infants of women with ICP.15,16 However, in clinical practice, a significant proportion of pregnant women with TBA ≥10 μmol/L still do not have clinical manifestations of ICP, namely asymptomatic hypercholanaemia of pregnancy (AHP), and there are still no relevant monitoring indicators for such groups of pregnant women. In addition, hypoxia-inducible factor-1α (HIF-1α), one of hypoxia-inducible factors, was highly expressed in placentas from ICP patients,17 and might influence the increase of P53 in placentas,18 and could inhibit placental angiogenesis through suppressing the expression of vascular endothelial growth factor, resulting in adverse pregnant outcomes.19 HIF-1α was also an essential indicator to evaluate the severity and prognosis of ICP.20
Herein, this study aimed to investigate the differences in different BA levels in women with AHP and ICP during pregnancy and probe into the changes in HIF-1α expression in the placenta of pregnant women, and to assess the significance of different BA levels on perinatal prognosis and correlation with HIF-1α. And 103 pregnant women with TBA ≥10 μmol/L in the the late pregnancy and 70 healthy pregnant women of the same gestational age admitted to our hospital between March 2020 and March 2021 were analyzed.
Materials and Methods
General Data
According to the previous study about the relationship between ICP and BA profiles,21 total sample size of 51 were obtained from the 3 groups whose means were to be compared given the 20% dropout rate using the PASS 15 Software (NCSS, LLC., USA), and achieved 90% power to detect a difference of at least 7.60 using the Tukey-Kramer (Pairwise) multiple comparison test at a 0.05 significance level. The common standard deviation within a group was assumed to be 0.80. A total of 103 pregnant women with TBA ≥10 μmol/L in middle and late pregnancy and 70 healthy pregnant women with TBA <10 μmol/L of the same gestational age were selected for the present retrospective analysis from March 2020 to March 2021. According to the existence of ICP symptoms, pregnant women with TBA ≥10 μmol/L were divided into the ICP group, with 42 cases, and the AHP group, with 61 cases, and 70 pregnant women of the same gestational age with normal physical examinations were enrolled as the control group. The differences in age, gestational age, body mass, cases of primipara/pluripara, times of pregnancies, and parity were not statistically significant among the three groups (P > 0.05), as shown in Table 1. The inclusion criteria were as follows: ① those with TBA <10 μmol/L in the late pregnancy were enrolled in the control group. Those with TBA ≥10 μmol/L in the late pregnancy were enrolled in the ICP and AHP groups, and ICP was diagnosed based on the criteria of the 9th edition of Chinese Obstetrics and Gynecology as fasting serum TBA ≥ 10 μmol/L with pruritus after 14 weeks of pregnancy.2 ② Enrolled pregnant women with singleton intrauterine fetuses and in the head position. ③ Other diseases causing TBA abnormalities were excluded, such as acute and chronic hepatitis, hepatitis B carriers, alcoholic hepatitis, extrahepatic bile duct obstruction disease, liver cirrhosis and obstructive jaundice. ④ Those who had established prenatal records and delivered at our hospital. The exclusion criteria were as follows: ① a history of gallbladder and liver system disease; ② other causes of itchy skin, such as skin diseases, systemic diseases and mental illness; ③ other pregnancy comorbidities and complications, such as gestational hypertension and anemia; ④ cardiac and renal dysfunction; ⑤ mental illness, communication difficulties, or inability to cooperate with the study. The Ethics Committee of the hospital approved the present study, and all subjects agreed to be enrolled and signed informed consent. The enrolled patients were monitored dynamically for TBA, and the therapeutic dose of ursodeoxycholic acid (UDCA) was administered at a daily dose of 10 c/kg orally when the fasting TBA was ≥15 μmol/L, and the treatment continued until termination of pregnancy.
 |
Table 1 Comparison of the General Characteristics Among the Three Groups |
Perinatal outcomes were monitored according to the assessment criteria of the 9th edition of Chinese Obstetrics and Gynecology,2 including: preterm birth, fetal distress, amniotic fluid and fecal contamination, neonatal asphyxia, and perinatal death. Premature delivery was those delivered beyond 28 weeks of gestation but less than 37 weeks. Intrauterine distress was a combination of symptoms in which the fetus endangers for health and life in utero due to acute or chronic hypoxia which were based on a comprehensive assessment of abnormal fetal heart rate at delivery, amniotic fluid and fetal contamination, abnormal fetal movement, and acidosis of fetal scalp blood through blood gas analysis. Amniotic fluid and fecal contamination referred to the fetus excreting meconium in utero and the simple cloudy amniotic fluid without abnormal fetal heart rate and abnormal fetal movement. Neonatal asphyxia: ①Apgar score ≤7 at 5 minutes and still no effective respiration was established; ② umbilical artery blood pH <7.15; ③ exclude other etiologies causing low Apgar score; ④ have prenatal high-risk factors that may lead to asphyxia; where ①-③ were necessary conditions and ④ was reference indicators.
Detection of the Serum Bile Acid Profile
Fasting blood specimens were collected from all study subjects using negative pressure ethylenediaminetetraacetic acid (EDTA) anticoagulation tubes, and 4 mL of peripheral anticoagulated venous blood was collected. Blood specimens were centrifuged at 2,000 r/min for 10 min, and the supernatant was taken and stored at –80°C. An ultra-performance liquid chromatography-tandem mass spectrometer ACQUITY UPLC I-Class IVD/Xevo TQ-S IVD System (Waters) was used to detect BA profiles, including bile acid (BA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA), UDCA, lithodeoxycholic acid (LCA), glycocholic acid (GCA), glycodeoxycholic acid (GDCA), glycylchenodeoxycholic acid (GCDCA), and glycinolithocholic acid (GLCA).
Detection of HIF-1α by Immunohistochemical Staining and Results Determination
Three pieces of 2.5 cm × 2.5 cm placental tissues were taken under aseptic conditions within 30 min after delivery of the study subjects, avoiding the calcified areas as well as the necrotic areas as much as possible. The tissue was fixed with 10% neutral formaldehyde, and paraffin-embedded sections were repaired. After dewaxing, hydration, and antibody repair, immunohistochemical staining was conducted. With serum blocking, HIF-1α antibody (Boster Biological Technology, Ltd.) was added and incubated overnight at 4°C. It was then washed with PBS three times × 5 min, and a secondary antibody was added (Guangzhou Anbipin Pharmaceutical Technology Co., Ltd.) and incubated at 37°C for 1 h. It was again washed with PBS three times × 5 min, and color development solution (Guangzhou Anbipin Pharmaceutical Technology Co., Ltd.) was added to develop color, seal the film, and observe under the microscope.
HIF-1α is mainly expressed in the cytoplasm and nucleus, and the positive coloring was yellow, brownish-yellow, and tan. According to the coloring degree, the score of staining degree was divided into the following: 0 for colorless, 1 for light yellow, 2 for brownish-yellow, 3 for brown. Based on the percentage of positive cells, the score was divided into 1 point for ≤10%, 2 points for 11–50%, 3 points for 51–75%, and 4 points for >75%. HIF-1α expression score = the score of staining degree × the score of percentage of positive cells, with a total score of 0~12. A total score of <3 was negative, indicated by “–,” a total score of 3–5 was weakly positive, indicated by “+,” a total score of 6–9 was positive, indicated by “++,” and a total score of >9 was strongly positive, indicated by “+++.”
Statistical Processing
The measurement data were expressed as mean ± standard deviation (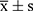 ), and one-way ANOVA was used for comparison of the differences among more than two groups, while the Least Significant Difference-t method was used for comparison of differences between two groups. The countable data were expressed as percentages (%), the chi-square test was used for comparison of the differences between groups, and the rank-sum test was adopted for comparison of perinatal prognosis. Pearson’s test was used for correlation analysis. The SPSS 17.0 software was used for statistical analysis, and P < 0.05 was considered statistically significant.
), and one-way ANOVA was used for comparison of the differences among more than two groups, while the Least Significant Difference-t method was used for comparison of differences between two groups. The countable data were expressed as percentages (%), the chi-square test was used for comparison of the differences between groups, and the rank-sum test was adopted for comparison of perinatal prognosis. Pearson’s test was used for correlation analysis. The SPSS 17.0 software was used for statistical analysis, and P < 0.05 was considered statistically significant.
Results
Comparison of Bile Acid Profiles Among Three Groups of Women in the Late Pregnancy
The levels of TBA and nine BA components were compared among the three groups, and it was found that the nine types of BA components were significantly higher in the ICP and AHP groups than in the control group (P < 0.05). The levels of CA and LCA in the AHP group were significantly lower than those in the ICP group, and the differences were statistically significant (P < 0.05). While differences in the other BA indicators were not statistically significant between the AHP and ICP groups (P > 0.05), as illustrated in Table 2.
 |
Table 2 Comparison of the Bile Acid Profiles Among the Three Groups of Study Object ( |
Comparison of the Diagnostic Predictive Efficacy of CA, LCA, and LCA+CA in Patients with ICP with TBA ≥10 μmol/L
The receiver operating characteristic (ROC) curve was adopted to analyze the predictive ability of each indicator for the study subjects, and it was revealed that the levels of CA, LCA, and LCA+CA were statistically significant (P < 0.05) in predicting ICP for those with TBA ≥10 μmol/L, with the largest area under the curve (AUC) of 0.923 for LCA+CA and a cut-off point of 3.28 μmol/L. This was followed by LCA (0.756) and CA (0.716), as shown in Figure 1.
 |
Figure 1 The ROC curves of CA, LCA, and CA+LCA for the prediction of ICP with TBA ≥10 μmol/L. |
Comparison of the Incidence of Adverse Perinatal Outcomes Between Patients with Different Levels of LCA+CA
The study population was subdivided into the high LCA+CA and low LCA+CA subgroups, based on the LCA+CA cut-off point in ROC to study the perinatal outcomes of pregnant women in both groups. The results showed that the incidences of perinatal preterm delivery, intrauterine distress, amniotic fluid and fecal contamination, and adverse outcomes were significantly higher in the high LCA+CA subgroup than in the low LCA+CA subgroup, and the differences were statistically significant (P < 0.05), as demonstrated in Table 3.
 |
Table 3 Differences in the Adverse Perinatal Outcomes Between the High LCA+CA Group and Low LCA+CA Group [Cases (%)] |
Comparison of Placental HIF-1α Expression Levels in Pregnant Women with Different Levels of LCA+CA
The placental HIF-1α expression levels in the pregnant women in the high LCA+CA and low LCA+CA subgroups were investigated by immunohistochemical staining (Figure 2). The results showed that the HIF-1α positivity in the high LCA+CA subgroup was 98.3%, of which 14 cases (24.1%) were weakly positive (+), and 43 cases (74.1%) were positive–strongly positive (++, ++++). The HIF-1α positivity in the low LCA+CA subgroup was 86.7%, of which 24 cases (53.4%) were weakly positive (+), and 15 cases (33.3%) were positive–strongly positive (++, ++++). The HIF-1α positivity was significantly higher in the high LCA+CA subgroup than in the low LCA+CA subgroup, and the difference was statistically significant (P < 0.05), as shown in Table 4.
 |
Table 4 Differences in the Protein Expression of Placental HIF-1α in Pregnant Women Between the High LCA+CA Group and Low LCA+CA Group [Cases (%)] |
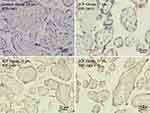 |
Figure 2 Detection of placental HIF-1α by immunohistochemical staining. Scale bar = 50 μm or 25 μm. |
Correlation Analysis of LCA, CA, and LCA+CA with HIF-1α Expression Levels
Pearson’s correlation analysis revealed that LCA (r = 0.473) and CA (r = 0.537) were moderately correlated with HIF-1α, and LCA+CA was strongly correlated with HIF-1α (r = 0.619) with r2 = 0.383. HIF-1α expression levels were positively correlated with LCA, CA, and LCA+CA, and the differences were all statistically significant (P < 0.05 in all).
Discussion
ICP is a liver dysfunction specific to pregnancy, occurring mainly between 16 and 35 weeks of gestation. Currently, ICP has become a common complication of pregnancy, which is closely correlated with the lack of awareness of the disease among pregnant women and the failure to make timely disease predictions, which in turn leads to worsening of ICP.22 It has been shown that there is a correlation between ICP and maternal TBA levels and that high levels of TBA are the leading cause of jaundice and pruritus in patients with ICP, and also leads to reduced fetal blood supply inducing intrauterine distress and poor pregnancy outcome, making TBA levels an essential indicator of the development of ICP.23 However, the specificity of TBA as a specific indicator reflecting liver function is poor. It is noteworthy that a considerable number of pregnant women with elevated TBA (TBA ≥10 μmol/L) in clinical practice do not have typical symptoms of ICP, and no fetal dysplasia is detected. Therefore, it is also of great interest to evaluate how to predict the risk of ICP development in this population. There are many categories of BAs, mainly including the hydrophilic BAs and hydrophobic BAs, among which hydrophobic BAs have the characteristic of destroying cell membrane structure, so hydrophobic BAs have high cytotoxicity. CA and LCA may be the subgroups of hydrophobic BAs that play an important role.24
In the present study, pregnant women with TBA ≥10 μmol/L in the late pregnancy were classified into the ICP and AHP groups, and the indicators that could predict and evaluate ICP in the presence of high levels of TBA were explored by comparing the two indicators with healthy pregnant women. First, the BA profiles, including nine types of BAs in the three groups, were detected, and it was found that there were significantly higher levels of CA and LCA in the ICP group, suggesting that CA and LCA might serve as risk predictors of ICP in pregnant women with high levels of TBA. In patients with ICP, elevated estrogen and other factors can lead to increased synthesis of CA. Studies have shown that CA levels are significantly elevated in patients with ICP, up to 10 times higher than in the controls and that elevated serum BAs are dominated by CA.25 Duan et al26 treated patients with ICP by UDCA and found that the reduction of CA might be correlated with improved perinatal outcome in ICP. LCA is produced by 7α-dehydroxylation of bound CDCA, and although the level in vivo is low, LCA is the most hydrophobic and, therefore, more cytotoxic than other BAs.27 However, in the present study, the elevation in the levels of CA and LCA was not so obvious, which might be correlated with the comprehensive investigation of those with ICP+AHP, together with the geographical and demographic factors. Du et al found that LCA could induce upregulation of placental tumor necrosis factor-α and apoptosis of syncytial trophoblast in ICP,28,29 suggesting a possible pathological mechanism for poor perinatal outcome in ICP. Li et al30 found that LCA levels were significantly elevated in patients with ICP and negatively correlated with the LCA detoxifying enzyme sulfotransferase 2A1, suggesting that the accumulation and abnormal metabolism of LCA might be an important cause of development in ICP. Chao et al31 revealed that the effector protein levels in the mTOR signaling pathway were elevated in the placenta of pregnant women with ICP, and pretreatment of LCA to human placental trophoblast cell lines could further activate this pathway, suggesting that LCA might contribute to the onset and development of ICP by activating mTOR signaling. In addition, the possibility of CA and LCA as risk predictors for ICP in pregnant women with high levels of TBA was explored in the present study, and it was found that the combined evaluation had the best efficacy. By observing the perinatal outcomes of patients with different levels of LCA+CA, it was found that LCA+CA could be used as an indicator to evaluate the occurrence of adverse perinatal outcomes. As a precursor substance of LCA, the combined assessment of the CA and LCA could represent the overall level of hydrophobic BAs to a certain extent. Yu et al32 suggested that CA might be of significance in distinguishing ICP from other liver function abnormalities and that elevated LCA might be correlated with poor prognosis of ICP. Another study also found a correlation between LCA and the severity of ICP.33
HIF-1α is a hypoxia-inducible factor, and the expression level can respond to the degree of hypoxia in tissues and is one of the initiators of hypoxic reperfusion.34 HIF-1α can accelerate placental vascular endothelial cell injury and induce micro thrombosis. In addition, HIF-1α can also affect BA metabolism in hepatocytes, which is an important factor in the deterioration of ICP. A study has shown that HIF-1α is also an essential indicator of the severity and prognosis of ICP.20 In the present study, it was found that there existed a high positivity of HIF-1α in pregnant women with high levels of LCA+CA, and by correlation analysis, it was suggested that CA and LCA were positively correlated with HIF-1α, and the highest correlation was found between the combination of the two indicators and HIF-1α. Yang et al35 found that placental HIF-1α levels were significantly elevated in patients with ICP and were associated with TBA. It has also been shown that S-adenosylmethionine combined with cholestatic drugs significantly downregulated the expression of HIF-1α in the placenta of patients with ICP with improvement in both symptoms and prognosis,36 which was consistent with the results of the present study whose result proved that LCA+CA was strongly correlated with HIF-1α. Therefore, the present study hypothesized that the accumulation of LCA and CA in pregnant women could involve the placenta and increase the expression level of HIF-1α, which could induce a series of hypoxic manifestations, such as vascular endothelial cell injury, and eventually lead to adverse outcomes, such as fetal distress. However, this hypothesis still needs to be confirmed by fundamental research.
However, there were still some limitations in the present study. First, this was a single-center study. In addition, the sample size was small because it was difficult to obtain the enrolled study subjects. The number of enrolled subjects should be increased with the conduction of multivariate correlation prediction analysis to provide more reliable data from the clinical trials.
In conclusion, the present study found that CA and LCA serum levels were higher in the ICP group than in the control and AHP group, and the ROC curve analysis showed that LCA, CA, and LCA+CA were all diagnostic indicators for ICP, and LCA+CA displayed the greatest diagnostic value. Subgroup analysis using the LCA+CA cut-off point (3.28 μmol/L) as the subgroup indicator proved that the incidence of adverse perinatal outcomes and the placental HIF-1α positivity were significantly higher in the high LCA+CA group than in the low LCA+CA group. Pearson correlation analysis revealed significant positive correlations of HIF-1α expression levels to LCA, CA and LCA+CA. In general, LCA and CA may be used as predictors for ICP in pregnant women with high levels of TBA, and the elevated levels suggested an increased risk of poor perinatal prognosis.
Data Sharing Statement
We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Dongguan Eighth People’s Hospital. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for Publication
All participants signed a document of informed consent.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
Dongguan Social Science and Technology Development (Key) Project in 2019. (Project No. 201950715028172).
Disclosure
The authors declare that they have no competing interests.
References
1. Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124(1):120–133. doi:10.1097/AOG.0000000000000346
2. Xing X, Wenli G, Tao D. Obstetrics and Gynecology.
3. Lee RH, Goodwin TM, Greenspoon J, Incerpi M. The prevalence of intrahepatic cholestasis of pregnancy in a primarily Latina Los Angeles population. J Perinatol. 2006;26(9):527–532. doi:10.1038/sj.jp.7211545
4. Reyes H, Ribalta J, González-Cerón M. Idiopathic cholestasis of pregnancy in a large kindred. Gut. 1976;17(9):709–713. doi:10.1136/gut.17.9.709
5. Reyes H, Simon FR. Intrahepatic cholestasis of pregnancy: an estrogen-related disease. Semin Liver Dis. 1993;13(3):289–301. doi:10.1055/s-2007-1007357
6. Dixon PH, Williamson C. The pathophysiology of intrahepatic cholestasis of pregnancy. Clin Res Hepatol Gastroenterol. 2016;40(2):141–153. doi:10.1016/j.clinre.2015.12.008
7. Torchinsky A, Markert UR, Toder V. TNF-alpha-mediated stress-induced early pregnancy loss: a possible role of leukemia inhibitory factor. Chem Immunol Allergy. 2005;89:62–71. doi:10.1159/000087913
8. Ovadia C, Seed PT, Sklavounos A, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393(10174):899–909. doi:10.1016/S0140-6736(18)31877-4
9. Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467–474. doi:10.1002/hep.20336
10. Tao JY, Liu SQ, Fang LJ. Analysis of total bile acid level and pregnancy outcome in patients with intrahepatic cholestasis of pregnancy. Matern Child Health Care China. 2019;34(18):4152–4154.
11. Germain AM, Kato S, Carvajal JA, et al. Bile acids increase response and expression of human myometrial oxytocin receptor. Am J Obstet Gynecol. 2003;189:577–582. doi:10.1067/S0002-9378(03)00545-3
12. Sepulveda WH, Gonzalez C, Cruz MA, Rudolph MI. Vasoconstrictive effect of bile acids on isolated human placental chorionic veins. Eur J Obstet Gynecol Reprod Biol. 1991;42(3):211–215. doi:10.1016/0028-2243(91)90222-7
13. Perez MJ, Macias RI, Duran C, Monte MJ, Gonzalez Buitrago JM, Marin JJ. Oxidative stress and apoptosis in fetal rat liver induced by maternal cholestasis. Protective effect of ursodeoxycholic acid . J Hepatol. 2005;43:324–332.
14. Williamson C, Gorelik J, Eaton BM, et al. The bile acid taurocholate impairs rat cardiomyocyte function: a proposed mechanism for intrauterine fetal death in obstetric cholestasis. Clin Sci. 2001;100:363–369. doi:10.1042/CS20000164
15. Herrera CA, Manuck TA, Stoddard GJ, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31(14):1913–1920. doi:10.1080/14767058.2017.1332036
16. Manzotti C, Casazza G, Stimac T, et al. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. Cochrane Database Syst Rev. 2019;7(7):CD012546. doi:10.1002/14651858
17. Wei W, Hu YY. Expression of hypoxia-regulated genes and glycometabolic genes in placenta from patients with intrahepatic cholestasis of pregnancy. Placenta. 2014;35(9):732–736. doi:10.1016/j.placenta.2014.06.372
18. Hu YY, Wang XD, Liu SY. The relationship between P53 and hypoxia-inducible transcription factor-1 alpha in the placenta of patient with intrahepatic cholestasis of pregnancy under acute hypoxic condition. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37(6):901–942.
19. Zeng YL, Xue S, Bi XL, et al. Expression of hypoxia-inducible factor-1 in mice infected with Toxoplasma gondii during pregnancy. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2021;33(6):615–622. doi:10.16250/j.32.1374.2021122
20. Bai YF.Hypoxia inducible factor HIF-1α in placental tissue of intrahepatic cholestasis of pregnancy in Xining Area Expression and its significance. Matern Child Health Care China. 2015;30(4):611–613.
21. Castaño G, Lucangioli S, Sookoian S, et al. Bile acid profiles by capillary electrophoresis in intrahepatic cholestasis of pregnancy. Clin Sci. 2006;110(4):459–465. doi:10.1042/CS20050302
22. Marschall HU. Management of intrahepatic cholestasis of pregnancy. Expert Rev Gastroenterol Hepatol. 2015;9(10):1273–1279. doi:10.1586/17474124.2015.1083857
23. Yan Y, Wang Q. Expression and significance of plasma CG and TBA in patients with intrahepatic cholestasis of pregnancy. J Clin Transfus Lab Med. 2019;21(6):592–595. doi:10.3969/j.issn.1671-2587.2019.06.010
24. Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol. 2017;313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
25. Li XP, Ouyang KQ, Cai SX. Characteristics of intrahepatic cholestasis of pregnancy (ICP). Matern Child Health Care China. 2002;17(11):703–705. doi:10.3969/j.issn.1001-4411.2002.11.028
26. Duan CY, Chen QS, Wang LM, et al. Efficacy of ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy. J Pract Hepatol. 2019;22(6):864–867. doi:10.3969/j.issn.1672-5069.2019.06.022
27. Masuno H, Kazui Y, Tanatani A, et al. Development of novel lithocholic acid derivatives as vitamin D receptor agonists. Bioorg Med Chem. 2019;27(16):3674–3681. doi:10.1016/j.bmc.2019.07.003
28. Qiaoling D, Zhang Y, Pan Y, et al. Lithocholic acid-induced placental tumor necrosis factor-α upregulation and syncytiotrophoblast cell apoptosis in intrahepatic cholestasis of pregnancy. Hepatol Res. 2014;44(5):532–541. doi:10.1111/hepr.12150
29. Carpentier PA, Dingman AL, Palmer TD. Placental TNFalpha signaling in illness-induced complications of pregnancy. Am J Pathol. 2011;178(6):2802–2810. doi:10.1016/j.ajpath.2011.02.042
30. Li M, Wei W. Expression of sulfate transferase 2A1 and its correlation with total bile acid, serum bilirubin, lithocholic acid and alanine aminotransferase in patients with intrahepatic cholestasis of pregnancy. Clin J Med Officer. 2019;47(8):835–836,839. doi:10.16680/j.1671-3826.2019.08.25
31. Chao S, Xiaojun L, Haizhen W, et al. Lithocholic acid activates mTOR signaling inducing endoplasmic reticulum stress in placenta during intrahepatic cholestasis of pregnancy. Life Sci. 2019;218:300–307. doi:10.1016/j.lfs.2018.12.050
32. Yu LM, Shi XY, Chen YM, et al. Detection of seven bile acids in intrahepatic cholestasis of pregnancy by high performance liquid chromatography tandem mass spectrometry and its clinical significance. Chin J Gen Pract. 2020;18(7):1153–1156. doi:10.16766/j.cnki.issn.1674-4152.001453
33. Wang XL, Shao Y. Detection of serum cholic acid in pregnant women with intrahepatic cholestasis of pregnancy by high performance liquid chromatography tandem mass spectrometry and its clinical significance. J Pract Obs Gynaecol. 2011;27(3):214–217.
34. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70(5):1469–1480. doi:10.1124/mol.106.027029
35. Yang L, Li HM, Zhu Y. Changes and significance of liver function and HSP70 and HIF-1α expression in placental tissues of patients with intrahepatic cholestasis of pregnancy. Chin J Reprod Health. 2019;30(4):367–370.
36. Ni HH, Xiao X, Li J, et al. Effect of S-adenosylmethionine combined with cholagogic drugs on intrahepatic cholestasis of pregnancy. China J Mod Med. 2018;28(26):100–103. doi:10.3969/j.issn.1005-8982.2018.26.019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

